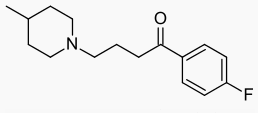
Melperone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Buronil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% (IM), 54% (Oral via syrup), 65% (Oral, tablet)[1] |
| Protein binding | 50% |
| Metabolism | Hepatic |
| Elimination half-life | 3–4 hours (oral)[1] 6 hours (IM) |
| Excretion | Renal, 70% as metabolites, 5.5-10.4% as unchanged drug[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H22FNO |
| Molar mass | 263.35 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Melperone |
|
Articles |
|---|
|
Most recent articles on Melperone |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Melperone at Clinical Trials.gov Clinical Trials on Melperone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Melperone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Melperone Discussion groups on Melperone Directions to Hospitals Treating Melperone Risk calculators and risk factors for Melperone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Melperone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Melperone (Bunil (PT), Buronil (AT, BE, CZ, DK, FL†, NL†, NO†, SE), Eunerpan (DE))[3] is an atypical antipsychotic of the butyrophenone chemical class, making it structurally related to the typical antipsychotic haloperidol. It first entered clinical use in 1960s.[4]
Marketing and indications
It has been tried in treatment-resistant cases of schizophrenia with some (albeit limited) success.[4][5][6][7] It has also been reported effective in the treatment of L-DOPA and other forms of psychosis in Parkinson's disease[8] (although a multicentre, double-blind, placebo-controlled study conducted in 2012 failed to support these findings[9]). It is also known to possess anxiolytic properties.[10] It is marketed in the following countries:[3]
Adverse effects
Melperone is reported to produce significantly less weight gain than clozapine and approximately as much weight gain as typical antipsychotics.[11] It is also purported to produce around as much prolactin secretion as clozapine (which is virtually nill).[12] It is also purported to produce sedative effects[13] and QT interval prolongation.[14] It is also known to produce less extrapyramidal side effects than the first-generation (typical) antipsychotic, thiothixene.[15] It can also produce (usually relatively mild) dry mouth.[16]
- Constipation
- Diarrhoea
- Nausea
- Vomiting
- Appetite loss
- Hypersalivation (drooling)
- Extrapyramidal side effects*
- Insomnia
- Agitation
- Headache
- Dizziness
- Fatigue
- Miosis
- Mydriasis
- Blurred vision
- Elevated liver enzymes (esp. ALT and GGTP)
* tremor, dystonia, hypokinesis, akathisia, dyskinesias
- Tardive dyskinesia
- Neuroleptic malignant syndrome
- Blood dyscrasias (pancytopaenia, agranulocytosis, leukopaenia, thrombocytopaenia, etc.)
- Seizures (probably rare/uncommon)
- Increased intraocular pressure
- Intrahepatic cholestasis (probably rare)
- Orthostatic hypotension (probably common)
- Arrhythmias
- Rash
- Hyperprolactinaemia**
- Weight gain
- Increased appetite
** which can lead to galactorrhoea, gynecomastia, etc.
Interactions
Melperone is reported to be a CYP2D6 inhibitor.[20][21][22]
Pharmacology
Melperone binds to the dopamine D2 receptor, just like all other clinically-utilised antipsychotics, but it does so with a very low affinity and hence may be liable to rapidly dissociate from the D2 receptor hence potentially giving it the profile of an atypical antipsychotic.[23]
| Receptor | Ki [nM][24] |
|---|---|
| 5-HT1A | 2,200 |
| 5-HT1D | 3,400 |
| 5-HT2A | 230 |
| 5-HT2C | 2,100 |
| 5-HT6 | 1,254 |
| 5-HT7 | 578 |
| α1 | 180 |
| α2 | 150 |
| M1 | >10,000 |
| M2 | 2,400 |
| M3 | >10,000 |
| M4 | 4,400 |
| M5 | >10,000 |
| D2 | 194 |
| D3 | 8.95 |
| D4 | 555 |
| H1 | 580 |
References
- ↑ 1.0 1.1 1.2 Borgström, L; Larsson, H; Molander, L (1982). "Pharmacokinetics of parenteral and oral melperone in man". European Journal of Clinical Pharmacology. 23 (2): 173–176. doi:10.1007/BF00545974. PMID 7140807.
- ↑ Product Information: Eunerpan(R), Melperonhydrochlorid. Knoll Deutschland GmbH, Ludwigshafen, 1995.
- ↑ 3.0 3.1 Melperone Hydrochloride. Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 30 January 2013. Retrieved 3 November 2013.
- ↑ 4.0 4.1 Röhricht, F; Gadhia, S; Alam, R; Willis, M (2012). "Auditing Clinical Outcomes after Introducing Off-Licence Prescribing of Atypical Antipsychotic Melperone for Patients with Treatment Refractory Schizophrenia". Scientific World Journal. 2012: 512047. doi:10.1100/2012/512047. PMC 3330679. PMID 22566771.
- ↑ Whiskey, E (February 2011). "Melperone in Treatment-Refractory Schizophrenia: A Case Series". Therapeutic Advances in Psychopharmacology. 1 (1): 19–23. doi:10.1177/2045125311399800. PMC 3736899. PMID 23983923. Unknown parameter
|coauthors=ignored (help) - ↑ Meltzer, HY; Sumiyoshi, T; Jayathilake, K (December 2001). "Melperone in the treatment of neuroleptic-resistant schizophrenia". Psychiatry Research. 105 (3): 201–209. doi:10.1016/s0165-1781(01)00346-8. PMID 11814539.
