Pramipexole
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pramipexole is a dopamine agonist that is FDA approved for the {{{indicationType}}} of idiopathic parkinson’s disease (PD), moderate-to-severe primary restless legs syndrome (RLS). Common adverse reactions include nausea, dizziness, somnolence, insomnia, constipation, asthenia, hallucinations, fatigue, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Parkinson's Disease
- Doses should be increased gradually from a starting dose of 0.375 mg/day given in three divided doses and should not be increased more frequently than every 5 to 7 days. A suggested ascending dosage schedule that was used in clinical studies is shown in the following table:
- Maintenance Treatment
- MIRAPEX tablets were effective and well tolerated over a dosage range of 1.5 to 4.5 mg/day administered in equally divided doses three times per day with or without concomitant levodopa (approximately 800 mg/day).
- In a fixed-dose study in early Parkinson's disease patients, doses of 3 mg, 4.5 mg, and 6 mg per day of MIRAPEX tablets were not shown to provide any significant benefit beyond that achieved at a daily dose of 1.5 mg/day. However, in the same fixed-dose study, the following adverse events were dose related: postural hypotension, nausea, constipation, somnolence, and amnesia. The frequency of these events was generally 2-fold greater than placebo for pramipexole doses greater than 3 mg/day. The incidence of somnolence reported with pramipexole at a dose of 1.5 mg/day was comparable to placebo.
- When MIRAPEX tablets are used in combination with levodopa, a reduction of the levodopa dosage should be considered. In a controlled study in advanced Parkinson's disease, the dosage of levodopa was reduced by an average of 27% from baseline.
Restless Legs Syndrome
- The recommended starting dose of MIRAPEX tablets is 0.125 mg taken once daily 2-3 hours before bedtime. For patients requiring additional symptomatic relief, the dose may be increased every 4-7 days (Table 3). Although the dose of MIRAPEX tablets was increased to 0.75 mg in some patients during long-term open-label treatment, there is no evidence that the 0.75 mg dose provides additional benefit beyond the 0.5 mg dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pramipexole in adult patients.
Non–Guideline-Supported Use
Depression
- Dosing Information
- Oral pramipexole (initial dose 0.125 milligrams (mg) twice daily, increased by 0.25 mg/day every 3 to 5 days to target dose of 1 to 2.5 mg/day).
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Pramipexole in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pramipexole in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pramipexole in pediatric patients.
Contraindications
- None
Warnings
Precautions
- Falling Asleep During Activities of Daily Living
- Patients treated with pramipexole have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles which sometimes resulted in accidents. Although many of these patients reported somnolence while on pramipexole tablets, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events had been reported as late as one year after the initiation of treatment.
- Somnolence is a common occurrence in patients receiving pramipexole at doses above 1.5 mg/day (0.5 mg TID) for Parkinson’s disease. In controlled clinical trials in RLS, patients treated with MIRAPEX tablets at doses of 0.25-0.75 mg once a day, the incidence of somnolence was 6% compared to an incidence of 3% for placebo-treated patients. Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
- Before initiating treatment with MIRAPEX tablets, advise patients of the potential to develop drowsiness and specifically asked about factors that may increase the risk with MIRAPEX tablets such as the use of concomitant sedating medications or alcohol, the presence of sleep disorders, and concomitant medications that increase pramipexole plasma levels (e.g., cimetidine). If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), MIRAPEX tablets should ordinarily be discontinued. If a decision is made to continue MIRAPEX tablets, advise patients not to drive and to avoid other potentially dangerous activities. While dose reduction reduces the degree of somnolence, there is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
- Symptomatic Orthostatic Hypotension
- Dopamine agonists, in clinical studies and clinical experience, appear to impair the systemic regulation of blood pressure, with resulting orthostatic hypotension, especially during dose escalation. Parkinson's disease patients, in addition, appear to have an impaired capacity to respond to an orthostatic challenge. For these reasons, both Parkinson's disease patients and RLS patients being treated with dopaminergic agonists ordinarily require careful monitoring for signs and symptoms of orthostatic hypotension, especially during dose escalation, and should be informed of this risk.
- In clinical trials of pramipexole, however, and despite clear orthostatic effects in normal volunteers, the reported incidence of clinically significant orthostatic hypotension was not greater among those assigned to pramipexole tablets than among those assigned to placebo. This result, especially with the higher doses used in Parkinson’s disease, is clearly unexpected in light of the previous experience with the risks of dopamine agonist therapy.
- While this finding could reflect a unique property of pramipexole, it might also be explained by the conditions of the study and the nature of the population enrolled in the clinical trials. Patients were very carefully titrated, and patients with active cardiovascular disease or significant orthostatic hypotension at baseline were excluded. Also, clinical trials in patients with RLS did not incorporate orthostatic challenges with intensive blood pressure monitoring done in close temporal proximity to dosing.
- Impulse Control/Compulsive Behaviors
- Case reports and the results of a cross-sectional study suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, binge eating, and/or other intense urges and the inability to control these urges while taking one or more of the medications, including MIRAPEX, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with MIRAPEX. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking MIRAPEX.
- In the three double-blind, placebo-controlled trials in early Parkinson's disease, hallucinations were observed in 9% (35 of 388) of patients receiving MIRAPEX tablets, compared with 2.6% (6 of 235) of patients receiving placebo. In the four double-blind, placebo-controlled trials in advanced Parkinson's disease, where patients received MIRAPEX tablets and concomitant levodopa, hallucinations were observed in 16.5% (43 of 260) of patients receiving MIRAPEX tablets compared with 3.8% (10 of 264) of patients receiving placebo. Hallucinations were of sufficient severity to cause discontinuation of treatment in 3.1% of the early Parkinson's disease patients and 2.7% of the advanced Parkinson's disease patients compared with about 0.4% of placebo patients in both populations.
- Age appears to increase the risk of hallucinations attributable to pramipexole. In the early Parkinson's disease patients, the risk of hallucinations was 1.9 times greater than placebo in patients younger than 65 years and 6.8 times greater than placebo in patients older than 65 years. In the advanced Parkinson's disease patients, the risk of hallucinations was 3.5 times greater than placebo in patients younger than 65 years and 5.2 times greater than placebo in patients older than 65 years.
- In the RLS clinical program, one pramipexole-treated patient (of 889) reported hallucinations; this patient discontinued treatment and the symptoms resolved.
- Mirapex tablets may potentiate the dopaminergic side effects of levodopa and may cause or exacerbate preexisting dyskinesia.
- Renal Impairment
- Since pramipexole is eliminated through the kidneys, caution should be exercised when prescribing MIRAPEX tablets to patients with renal impairment.
- A single case of rhabdomyolysis occurred in a 49-year-old male with advanced Parkinson's disease treated with MIRAPEX tablets. The patient was hospitalized with an elevated CPK (10,631 IU/L). The symptoms resolved with discontinuation of the medication.
- Retinal Pathology
- Human Data
- A two-year open-label, randomized, parallel-group safety study of retinal deterioration and vision compared MIRAPEX tablets and immediate-release ropinirole. Two hundred thirty four Parkinson’s disease patients (115 on pramipexole, mean dose 3.0 mg/day and 119 on ropinirole, mean dose 9.5 mg/day) were evaluated using a panel of clinical ophthalmological assessments. Of 234 patients who were evaluable, 196 had been treated for two years and 29 were judged to have developed clinical abnormalities that were considered meaningful (19 patients in each treatment arm had received treatment for less than two years). There was no statistical difference in retinal deterioration between the treatment arms; however, the study was only capable of detecting a very large difference between treatments. In addition, because the study did not include an untreated comparison group (placebo treated), it is unknown whether the findings reported in patients treated with either drug are greater than the background rate in an aging population.
