Lobeline
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
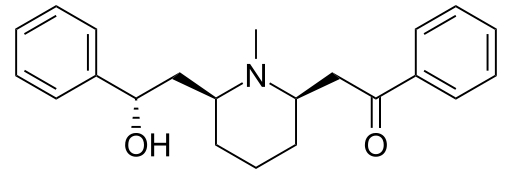
| Formula | C22H27NO2 |
| Molar mass | 337.455 |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Lobeline |
|
Articles |
|---|
|
Most recent articles on Lobeline |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Lobeline at Clinical Trials.gov Clinical Trials on Lobeline at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Lobeline
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Lobeline Risk calculators and risk factors for Lobeline
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Lobeline |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Lobeline is an alkaloid found in "Indian tobacco" (Lobelia inflata), "Devil's tobacco" (Lobelia tupa), "cardinal flower" (Lobelia cardinalis), "great lobelia" (Lobelia siphilitica), and Hippobroma longiflora. Additionally, it is also found in Lobelia chinensis. In its pure form it is a white amorphous powder which is freely soluble in water.
Potential uses
Lobeline has been studied for its potential use as a smoking cessation aid,[1][2][3] and may have application in the treatment of other drug addictions such as addiction to amphetamines,[4][5] cocaine,[6] or alcohol.[7]
Pharmacology
Lobeline has multiple mechanisms of action, acting as a VMAT2 ligand,[8][9][10] which stimulates dopamine release to a moderate extent when administered alone, but reduces the dopamine release caused by methamphetamine.[11][12] It also inhibits the reuptake of dopamine and serotonin,[13] and acts as a mixed agonist–antagonist at nicotinic acetylcholine receptors[14][15] to which it binds at the subunit interfaces of the extracellular domain.[16] It is also an antagonist at μ-opioid receptors.[17]
References
- ↑ Stead LF, Hughes JR; Hughes (2012). "Lobeline for smoking cessation". Cochrane Database Syst Rev. 2: CD000124. doi:10.1002/14651858.CD000124.pub2. PMID 22336780.
- ↑ Marlow, S. P.; Stoller, J. K. (2003). "Smoking cessation". Respiratory care. 48 (12): 1238–54, discussion 1254–6. PMID 14651764.
- ↑ Buchhalter, A. R.; Fant, R. V.; Henningfield, J. E. (2008). "Novel pharmacological approaches for treating tobacco dependence and withdrawal: Current status". Drugs. 68 (8): 1067–88. PMID 18484799.
- ↑ Neugebauer, N. M.; Harrod, S. B.; Stairs, D. J.; Crooks, P. A.; Dwoskin, L. P.; Bardo, M. T. (2007). "Lobelane decreases methamphetamine self-administration in rats". European Journal of Pharmacology. 571 (1): 33–8. doi:10.1016/j.ejphar.2007.06.003. PMC 2104779. PMID 17612524.
- ↑ Eyerman, D. J.; Yamamoto, B. K. (2005). "Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum". Journal of Pharmacology and Experimental Therapeutics. 312 (1): 160–9. doi:10.1124/jpet.104.072264. PMID 15331654.
- ↑ Polston, J. E.; Cunningham, C. S.; Rodvelt, K. R.; Miller, D. K. (2006). "Lobeline augments and inhibits cocaine-induced hyperactivity in rats". Life Sciences. 79 (10): 981–90. doi:10.1016/j.lfs.2006.05.006. PMID 16765386.
- ↑ Farook, J. M.; Lewis, B; Gaddis, J. G.; Littleton, J. M.; Barron, S (2009). "Lobeline, a nicotinic partial agonist attenuates alcohol consumption and preference in male C57BL/6J mice". Physiology & Behavior. 97 (3–4): 503–6. doi:10.1016/j.physbeh.2009.02.031. PMID 19268674.
- ↑ Zheng, G; Dwoskin, L. P.; Crooks, P. A. (2006). "Vesicular monoamine transporter 2: Role as a novel target for drug development". The AAPS Journal. 8 (4): E682–92. doi:10.1208/aapsj080478. PMC 2751365. PMID 17233532.
- ↑ Zheng, F; Zheng, G; Deaciuc, A. G.; Zhan, C. G.; Dwoskin, L. P.; Crooks, P. A. (2007). "Computational neural network analysis of the affinity of lobeline and tetrabenazine analogs for the vesicular monoamine transporter-2". Bioorganic & Medicinal Chemistry. 15 (8): 2975–92. doi:10.1016/j.bmc.2007.02.013. PMC 2001191. PMID 17331733.
- ↑ Zheng, G; Dwoskin, L. P.; Deaciuc, A. G.; Norrholm, S. D.; Crooks, P. A. (2005). "Defunctionalized lobeline analogues: Structure-activity of novel ligands for the vesicular monoamine transporter". Journal of Medicinal Chemistry. 48 (17): 5551–60. doi:10.1021/jm0501228. PMC 3617589. PMID 16107155.
- ↑ Wilhelm, C. J.; Johnson, R. A.; Eshleman, A. J.; Janowsky, A (2008). "Lobeline effects on tonic and methamphetamine-induced dopamine release". Biochemical Pharmacology. 75 (6): 1411–5. doi:10.1016/j.bcp.2007.11.019. PMC 2435375. PMID 18191815.
- ↑ Wilhelm, C. J.; Johnson, R. A.; Lysko, P. G.; Eshleman, A. J.; Janowsky, A (2004). "Effects of methamphetamine and lobeline on vesicular monoamine and dopamine transporter-mediated dopamine release in a cotransfected model system". Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1142–51. doi:10.1124/jpet.104.067314. PMID 15102929.
- ↑ Zheng, G; Horton, D. B.; Deaciuc, A. G.; Dwoskin, L. P.; Crooks, P. A. (2006). "Des-keto lobeline analogs with increased potency and selectivity at dopamine and serotonin transporters". Bioorganic & Medicinal Chemistry Letters. 16 (19): 5018–21. doi:10.1016/j.bmcl.2006.07.070. PMC 3934794. PMID 16905316.
- ↑ Damaj, M. I.; Patrick, G. S.; Creasy, K. R.; Martin, B. R. (1997). "Pharmacology of lobeline, a nicotinic receptor ligand". The Journal of pharmacology and experimental therapeutics. 282 (1): 410–9. PMID 9223582.
- ↑ Miller, D. K.; Harrod, S. B.; Green, T. A.; Wong, M. Y.; Bardo, M. T.; Dwoskin, L. P. (2003). "Lobeline attenuates locomotor stimulation induced by repeated nicotine administration in rats". Pharmacology, biochemistry, and behavior. 74 (2): 279–86. PMID 12479946.
- ↑ PDB entry 2bys. Hansen, S. B.; Sulzenbacher, G; Huxford, T; Marchot, P; Taylor, P; Bourne, Y (2005). "Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations". The EMBO Journal. 24 (20): 3635–46. doi:10.1038/sj.emboj.7600828. PMC 1276711. PMID 16193063.
- ↑ Miller, D. K.; Lever, J. R.; Rodvelt, K. R.; Baskett, J. A.; Will, M. J.; Kracke, G. R. (2007). "Lobeline, a potential pharmacotherapy for drug addiction, binds to mu opioid receptors and diminishes the effects of opioid receptor agonists". Drug and Alcohol Dependence. 89 (2–3): 282–91. doi:10.1016/j.drugalcdep.2007.02.003. PMID 17368966.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Alkaloids
- Antidepressants
- Piperidines
- Nicotinic agonists
- Ketones
- Opioid antagonists
- VMAT inhibitors