- ↑ Sumiyoshi, T; Meltzer, HY; Jayathilake, K (2004). "Melperone, an atypical antipsychotic drug, in the treatment of schizophrenia: dose-response analysis on effectiveness and tolerability, and efficacy for treatment-resistant schizophrenia and cognitive function". International Clinical Psychopharmacology. 19 (3): 184. doi:10.1097/00004850-200405000-00039.
- ↑ Barbato L, Monge A, Stocchi F, Nordera G. Melperone in the treatment of iatrogenic psychosis in Parkinson’s disease. Funct Neurol. 1996 Aug;11(4):201–7.
- ↑ Friedman, JH (May 2012). "Melperone is ineffective in treating Parkinson's disease psychosis". Movement Disorders. 27 (6): 803–804. doi:10.1002/mds.24942. PMID 22362330.
- ↑ Pöldinger, WJ (1984). "Melperone in low doses in anxious neurotic patients. A double-blind placebo-controlled clinical study". Neuropsychobiology. 11 (3): 181–186. doi:10.1159/000118074. PMID 6147789.
- ↑ Bobo, WV; Jayathilake, K; Lee, MA; Meltzer HY (April 2010). "Changes in weight and body mass index during treatment with melperone, clozapine and typical neuroleptics". Psychiatry Research. 176 (2–3): 114–119. doi:10.1016/j.psychres.2009.03.026. PMID 20199813.
- ↑ Bobo, WV; Jayathilake, K; Lee, MA; Meltzer, HY (July 2009). "Melperone, an aytpical antipsychotic drug with clozapine-like effect on plasma prolactin: contrast with typical neuroleptics". Human Psychopharmacology: Clinical and Experimental. 24 (5): 415–422. doi:10.1002/hup.1036. PMID 19551763.
- ↑ Molander, L; Borgström, L (1983). "Sedative effects and prolactin response to single oral doses of melperone". Psychopharmacology. 79 (2–3): 142–147. doi:10.1007/bf00427801. PMID 6133301.
- ↑ Hui, WK; Mitchell, LB; Kavanagh, KM; Gillis, AM; Wyse, DG; Manyari, DE; Duff, HJ (January 1990). "Melperone: electrophysiologic and antiarrhythmic activity in humans". Journal of Cardiovascular Pharmacology. 15 (1): 144–149. doi:10.1097/00005344-199001000-00023. PMID 1688972.
- ↑ Bjerkenstedt, L (1989). "Melperone in the treatment of schizophrenia". Acta Psychiatrica Scandinavica Supplementum. 352: 35–39. PMID 2479227.
- ↑ Molander, L; Birkhed, D (1981). "Effect of single oral doses of various neuroleptic drugs on salivary secretion rate, pH, and buffer capacity in healthy subjects". Psychopharmacology. 75 (2): 114–118. doi:10.1007/bf00432171. PMID 6119724.
- ↑ 17.0 17.1 17.2 "Product Information: Eunerpan(R), Melperonhydrochlorid". Knoll Deutschland GmbH, Ludwigshafen. 1995. Missing or empty
|url=(help) - ↑ 18.0 18.1 18.2 Kirkegaard, A; Kirkegaard, G; Geismar, L (1981). "Additional studies on side effects of melperone in long-term therapy for 1 to 15 years in psychiatric patients". Arzneimittel-Forschung. 31 (4): 737–740. PMID 6113835.
- ↑ 19.0 19.1 19.2 Christensen, I; Geismar, L; Kirkegaard, A; Kirkegaard, G (May 1986). "Additional studies on side effects of melperone in long-term therapy for 1-20 years in psychiatric patients". Arzneimittel-Forschung. 36 (5): 855–860. PMID 2873821.
- ↑ Gahr, M; Gastl, R; Kölle, MA; Schönfeldt-Lecuona, C; Freudenmann, RW (2012). "Successful treatment of schizophrenia with melperone augmentation in a patient with phenotypic CYP2D6 ultrarapid metabolization: a case report". Journal of Medical Case Reports. 6 (1): 49. doi:10.1186/1752-1947-6-49. PMC 3298719. PMID 22309430.
- ↑ Köhnke, MD; Lutz, U; Wiatr, G; Schwärzler, F; Weller, B; Schott, K; Buchkremer, G (April 2006). "Cytochrome P450 2D6 dependent metabolization of risperidone is inhibited by melperone". European Journal of Clinical Pharmacology. 62 (4): 333–334. doi:10.1007/s00228-006-0098-y. PMID 16534635.
- ↑ Grözinger, M; Dragicevic, A; Hiemke, C; Shams, M; Müller, MJ; Härtter, S (January 2003). "Melperone is an inhibitor of the CYP2D6 catalyzed O-demethylation of venlafaxine". Pharmacopsychiatry. 36 (1): 3–6. doi:10.1055/s-2003-38084. PMID 12649767.
- ↑ Seeman, P (January 2004). "Atypical Antipsychotics: Mechanism of Action" (PDF). FOCUS: The Journal of Lifelong Learning in Psychiatry. 2 (1): 48–58.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 2013-10-14.
External links
- PubChem Substance
- Clinical trial number NCT00125138 at ClinicalTrials.gov
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages using web citations with no URL
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Pages using div col with unknown parameters
- Drug
- Organofluorides
- Aromatic ketones
- Piperidines
- Butyrophenone antipsychotics
- Dopamine antagonists
- Serotonin antagonists