- Animal Data
- Pathologic changes (degeneration and loss of photoreceptor cells) were observed in the retina of albino rats in the 2-year carcinogenicity study. While retinal degeneration was not diagnosed in pigmented rats treated for 2 years, a thinning in the outer nuclear layer of the retina was slightly greater in rats given drug compared with controls. Evaluation of the retinas of albino mice, monkeys, and minipigs did not reveal similar changes. The potential significance of this effect in humans has not been established, but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (i.e., disk shedding) may be involved.
- Events Reported with Dopaminergic Therapy
- Although the events enumerated below may not have been reported in association with the use of pramipexole in its development program, they are associated with the use of other dopaminergic drugs. The expected incidence of these events, however, is so low that even if pramipexole caused these events at rates similar to those attributable to other dopaminergic therapies, it would be unlikely that even a single case would have occurred in a cohort of the size exposed to pramipexole in studies to date.
- Withdrawal-Emergent Hyperpyrexia and Confusion
- Although not reported with pramipexole in the clinical development program, a symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
- Fibrotic Complications
- Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, pericarditis, and cardiac valvulopathy have been reported in patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur.
- Although these adverse events are believed to be related to the ergoline structure of these compounds, whether other, nonergot-derived dopamine agonists can cause them is unknown.
- Cases of possible fibrotic complications, including peritoneal fibrosis, pleural fibrosis, and pulmonary fibrosis have been reported in the post marketing experience with MIRAPEX tablets. While the evidence is not sufficient to establish a causal relationship between MIRAPEX tablets and these fibrotic complications, a contribution of MIRAPEX tablets cannot be completely ruled out.
- Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the observed increased risk was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
- For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using MIRAPEX tablets for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
- Rebound and Augmentation in RLS
- Reports in the literature indicate treatment of RLS with dopaminergic medications can result in rebound: a worsening of symptoms following treatment cessation with greater intensity than described before starting treatment. In a 26 week placebo controlled clinical trial in patients with RLS, a worsening of symptoms scores (IRLS) beyond their untreated baseline levels was reported more frequently by patients suddenly withdrawn from MIRAPEX (up to 0.75 mg once daily) compared to the group assigned to placebo (10% vs. 2%, respectively). The worsening of RLS symptoms was considered generally mild.
- Augmentation has also been described during therapy for RLS. Augmentation refers to the earlier onset of symptoms in the evening (or even the afternoon), increase in symptoms, and spread of symptoms to involve other extremities. In a 26 week placebo controlled clinical trial in patients with RLS, augmentation was reported with greater frequency by patients treated with MIRAPEX (up to 0.75 mg once daily) compared to patients who received placebo (12% vs. 9%, respectively). The incidence of augmentation increased with increasing duration of exposure to MIRAPEX and to placebo.
- The frequency and severity of augmentation and/or rebound after longer-term use of MIRAPEX tablets and the appropriate management of these events have not been adequately evaluated in controlled clinical trials.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Parkinson's Disease
- During the premarketing development of pramipexole, patients with either early or advanced Parkinson's disease were enrolled in clinical trials. Apart from the severity and duration of their disease, the two populations differed in their use of concomitant levodopa therapy. Patients with early disease did not receive concomitant levodopa therapy during treatment with pramipexole; those with advanced Parkinson's disease all received concomitant levodopa treatment. Because these two populations may have differential risks for various adverse events, this section will, in general, present adverse-event data for these two populations separately.
- Because the controlled trials performed during premarketing development all used a titration design, with a resultant confounding of time and dose, it was impossible to adequately evaluate the effects of dose on the incidence of adverse events.
- Early Parkinson's Disease
- In the three double-blind, placebo-controlled trials of patients with early Parkinson's disease, the most commonly observed adverse events (>5%) that were numerically more frequent in the group treated with MIRAPEX tablets were nausea, dizziness, somnolence, insomnia, constipation, asthenia, and hallucinations.
- Approximately 12% of 388 patients with early Parkinson's disease and treated with MIRAPEX tablets who participated in the double-blind, placebo-controlled trials discontinued treatment due to adverse events compared with 11% of 235 patients who received placebo. The adverse events most commonly causing discontinuation of treatment were related to the nervous system (hallucinations [3.1% on MIRAPEX tablets vs 0.4% on placebo]; dizziness [2.1% on MIRAPEX tablets vs 1% on placebo]; somnolence [1.6% on MIRAPEX tablets vs 0% on placebo]; extrapyramidal syndrome [1.6% on MIRAPEX tablets vs 6.4% on placebo]; headache and confusion [1.3% and 1.0%, respectively, on MIRAPEX tablets vs 0% on placebo]); and gastrointestinal system (nausea [2.1% on MIRAPEX tablets vs 0.4% on placebo]).
- Adverse-event Incidence in Controlled Clinical Studies in Early Parkinson's Disease: Table 4 lists treatment-emergent adverse events that occurred in the double-blind, placebo-controlled studies in early Parkinson's disease that were reported by ≥1% of patients treated with MIRAPEX tablets and were numerically more frequent than in the placebo group. In these studies, patients did not receive concomitant levodopa. Adverse events were usually mild or moderate in intensity.
- The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the adverse-event incidence rate in the population studied.
- Other events reported by 1% or more of patients with early Parkinson's disease and treated with MIRAPEX tablets but reported equally or more frequently in the placebo group were infection, accidental injury, headache, pain, tremor, back pain, syncope, postural hypotension, hypertonia, depression, abdominal pain, anxiety, dyspepsia, flatulence, diarrhea, rash, ataxia, dry mouth, extrapyramidal syndrome, leg cramps, twitching, pharyngitis, sinusitis, sweating, rhinitis, urinary tract infection, vasodilation, flu syndrome, increased saliva, tooth disease, dyspnea, increased cough, gait abnormalities, urinary frequency, vomiting, allergic reaction, hypertension, pruritus, hypokinesia, increased creatine PK, nervousness, dream abnormalities, chest pain, neck pain, paresthesia, tachycardia, vertigo, voice alteration, conjunctivitis, paralysis, accommodation abnormalities, tinnitus, diplopia, and taste perversions.
- In a fixed-dose study in early Parkinson's disease, occurrence of the following events increased in frequency as the dose increased over the range from 1.5 mg/day to 6 mg/day: postural hypotension, nausea, constipation, somnolence, and amnesia. The frequency of these events was generally 2-fold greater than placebo for pramipexole doses greater than 3 mg/day. The incidence of somnolence with pramipexole at a dose of 1.5 mg/day was comparable to that reported for placebo.
- Advanced Parkinson's Disease
- In the four double-blind, placebo-controlled trials of patients with advanced Parkinson's disease, the most commonly observed adverse events (>5%) that were numerically more frequent in the group treated with MIRAPEX tablets and concomitant levodopa were postural orthostatic hypotension, dyskinesia, extrapyramidal syndrome, insomnia, dizziness, hallucinations, accidental injury, dream abnormalities, confusion, constipation, asthenia, somnolence, dystonia, gait abnormality, hypertonia, dry mouth, amnesia, and urinary frequency.
- Approximately 12% of 260 patients with advanced Parkinson's disease who received MIRAPEX tablets and concomitant levodopa in the double-blind, placebo-controlled trials discontinued treatment due to adverse events compared with 16% of 264 patients who received placebo and concomitant levodopa. The events most commonly causing discontinuation of treatment were related to the nervous system (hallucinations [2.7% on MIRAPEX tablets vs 0.4% on placebo]; dyskinesia [1.9% on MIRAPEX tablets vs 0.8% on placebo]; extrapyramidal syndrome [1.5% on MIRAPEX tablets vs 4.9% on placebo]; dizziness [1.2% on MIRAPEX tablets vs 1.5% on placebo]; confusion [1.2% on MIRAPEX tablets vs 2.3% on placebo]); and cardiovascular system (postural orthostatic hypotension [2.3% on MIRAPEX tablets vs 1.1% on placebo]).
- Adverse-event Incidence in Controlled Clinical Studies in Advanced Parkinson's Disease: Table 5 lists treatment-emergent adverse events that occurred in the double-blind, placebo-controlled studies in advanced Parkinson's disease that were reported by ≥1% of patients treated with MIRAPEX tablets and were numerically more frequent than in the placebo group. In these studies, MIRAPEX tablets or placebo was administered to patients who were also receiving concomitant levodopa. Adverse events were usually mild or moderate in intensity.
- The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the adverse-events incidence rate in the population studied.
- Other events reported by 1% or more of patients with advanced Parkinson's disease and treated with MIRAPEX tablets but reported equally or more frequently in the placebo group were nausea, pain, infection, headache, depression, tremor, hypokinesia, anorexia, back pain, dyspepsia, flatulence, ataxia, flu syndrome, sinusitis, diarrhea, myalgia, abdominal pain, anxiety, rash, paresthesia, hypertension, increased saliva, tooth disorder, apathy, hypotension, sweating, vasodilation, vomiting, increased cough, nervousness, pruritus, hypesthesia, neck pain, syncope, arthralgia, dysphagia, palpitations, pharyngitis, vertigo, leg cramps, conjunctivitis, and lacrimation disorders.
Restless Legs Syndrome
- MIRAPEX tablets for treatment of RLS have been evaluated for safety in 889 patients, including 427 treated for over six months and 75 for over one year.
- The overall safety assessment focuses on the results of three double-blind, placebo-controlled trials, in which 575 patients with RLS were treated with MIRAPEX tablets for up to 12 weeks. The most commonly observed adverse events with MIRAPEX tablets in the treatment of RLS (observed in >5% of pramipexole-treated patients and at a rate at least twice that observed in placebo-treated patients) were nausea and somnolence. Occurrences of nausea and somnolence in clinical trials were generally mild and transient.
- Approximately 7% of 575 patients treated with MIRAPEX tablets during the double-blind periods of three placebo-controlled trials discontinued treatment due to adverse events compared to 5% of 223 patients who received placebo. The adverse event most commonly causing discontinuation of treatment was nausea (1%).
- Table 6 lists treatment-emergent events that occurred in three double-blind, placebo-controlled studies in RLS patients that were reported by ≥2% of patients treated with MIRAPEX tablets and were numerically more frequent than in the placebo group.
- The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the adverse-event incidence rate in the population studied.
- Other events reported by 2% or more of RLS patients treated with MIRAPEX tablets but reported equally or more frequently in the placebo group, were: vomiting, nasopharyngitis, back pain, pain in extremity, dizziness, and insomnia.
- Table 7 summarizes data for adverse events that appeared to be dose related in the 12-week fixed dose study.
- General
- Adverse Events: Relationship to Age, Gender, and Race
- Among the treatment-emergent adverse events in patients treated with MIRAPEX tablets, hallucination appeared to exhibit a positive relationship to age in patients with Parkinson’s disease. Although no gender-related differences were observed in Parkinson’s disease patients, nausea and fatigue, both generally transient, were more frequently reported by female than male RLS patients. Less than 4% of patients enrolled were non-Caucasian: therefore, an evaluation of adverse events related to race is not possible.
- Laboratory Tests
- During the development of MIRAPEX tablets, no systematic abnormalities on routine laboratory testing were noted. Therefore, no specific guidance is offered regarding routine monitoring; the practitioner retains responsibility for determining how best to monitor the patient in his or her care.
- Other Adverse Events Observed During Phase 2 and 3 Clinical Trials
- MIRAPEX tablets have been administered to 1620 Parkinson’s disease patients and to 889 RLS patients in Phase 2 and 3 clinical trials. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing; similar types of events were grouped into a smaller number of standardized categories using MedDRA dictionary terminology. These categories are used in the listing below. Adverse events which are not listed above but occurred on at least two occasions (one occasion if the event was serious) in the 2509 individuals exposed to MIRAPEX tablets are listed below. The reported events below are included without regard to determination of a causal relationship to MIRAPEX tablets.
Blood and lymphatic system disorders
Anemia, iron deficiency anemia, leukocytosis, leukopenia, lymphadenitis, lymphadenopathy, thrombocythaemia, thrombocytopenia
Cardiac disorders
Angina pectoris, supraventricular arrhythmia, atrial fibrillation, first degree atrioventricular block, second degree atrioventricular block, bradycardia, bundle branch block, cardiac arrest, cardiac failure, congestive cardiac failure, cardiomegaly, coronary artery occlusion, cyanosis, extrasystoles, left ventricular failure, myocardial infarction, nodal arrhythmia, sinus arrhythmia, sinus bradycardia, sinus tachycardia, supraventricular extrasystoles, supraventricular tachycardia, tachycardia, ventricular fibrillation, ventricular extrasystoles, ventricular hypertrophy
Congenital, familial and genetic disorders
Atrial septal defect, congenital foot malformation, spine malformation
Ear and labyrinth disorders
Deafness, ear pain, hearing impaired, hypoacusis, motion sickness, vestibular ataxia
Endocrine disorders
Goiter, hyperthyroidism, hypothyroidism
Eye disorders
Amaurosis fugax, blepharitis, blepharospasm, cataract, dacryostenosis acquired, dry eye, eye hemorrhage, eye irritation, eye pain, eyelid edema, eyelid ptosis, glaucoma, keratitis, macular degeneration, myopia, photophobia, retinal detachment, retinal vascular disorder, scotoma, vision blurred, visual acuity reduced, vitreous floaters
Gastrointestinal disorders
Abdominal discomfort, abdominal distension, aphthous stomatitis, ascites, cheilitis, colitis, ulcerative colitis, duodenal ulcer, duodenal ulcer hemorrhage, enteritis, eructation, fecal incontinence, gastric ulcer, gastric ulcer hemorrhage, gastritis, gastrointestinal hemorrhage, gastroesophageal reflux disease, gingivitis, haematemesis, haematochezia, hemorrhoids, hiatus hernia, hyperchlorhydria, ileus, inguinal hernia, intestinal obstruction, irritable bowel syndrome, esophageal spasm, esophageal stenosis, esophagitis, pancreatitis, periodontitis, rectal hemorrhage, reflux esophagitis, tongue edema, tongue ulceration, toothache, umbilical hernia
General disorders
Chest discomfort, chills, death, drug withdrawal syndrome, face edema, feeling cold, feeling hot, feeling jittery, gait disturbance, impaired healing, influenza-like illness, irritability, localized edema, edema, pitting edema, thirst
Hepatobiliary disorders
Biliary colic, cholecystitis, cholecystitis chronic, cholelithiasis
Immune system disorders
Drug hypersensitivity
Infections and infestations
Abscess, acute tonsillitis, appendicitis, bronchiolitis, bronchitis, bronchopneumonia, cellulitis, cystitis, dental caries, diverticulitis, ear infection, eye infection, folliculitis, fungal infection, furuncle, gangrene, gastroenteritis, gingival infection, herpes simplex, herpes zoster, hordeolum, intervertebral discitis, laryngitis, lobar pneumonia, nail infection, onychomycosis, oral candidiasis, orchitis, osteomyelitis, otitis externa, otitis media, paronychia, pyelonephritis, pyoderma, sepsis, skin infection, tonsillitis, tooth abscess, tooth infection, upper respiratory tract infection, urethritis, vaginal candidiasis, vaginal infection, viral infection, wound infection
Injury, poisoning and procedural complications
Accidental falls, drug toxicity, epicondylitis, road traffic accident, sunburn, tendon rupture
Metabolism and nutrition disorders
Cachexia, decreased appetite, dehydration, diabetes mellitus, fluid retention, gout, hypercholesterolemia, hyperglycemia, hyperlipidemia, hyperuricemia, hypocalcemia, hypoglycemia, hypokalemia, hyponatremia, hypovitaminosis, increased appetite, metabolic alkalosis
Musculoskeletal and connective tissue disorders
Bone pain, fasciitis, flank pain, intervertebral disc disorder, intervertebral disc protrusion, joint effusion, joint stiffness, joint swelling, monarthritis, muscle rigidity, muscle spasms, musculoskeletal stiffness, myopathy, myositis, nuchal rigidity, osteoarthritis, osteonecrosis, osteoporosis, polymyalgia, rheumatoid arthritis, shoulder pain, spinal osteoarthritis, tendonitis, tenosynovitis
Neoplasms benign, malignant and unspecified
Abdominal neoplasm, adenocarcinoma, adenoma benign, basal cell carcinoma, bladder cancer, breast cancer, breast neoplasm, chronic lymphocytic leukemia, colon cancer, colorectal cancer, endometrial cancer, gallbladder cancer, gastric cancer, gastrointestinal neoplasm, hemangioma, hepatic neoplasm, hepatic neoplasm malignant, lip and/or oral cavity cancer, lung neoplasm malignant, lung cancer metastatic, lymphoma, malignant melanoma, melanocytic naevus, metastases to lung, multiple myeloma, oral neoplasm benign, neoplasm, neoplasm malignant, neoplasm prostate, neoplasm skin, neuroma, ovarian cancer, prostate cancer, prostatic adenoma, pseudo lymphoma, renal neoplasm, skin cancer, skin papilloma, squamous cell carcinoma, thyroid neoplasm, uterine leiomyoma
Nervous system disorders
Ageusia, akinesia, anticholinergic syndrome, aphasia, balance disorder, brain edema, carotid artery occlusion, carpal tunnel syndrome, cerebral artery embolism, cerebral hemorrhage, cerebral infarction, cerebral ischemia, chorea, cognitive disorder, coma, convulsion, coordination abnormal, dementia, depressed level of consciousness, disturbance in attention, dizziness postural, dysarthria, dysgraphia, facial palsy, grand mal convulsion, hemiplegia, hyperaesthesia, hyperkinesia, hyperreflexia, hyporeflexia, hypotonia, lethargy, loss of consciousness, memory impairment, migraine, muscle contractions involuntary, narcolepsy, neuralgia, neuropathy, nystagmus, parosmia, psychomotor hyperactivity, sciatica, sedation, sensory disturbance, sleep phase rhythm disturbance, sleep talking, stupor, vasovagal syncope, tension headache
Psychiatric disorders
Affect lability, aggression, agitation, bradyphrenia, bruxism, suicide, delirium, delusional disorder persecutory type, disorientation, dissociation, emotional distress, euphoric mood, hallucination auditory, hallucination visual, initial insomnia, libido increased, mania, middle insomnia, mood altered, nightmare, obsessive thoughts, obsessive-compulsive disorder, panic reaction, parasomnia, personality disorder, psychotic disorder, restlessness, sleep walking, suicidal ideation
Renal and urinary disorders
Chromaturia, dysuria, glycosuria, hematuria, urgency, nephrolithiasis, neurogenic bladder, nocturia, oliguria, pollakiuria, proteinuria, renal artery stenosis, renal colic, renal cyst, renal failure, renal impairment, urinary retention
Reproductive system and breast disorders
Amenorrhea, breast pain, dysmenorrhea, epididymitis, gynaecomastia, menopausal symptoms, menorrhagia, metrorrhagia, ovarian cyst, priapism, prostatitis, sexual dysfunction, uterine hemorrhage, vaginal discharge, vaginal hemorrhage
Respiratory, thoracic and mediastinal disorders
Apnea, aspiration, asthma, choking, chronic obstructive pulmonary disease, dry throat, dysphonia, exertional dyspnea, epistaxis, haemoptysis, hiccups, hyperventilation, increased bronchial secretion, laryngospasm, nasal dryness, nasal polyps, obstructive airways disorder, pharyngolaryngeal pain, pleurisy, aspiration pneumonia, pneumothorax, postnasal drip, productive cough, pulmonary embolism, pulmonary edema, respiratory alkalosis, respiratory distress, respiratory failure, respiratory tract congestion, allergic rhinitis, rhinorrhea, sinus congestion, sleep apnoea syndrome, sneezing, snoring, tachypnea, wheezing
Skin and subcutaneous tissue disorders
Acne, alopecia, cold sweat, dermal cyst, dermatitis, dermatitis bullous, dermatitis contact, dry skin, ecchymosis, eczema, erythema, hyperkeratosis, livedo reticularis, night sweats, periorbital edema, petechiae, photosensitivity allergic reaction, psoriasis, purpura, rash erythematous, rash maculo-papular, rash papular, rosacea, seborrhea, seborrheic dermatitis, skin burning sensation, skin discoloration, skin exfoliation, skin hyperpigmentation, skin hypertrophy, skin irritation, skin nodule, skin odor abnormal, skin ulcer, urticaria
Vascular disorders
Aneurysm, angiopathy, arteriosclerosis, circulatory collapse, deep vein thrombosis, embolism, hematoma, hot flush, hypertensive crisis, lymphoedema, pallor, phlebitis, Raynaud’s phenomenon, shock, thrombophlebitis, thrombosis, varicose vein.
Postmarketing Experience
- In addition to the adverse events reported during clinical trials, the following adverse reactions have been identified during post-approval use of MIRAPEX tablets, primarily in Parkinson’s disease patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these events in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to pramipexole tablets. Similar types of reactions were grouped into a smaller number of standardized categories using the MedDRA terminology: abnormal behavior, abnormal dreams, accidents (including fall), blackouts, compulsive shopping, fatigue, hallucinations (all kinds), headache, hypotension (including postural hypotension), syndrome of inappropriate antidiuretic hormone secretion (SIADH), increased eating (including binge eating, compulsive eating, and hyperphagia), libido disorders (including increased and decreased libido, and hypersexuality), pathological gambling, pruritus, syncope, vomiting, and weight increase.
Drug Interactions
- Dopamine Antagonists
- Since pramipexole is a dopamine agonist, it is possible that dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of MIRAPEX tablets.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well-controlled studies in pregnant women. MIRAPEX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- When pramipexole was given to female rats throughout pregnancy, implantation was inhibited at a dose of 2.5 mg/kg/day (5 times the maximum recommended human dose (MRHD) for Parkinson’s disease of 4.5 mg/day on a body surface area (mg/m2) basis). Administration of 1.5 mg/kg/day of pramipexole to pregnant rats during the period of organogenesis (gestation days 7 through 16) resulted in a high incidence of total resorption of embryos. The plasma AUC in rats at this dose was 4 times the AUC in humans at the MRHD. These findings are thought to be due to the prolactin-lowering effect of pramipexole, since prolactin is necessary for implantation and maintenance of early pregnancy in rats (but not rabbits or humans). Because of pregnancy disruption and early embryonic loss in these studies, the teratogenic potential of pramipexole could not be adequately evaluated. There was no evidence of adverse effects on embryo-fetal development following administration of up to 10 mg/kg/day to pregnant rabbits during organogenesis (plasma AUC was 70 times that in humans at the MRHD). Postnatal growth was inhibited in the offspring of rats treated with 0.5 mg/kg/day (approximately equivalent to the MRHD on a mg/m2 basis) or greater during the latter part of pregnancy and throughout lactation.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pramipexole in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pramipexole during labor and delivery.
Nursing Mothers
Studies have shown that pramipexole treatment resulted in an inhibition of prolactin secretion in humans and rats.
- A single-dose, radio-labeled study showed that drug-related material was present in rat milk at concentrations three to six times higher than those in plasma at equivalent time points. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from MIRAPEX, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness of MIRAPEX in pediatric patients has not been established.
Geriatic Use
- Pramipexole total oral clearance is approximately 30% lower in subjects older than 65 years compared with younger subjects, because of a decline in pramipexole renal clearance due to an age-related reduction in renal function. This resulted in an increase in elimination half-life from approximately 8.5 hours to 12 hours.
- In clinical studies with Parkinson’s disease patients, 38.7% of patients were older than 65 years. There were no apparent differences in efficacy or safety between older and younger patients, except that the relative risk of hallucination associated with the use of MIRAPEX tablets was increased in the elderly.
- In clinical studies with RLS patients, 22% of patients were at least 65 years old. There were no apparent differences in efficacy or safety between older and younger patients.
Gender
There is no FDA guidance on the use of Pramipexole with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pramipexole with respect to specific racial populations.
Renal Impairment
- The elimination of pramipexole is dependent on renal function. Pramipexole clearance is extremely low in dialysis patients, as a negligible amount of pramipexole is removed by dialysis. Caution should be exercised when administering MIRAPEX tablets to patients with renal disease.
Hepatic Impairment
There is no FDA guidance on the use of Pramipexole in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pramipexole in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pramipexole in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Pramipexole in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Pramipexole in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- There is no clinical experience with significant overdosage. One patient took 11 mg/day of pramipexole for 2 days in a clinical trial for an investigational use. Blood pressure remained stable although pulse rate increased to between 100 and 120 beats/minute. No other adverse events were reported related to the increased dose.
Management
- There is no known antidote for overdosage of a dopamine agonist. If signs of central nervous system stimulation are present, a phenothiazine or other butyrophenone neuroleptic agent may be indicated; the efficacy of such drugs in reversing the effects of overdosage has not been assessed. Management of overdose may require general supportive measures along with gastric lavage, intravenous fluids, and electrocardiogram monitoring.
Chronic Overdose
There is limited information regarding Chronic Overdose of Pramipexole in the drug label.
Pharmacology
| |

| |
Pramipexole
| |
| Systematic (IUPAC) name | |
| (S)-N 6-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | N04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 211.324 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Protein binding | 15% |
| Metabolism | ? |
| Half life | 8–12 hours |
| Excretion | Urine (90%), Feces (2%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
- Pramipexole is a non-ergot dopamine agonist with high relative in vitro specificity and full intrinsic activity at the D2 subfamily of dopamine receptors, binding with higher affinity to D3 than to D2 or D4 receptor subtypes.
- The precise mechanism of action of pramipexole as a treatment for Parkinson's disease is unknown, although it is believed to be related to its ability to stimulate dopamine receptors in the striatum. This conclusion is supported by electrophysiologic studies in animals that have demonstrated that pramipexole influences striatal neuronal firing rates via activation of dopamine receptors in the striatum and the substantia nigra, the site of neurons that send projections to the striatum. The relevance of D3 receptor binding in Parkinson’s disease is unknown.
- Restless Legs Syndrome (RLS)
- The precise mechanism of action of MIRAPEX tablets as a treatment for RLS is unknown. Although the pathophysiology of RLS is largely unknown, neuropharmacological evidence suggests primary dopaminergic system involvement. Positron Emission Tomographic (PET) studies suggest that a mild striatal presynaptic dopaminergic dysfunction may be involved in the pathogenesis of RLS.
Structure
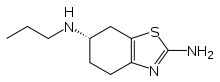
- MIRAPEX tablets contain pramipexole, a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S · 2HCl · H2O, and its molecular weight is 302.26.
- The structural formula is:
- Pramipexole dihydrochloride is a white to off-white powder substance. Melting occurs in the range of 296°C to 301°C, with decomposition. Pramipexole dihydrochloride is more than 20% soluble in water, about 8% in methanol, about 0.5% in ethanol, and practically insoluble in dichloromethane.
- MIRAPEX tablets, for oral administration, contain 0.125 mg, 0.25 mg, 0.5 mg, 0.75 mg, 1 mg, or 1.5 mg of pramipexole dihydrochloride monohydrate. Inactive ingredients consist of mannitol, corn starch, colloidal silicon dioxide, povidone, and magnesium stearate.
Pharmacodynamics
- The effect of pramipexole on the QT interval of the ECG was investigated in a clinical study in 60 healthy male and female volunteers. All subjects initiated treatment with 0.375 mg extended release pramipexole tablets administered once daily, and were up-titrated every 3 days to 2.25 mg and 4.5 mg daily. No dose- or exposure-related effect on mean QT intervals was observed; however the study did not have a valid assessment of assay sensitivity. The effect of pramipexole on QTc intervals at higher exposures achieved either due to drug interactions (e.g., with cimetidine), renal impairment, or at higher doses has not been systematically evaluated.
- Although mean values remained within normal reference ranges throughout the study, supine systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate for subjects treated with pramipexole generally increased during the rapid up-titration phase, by 10 mmHg, 7 mmHg, and 10 bpm higher than placebo, respectively. Higher SBP, DBP, and pulse rates compared to placebo were maintained until the pramipexole doses were tapered; values on the last day of tapering were generally similar to baseline values. Such effects have not been observed in clinical studies with Parkinson’s disease patients, who were titrated according to labeled recommendations.
Pharmacokinetics
- Pramipexole displays linear pharmacokinetics over the clinical dosage range. Its terminal half-life is about 8 hours in young healthy volunteers and about 12 hours in elderly volunteers. Steady-state concentrations are achieved within 2 days of dosing.
- Absorption
- Pramipexole is rapidly absorbed, reaching peak concentrations in approximately 2 hours. The absolute bioavailability of pramipexole is greater than 90%, indicating that it is well absorbed and undergoes little presystemic metabolism. Food does not affect the extent of pramipexole absorption, although the time of maximum plasma concentration (Tmax) is increased by about 1 hour when the drug is taken with a meal.
- Distribution
- Pramipexole is extensively distributed, having a volume of distribution of about 500 L (coefficient of variation [CV]=20%). It is about 15% bound to plasma proteins. Pramipexole distributes into red blood cells as indicated by an erythrocyte-to-plasma ratio of approximately 2.
- Metabolism
- Pramipexole is metabolized only to a negligible extent (<10%). No specific active metabolite has been identified in human plasma or urine.
- Elimination
- Urinary excretion is the major route of pramipexole elimination, with 90% of a pramipexole dose recovered in urine, almost all as unchanged drug. The renal clearance of pramipexole is approximately 400 mL/min (CV=25%), approximately three times higher than the glomerular filtration rate. Thus, pramipexole is secreted by the renal tubules, probably by the organic cation transport system.
- Pharmacokinetics in Specific Populations
- Because therapy with MIRAPEX tablets is initiated at a low dose and gradually titrated upward according to clinical tolerability to obtain the optimum therapeutic effect, adjustment of the initial dose based on gender, weight, race, or age is not necessary. However, renal insufficiency, which can cause a large decrease in the ability to eliminate pramipexole, may necessitate dosage adjustment.
- Gender
- Pramipexole clearance is about 30% lower in women than in men, but this difference can be accounted for by differences in body weight. There is no difference in half-life between males and females.
- Age
- Pramipexole clearance decreases with age as the half-life and clearance are about 40% longer and 30% lower, respectively, in elderly (aged 65 years or older) compared with young healthy volunteers (aged less than 40 years). This difference is most likely due to the reduction in renal function with age, since pramipexole clearance is correlated with renal function, as measured by creatinine clearance.
- Race
- No racial differences in metabolism and elimination have been identified.
- Parkinson's Disease Patients
- A cross-study comparison of data suggests that the clearance of pramipexole may be reduced by about 30% in Parkinson's disease patients compared with healthy elderly volunteers. The reason for this difference appears to be reduced renal function in Parkinson's disease patients, which may be related to their poorer general health. The pharmacokinetics of pramipexole were comparable between early and advanced Parkinson's disease patients.
- Restless Legs Syndrome Patients
- A cross-study comparison of data suggests that the pharmacokinetic profile of pramipexole administered once daily in RLS patients is similar to the pharmacokinetic profile of pramipexole in healthy volunteers.
- Hepatic Impairment
- The influence of hepatic insufficiency on pramipexole pharmacokinetics has not been evaluated. Because approximately 90% of the recovered dose is excreted in the urine as unchanged drug, hepatic impairment would not be expected to have a significant effect on pramipexole elimination.
- Renal Impairment
- Clearance of pramipexole was about 75% lower in patients with severe renal impairment (creatinine clearance approximately 20 mL/min) and about 60% lower in patients with moderate impairment (creatinine clearance approximately 40 mL/min) compared with healthy volunteers. In patients with varying degrees of renal impairment, pramipexole clearance correlates well with creatinine clearance. Therefore, creatinine clearance can be used as a predictor of the extent of decrease in pramipexole clearance.
- Drug Interactions
- Carbidopa/levodopa: Carbidopa/levodopa did not influence the pharmacokinetics of pramipexole in healthy volunteers (N=10). Pramipexole did not alter the extent of absorption (AUC) or the elimination of carbidopa/levodopa, although it caused an increase in levodopa Cmax by about 40% and a decrease in Tmax from 2.5 to 0.5 hours.
- Selegiline: In healthy volunteers (N=11), selegiline did not influence the pharmacokinetics of pramipexole.
- Amantadine: Population pharmacokinetic analyses suggest that amantadine may slightly decrease the oral clearance of pramipexole.
- Cimetidine: Cimetidine, a known inhibitor of renal tubular secretion of organic bases via the cationic transport system, caused a 50% increase in pramipexole AUC and a 40% increase in half-life (N=12).
- Probenecid: Probenecid, a known inhibitor of renal tubular secretion of organic acids via the anionic transporter, did not noticeably influence pramipexole pharmacokinetics (N=12).
- Other drugs eliminated via renal secretion: Population pharmacokinetic analysis suggests that coadministration of drugs that are secreted by the cationic transport system (e.g., cimetidine, ranitidine, diltiazem, triamterene, verapamil, quinidine, and quinine) decreases the oral clearance of pramipexole by about 20%, while those secreted by the anionic transport system (e.g., cephalosporins, penicillins, indomethacin, hydrochlorothiazide, and chlorpropamide) are likely to have little effect on the oral clearance of pramipexole. Other known organic cation transport substrates and/or inhibitors (e.g., cisplatin and procainamide) may also decrease the clearance of pramipexole.
- CYP interactions: Inhibitors of cytochrome P450 enzymes would not be expected to affect pramipexole elimination because pramipexole is not appreciably metabolized by these enzymes in vivo or in vitro. Pramipexole does not inhibit CYP enzymes CYP1A2, CYP2C9, CYP2C19, CYP2E1, and CYP3A4. Inhibition of CYP2D6 was observed with an apparent Ki of 30 µM, indicating that pramipexole will not inhibit CYP enzymes at plasma concentrations observed following the clinical dose of 4.5 mg/day (1.5 mg TID).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Two-year carcinogenicity studies with pramipexole have been conducted in mice and rats. Pramipexole was administered in the diet to mice at doses up to 10 mg/kg/day (or approximately 10 times the maximum recommended human dose (MRHD) for Parkinson’s disease of 4.5 mg/day on a mg/m2 basis). Pramipexole was administered in the diet to rats at doses up to 8 mg/kg/day. These doses were associated with plasma AUCs up to approximately 12 times that in humans at the MRHD. No significant increases in tumors occurred in either species.
- Pramipexole was not mutagenic or clastogenic in a battery of in vitro (bacterial reverse mutation, V79/HGPRT gene mutation, chromosomal aberration in CHO cells) and in vivo (mouse micronucleus) assays.
- In rat fertility studies, pramipexole at a dose of 2.5 mg/kg/day (5 times the MRHD on a mg/m2 basis), prolonged estrus cycles and inhibited implantation. These effects were associated with reductions in serum levels of prolactin, a hormone necessary for implantation and maintenance of early pregnancy in rats.
Animal Toxicology and/or Pharmacology
- Retinal Pathology in Rats
- Pathologic changes (degeneration and loss of photoreceptor cells) were observed in the retina of albino rats in the 2-year carcinogenicity study with pramipexole. These findings were first observed during week 76 and were dose-dependent in animals receiving 2 or 8 mg/kg/day (plasma AUCs equal to 2.5 and 12.5 times that in humans at the MRHD). In a similar study of pigmented rats with 2 years exposure to pramipexole at 2 or 8 mg/kg/day, retinal degeneration was not observed. Animals given drug had thinning in the outer nuclear layer of the retina that was only slightly greater (by morphometric analysis) than that seen in control rats.
- Investigative studies demonstrated that pramipexole reduced the rate of disk shedding from the photoreceptor rod cells of the retina in albino rats, which was associated with enhanced sensitivity to the damaging effects of light. In a comparative study, degeneration and loss of photoreceptor cells occurred in albino rats after 13 weeks of treatment with 25 mg/kg/day of pramipexole (54 times the MRHD on a mg/m2 basis) and constant light (100 lux) but not in pigmented rats exposed to the same dose and higher light intensities (500 lux). Thus, the retina of albino rats is considered to be uniquely sensitive to the damaging effects of pramipexole and light. Similar changes in the retina did not occur in a 2-year carcinogenicity study in albino mice treated with 0.3, 2, or 10 mg/kg/day (0.3, 2.2 and 11 times the MRHD on a mg/m2 basis). Evaluation of the retinas of monkeys given 0.1, 0.5, or 2.0 mg/kg/day of pramipexole (0.4, 2.2, and 8.6 times the MRHD on a mg/m2 basis) for 12 months and minipigs given 0.3, 1, or 5 mg/kg/day of pramipexole for 13 weeks also detected no changes.
- The potential significance of this effect in humans has not been established, but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (i.e., disk shedding) may be involved.
- Fibro-osseous Proliferative Lesions in Mice
- An increased incidence of fibro-osseous proliferative lesions occurred in the femurs of female mice treated for 2 years with 0.3, 2.0, or 10 mg/kg/day (0.3, 2.2, and 11 times the MRHD on a mg/m2 basis). Similar lesions were not observed in male mice or rats and monkeys of either sex that were treated chronically with pramipexole. The significance of this lesion to humans is not known.
Clinical Studies
Parkinson's Disease
- The effectiveness of MIRAPEX tablets in the treatment of Parkinson's disease was evaluated in a multinational drug development program consisting of seven randomized, controlled trials. Three were conducted in patients with early Parkinson's disease who were not receiving concomitant levodopa, and four were conducted in patients with advanced Parkinson's disease who were receiving concomitant levodopa. Among these seven studies, three studies provide the most persuasive evidence of pramipexole's effectiveness in the management of patients with Parkinson's disease who were and were not receiving concomitant levodopa. Two of these three trials enrolled patients with early Parkinson's disease (not receiving levodopa), and one enrolled patients with advanced Parkinson's disease who were receiving maximally tolerated doses of levodopa.
- In all studies, the Unified Parkinson's Disease Rating Scale (UPDRS), or one or more of its subparts, served as the primary outcome assessment measure. The UPDRS is a four-part multi-item rating scale intended to evaluate mentation (part I), Activities of Daily Living (ADL) (part II), motor performance (part III), and complications of therapy (part IV).
- Part II of the UPDRS contains 13 questions relating to ADL, which are scored from 0 (normal) to 4 (maximal severity) for a maximum (worst) score of 52. Part III of the UPDRS contains 27 questions (for 14 items) and is scored as described for part II. It is designed to assess the severity of the cardinal motor findings in patients with Parkinson's disease (e.g., tremor, rigidity, bradykinesia, postural instability, etc.), scored for different body regions, and has a maximum (worst) score of 108.
- Studies in Patients with Early Parkinson's Disease
- Patients (N=599) in the two studies of early Parkinson's disease had a mean disease duration of 2 years, limited or no prior exposure to levodopa (generally none in the preceding 6 months), and were not experiencing the "on-off" phenomenon and dyskinesia characteristic of later stages of the disease.
- One of the two early Parkinson's disease studies (N=335) was a double-blind, placebo-controlled, parallel trial consisting of a 7-week dose-escalation period and a 6-month maintenance period. Patients could be on selegiline, anticholinergics, or both, but could not be on levodopa products or amantadine. Patients were randomized to MIRAPEX tablets or placebo. Patients treated with MIRAPEX tablets had a starting daily dose of 0.375 mg and were titrated to a maximally tolerated dose, but no higher than 4.5 mg/day in three divided doses. At the end of the 6-month maintenance period, the mean improvement from baseline on the UPDRS part II (ADL) total score was 1.9 in the group receiving MIRAPEX tablets and -0.4 in the placebo group, a difference that was statistically significant. The mean improvement from baseline on the UPDRS part III total score was 5.0 in the group receiving MIRAPEX tablets and -0.8 in the placebo group, a difference that was also statistically significant. A statistically significant difference between groups in favor of MIRAPEX tablets was seen beginning at week 2 of the UPDRS part II (maximum dose 0.75 mg/day) and at week 3 of the UPDRS part III (maximum dose 1.5 mg/day).
- The second early Parkinson's disease study (N=264) was a double-blind, placebo-controlled, parallel trial consisting of a 6-week dose-escalation period and a 4-week maintenance period. Patients could be on selegiline, anticholinergics, amantadine, or any combination of these, but could not be on levodopa products. Patients were randomized to 1 of 4 fixed doses of MIRAPEX tablets (1.5 mg, 3.0 mg, 4.5 mg, or 6.0 mg per day) or placebo. At the end of the 4-week maintenance period, the mean improvement from baseline on the UPDRS part II total score was 1.8 in the patients treated with MIRAPEX tablets, regardless of assigned dose group, and 0.3 in placebo-treated patients. The mean improvement from baseline on the UPDRS part III total score was 4.2 in patients treated with MIRAPEX tablets and 0.6 in placebo-treated patients. No dose-response relationship was demonstrated. The between-treatment differences on both parts of the UPDRS were statistically significant in favor of MIRAPEX tablets for all doses.
- No differences in effectiveness based on age or gender were detected. There were too few non-Caucasian patients to evaluate the effect of race. Patients receiving selegiline or anticholinergics had responses similar to patients not receiving these drugs.
- Studies in Patients with Advanced Parkinson's Disease
- In the advanced Parkinson's disease study, the primary assessments were the UPDRS and daily diaries that quantified amounts of "on" and "off" time.
- Patients in the advanced Parkinson's disease study (N=360) had a mean disease duration of 9 years, had been exposed to levodopa for long periods of time (mean 8 years), used concomitant levodopa during the trial, and had "on-off" periods.
- The advanced Parkinson's disease study was a double-blind, placebo-controlled, parallel trial consisting of a 7-week dose-escalation period and a 6-month maintenance period. Patients were all treated with concomitant levodopa products and could additionally be on concomitant selegiline, anticholinergics, amantadine, or any combination. Patients treated with MIRAPEX tablets had a starting dose of 0.375 mg/day and were titrated to a maximally tolerated dose, but no higher than 4.5 mg/day in three divided doses. At selected times during the 6-month maintenance period, patients were asked to record the amount of "off," "on," or "on with dyskinesia" time per day for several sequential days. At the end of the 6-month maintenance period, the mean improvement from baseline on the UPDRS part II total score was 2.7 in the group treated with MIRAPEX tablets and 0.5 in the placebo group, a difference that was statistically significant. The mean improvement from baseline on the UPDRS part III total score was 5.6 in the group treated with MIRAPEX tablets and 2.8 in the placebo group, a difference that was statistically significant. A statistically significant difference between groups in favor of MIRAPEX tablets was seen at week 3 of the UPDRS part II (maximum dose 1.5 mg/day) and at week 2 of the UPDRS part III (maximum dose 0.75 mg/day). Dosage reduction of levodopa was allowed during this study if dyskinesia (or hallucinations) developed; levodopa dosage reduction occurred in 76% of patients treated with MIRAPEX tablets versus 54% of placebo patients. On average, the levodopa dose was reduced 27%.
- The mean number of "off" hours per day during baseline was 6 hours for both treatment groups. Throughout the trial, patients treated with MIRAPEX tablets had a mean of 4 "off" hours per day, while placebo-treated patients continued to experience 6 "off" hours per day.
- No differences in effectiveness based on age or gender were detected. There were too few non-Caucasian patients to evaluate the effect of race.
Restless Legs Syndrome
- The efficacy of MIRAPEX tablets in the treatment of RLS was evaluated in a multinational drug development program consisting of 4 randomized, double-blind, placebo-controlled trials. This program included approximately 1000 patients with moderate to severe RLS; patients with RLS secondary to other conditions (e.g., pregnancy, renal failure, and anemia) were excluded. All patients were administered MIRAPEX tablets (0.125 mg, 0.25 mg, 0.5 mg, or 0.75 mg) or placebo once daily 2-3 hours before going to bed. Across the 4 studies, the mean duration of RLS was 4.6 years (range of 0 to 56 years), mean age was approximately 55 years (range of 18 to 81 years), and approximately 66.6% were women.
- Key diagnostic criteria for RLS are: an urge to move the legs usually accompanied or caused by uncomfortable and unpleasant leg sensations; symptoms begin or worsen during periods of rest or inactivity such as lying or sitting; symptoms are partially or totally relieved by movement such as walking or stretching at least as long as the activity continues; and symptoms are worse or occur only in the evening or night. Difficulty falling asleep may frequently be associated with symptoms of RLS.
- The two outcome measures used to assess the effect of treatment were the International RLS Rating Scale (IRLS Scale) and a Clinical Global Impression - Improvement (CGI-I) assessment. The IRLS Scale contains 10 items designed to assess the severity of sensory and motor symptoms, sleep disturbance, daytime somnolence, and impact on activities of daily living and mood associated with RLS. The range of scores is 0 to 40, with 0 being absence of RLS symptoms and 40 the most severe symptoms. The CGI-I is designed to assess clinical progress (global improvement) on a 7-point scale.
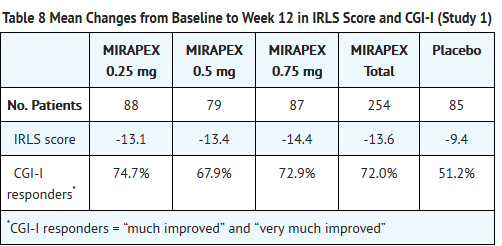
- In Study 1, fixed doses of MIRAPEX tablets were compared to placebo in a study of 12 weeks duration. A total of 344 patients were randomized equally to the 4 treatment groups. Patients treated with MIRAPEX tablets (n=254) had a starting dose of 0.125 mg/day and were titrated to one of the three randomized doses (0.25, 0.5, 0.75 mg/day) in the first three weeks of the study. The mean improvement from baseline on the IRLS Scale total score and the percentage of CGI-I responders for each of the MIRAPEX tablets treatment groups compared to placebo are summarized in Table 8. All treatment groups reached statistically significant superiority compared to placebo for both endpoints. There was no clear evidence of a dose-response across the 3 randomized dose groups.
- Study 2 was a randomized-withdrawal study, designed to demonstrate the sustained efficacy of pramipexole for treatment of RLS after a period of six months. RLS patients who responded to MIRAPEX tablets treatment in a preceding 6-month open-label treatment phase (defined as having a CGI-I rating of “very much improved” or “much improved” compared to baseline and an IRLS score of 15 or below) were randomized to receive either continued active treatment (n=78) or placebo (n=69) for 12 weeks. The primary endpoint of this study was time to treatment failure, defined as any worsening on the CGI-I score along with an IRLS Scale total score above 15.
- In patients who had responded to 6-month open label treatment with MIRAPEX tablets, the administration of placebo led to a rapid decline in their overall conditions and return of their RLS symptoms. At the end of the 12-week observation period, 85% of patients treated with placebo had failed treatment, compared to 21% treated with blinded pramipexole, a difference that was highly statistically significant. The majority of treatment failures occurred within 10 days of randomization. For the patients randomized, the distribution of doses was: 7 on 0.125 mg, 44 on 0.25 mg, 47 on 0.5 mg, and 49 on 0.75 mg.
- Study 3 was a 6-week study, comparing a flexible dose of MIRAPEX tablets to placebo. In this study, 345 patients were randomized in a 2:1 ratio to MIRAPEX tablets or placebo. The mean improvement from baseline on the IRLS Scale total score was -12 for MIRAPEX-treated patients and -6 for placebo-treated patients. The percentage of CGI-I responders was 63% for MIRAPEX-treated patients and 32% for placebo-treated patients. The between-group differences were statistically significant for both outcome measures. For the patients randomized to MIRAPEX tablets, the distribution of achieved doses was: 35 on 0.125 mg, 51 on 0.25 mg, 65 on 0.5 mg, and 69 on 0.75 mg.
- Study 4 was a 3-week study, comparing 4 fixed doses of MIRAPEX tablets, 0.125 mg, 0.25 mg, 0.5 mg, and 0.75 mg, to placebo. Approximately 20 patients were randomized to each of the 5 dose groups. The mean improvement from baseline on the IRLS Scale total score and the percentage of CGI-I responders for each of the MIRAPEX tablets treatment groups compared to placebo are summarized in Table 9. In this study, the 0.125 mg dose group was not significantly different from placebo. On average, the 0.5 mg dose group performed better than the 0.25 mg dose group, but there was no difference between the 0.5 mg and 0.75 mg dose groups.
- No differences in effectiveness based on age or gender were detected. There were too few non-Caucasian patients to evaluate the effect of race.
How Supplied
- MIRAPEX tablets are available as follows:
- 0.125 mg: white, round, tablet with "BI" on one side and "83" on the reverse side.
- Bottles of 90 NDC 0597-0183-90
- 0.25 mg: white, oval, scored tablet with "BI BI" on one side and "84 84" on the reverse side.
- Bottles of 90 NDC 0597-0184-90
- Unit dose packages of 100 NDC 0597-0184-61
- 0.5 mg: white, oval, scored tablet with "BI BI" on one side and "85 85" on the reverse side.
- Bottles of 90 NDC 0597-0185-90
- Unit dose packages of 100 NDC 0597-0185-61
- 0.75 mg: white, oval, debossed tablet with "BI” on one side and "101" on the reverse side.
- Bottles of 90 NDC 0597-0101-90
- 1 mg: white, round, scored tablet with "BI BI" on one side and "90 90" on the reverse side.
- Bottles of 90 NDC 0597-0190-90
- Unit dose packages of 100 NDC 0597-0190-61
- 1.5 mg: white, round, scored tablet with "BI BI" on one side and "91 91" on the reverse side.
- Bottles of 90 NDC 0597-0191-90
- Unit dose packages of 100 NDC 0597-0191-61
- Storage
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
- Protect from light.
- Store in a safe place out of the reach of children.
Storage
There is limited information regarding Pramipexole Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pramipexole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pramipexole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Dosing Instructions
- Instruct patients to take MIRAPEX tablets only as prescribed. If a dose is missed, advise patients not to double their next dose.
- MIRAPEX tablets can be taken with or without food. If patients develop nausea, they should be advised that taking MIRAPEX tablets with food may reduce the occurrence of nausea.
- Pramipexole is the active ingredient that is in both MIRAPEX tablets and extended-release pramipexole tablets. Ensure that patients do not take both extended-release pramipexole and MIRAPEX.
- Sedating Effects
- Patients should be alerted to the potential sedating effects associated with MIRAPEX tablets, including somnolence and the possibility of falling asleep while engaged in activities of daily living. Since somnolence is a frequent adverse event with potentially serious consequences, patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with MIRAPEX tablets to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., conversations or eating) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Because of possible additive effects, caution should be advised when patients are taking other sedating medications or alcohol in combination with MIRAPEX tablets and when taking concomitant medications that increase plasma levels of pramipexole (e.g., cimetidine).
- Impulse Control Symptoms Including Compulsive Behaviors
- Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges, binge eating and/or other intense urges and the inability to control these urges while taking MIRAPEX.
- Hallucinations
- Patients should be informed that hallucinations can occur and that the elderly are at a higher risk than younger patients with Parkinson's disease. In clinical trials, patients with RLS treated with pramipexole rarely reported hallucinations.
- Postural (Orthostatic) Hypotension
- Advise patients that they may develop orthostatic hypotension, with or without symptoms such as dizziness, nausea, fainting or blackouts, and sometimes, sweating. Hypotension may occur more frequently during initial therapy. Accordingly, caution patients against rising rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with MIRAPEX tablets.
- Pregnancy
- Because the teratogenic potential of pramipexole has not been completely established in laboratory animals, and because experience in humans is limited, advise women to notify their physicians if they become pregnant or intend to become pregnant during therapy.
- Nursing Mothers
- Because of the possibility that pramipexole may be excreted in breast milk, advise women to notify their physicians if they intend to breast-feed or are breast-feeding an infant.
Precautions with Alcohol
- Alcohol-Pramipexole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MIRAPEX®[1]
Look-Alike Drug Names
- Mirapex® — Hiprex®[2]
- Mirapex® — Miralax®[2]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "MIRAPEX- pramipexole dihydrochloride tablet".
- ↑ 2.0 2.1 "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Pramipexole |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole11.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole12.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole13.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole14.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole15.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole16.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole17.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole18.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole19.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole20.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole21.png
}}
{{#subobject:
|Label Page=Pramipexole |Label Name=Pramipexole21.png
}}