Agomelatine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Melitor, Thymanax, Valdoxan |
| AHFS/Drugs.com | International Drug Names |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80%[1] |
| Protein binding | 95%[1] |
| Metabolism | hepatic (90% CYP1A2 and 10% CYP2C9)[1] |
| Elimination half-life | 1-2 hours[1] |
| Excretion | Renal (80%, mostly as metabolites)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
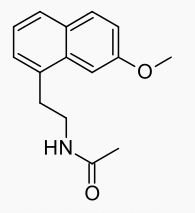
| Formula | C15H17NO2 |
| Molar mass | 243.301 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Agomelatine (BAN, rINN; trade names Valdoxan, Melitor, Thymanax) is a melatonergic antidepressant developed by the pharmaceutical company Servier. It is marketed for the treatment of major depressive disorder and has been reported not to produce discontinuation syndrome or sexual side effects (compared to SSRIs, SNRIs and the older tricyclic antidepressants). Agomelatine may also have positive[clarification needed] effects on sleep and cognition.
History
Agomelatine was discovered and developed by the European pharmaceutical company Servier Laboratories Ltd. Servier continued to develop the drug and conduct phase III trials in the European Union.
In March 2005, Servier submitted agomelatine to the European Medicines Agency (EMA) under the trade names Valdoxan and Thymanax.[2] On 27 July 2006, the Committee for Medical Products for Human Use (CHMP) of the EMA recommended a refusal of the marketing authorisation of Valdoxan/Thymanax (agomelatine). The major concern was that efficacy had not been sufficiently shown. The CHMP had no special concerns about side effects.[2] In September 2007, Servier submitted a new marketing application for Valdoxan (agomelatine) to the EMA.[3]
In March 2006, Servier announced it had sold the rights to market agomelatine in the United States to Novartis.[4] It was undergoing several phase III clinical trials in the US, and until October 2011 Novartis listed the drug as scheduled for submission to the FDA no earlier than 2012.[5] However, the development for the US market was discontinued in October 2011, when the results from the last of those trials became available.[6]
It received EMA approval for marketing in the European Union in February 2009[7] and TGA approval for marketing in Australia in August 2010.[1]
Medical uses
Major depressive disorder
Agomelatine is indicated for the treatment of major depressive episodes in adults.[7] Ten placebo controlled trials have been performed to investigate the short term efficacy of agomelatine in major depressive disorder. At the end of treatment, significant efficacy was demonstrated in six of the ten short-term double-blind placebo-controlled studies.[7] Two were considered "failed" trials, as comparators of established efficacy failed to differentiated from placebo. Efficacy was also observed in more severely depressed patients in all positive placebo-controlled studies.[7] The maintenance of antidepressant efficacy was demonstrated in a relapse prevention study.[7]
Results of the meta-analysis of three positive, randomized, double-blind, placebo controlled studies in 357 patients treated with agomelatine and 360 patients treated with placebo show that agomelatine is effective in treating severe depression. Its antidepressant effect is greater for more severe depression. In patients with a greater baseline score (>30 on HAMD17 scale), the agomelatine-placebo difference was of 4.53 points.[8] Controlled studies in humans have shown that agomelatine is at least as effective as the SSRI antidepressants paroxetine, sertraline, escitalopram, venlafaxine and fluoxetine in the treatment of major depression.[9][10][11]
A large meta-analysis of 20 trials with 7460 participants found agomelatine to be as effective as standard antidepressants.[12]
A small open-label study has suggested efficacy in the treatment of atypical and melancholic depression.[13] Well-designed clinical trials have demonstrated efficacy in the treatment of anxious depression.[14][15] Agomelatine’s onset of action has been reported to occur as early as the first week of treatment.[16]
Investigational
Additionally, possibly because of its action on melatonin receptors, agomelatine appears to improve sleep quality, with no reported daytime drowsiness.[17] Agomelatine has demonstrated anxiolytic properties in rodents.[18] It has been found significantly more effective than placebo in the treatment of generalised anxiety disorder.[19] There is tentative evidence to suggest the efficacy of agomelatine as either a monotherapy or adjunct in the treatment of obsessive-compulsive disorder.[20][21][22][23] A case report documenting the efficacy of agomelatine in the treatment of social anxiety disorder has been published.[24] An open-label study has found agomelatine to be efficacious in the treatment of seasonal affective disorder.[25] Open-label studies have suggested efficacy of adjunctive agomelatine in bipolar depression.[26][27] A study in mice found that protected against pentylenetetrazole- and pilocarpine-induced seizures.[28] A small placebo-controlled trial found some benefit of agomelatine in ADHD.[29]
Use in special populations
It is not recommended for use in children and adolescents below 18 years of age due to a lack of data on safety and efficacy.[7] Only limited clinical data is available on the use of agomelatine in elderly patients ≥ 65 years old with major depressive episodes. Therefore, caution should be exercised when prescribing it to these patients.[7]
Adverse effects
Agomelatine does not alter daytime vigilance and memory in healthy volunteers. In depressed patients, treatment with the drug increased slow wave sleep without modification of REM (Rapid Eye Movement) sleep amount or REM latency. Agomelatine also induced an advance of the time of sleep onset and of minimum heart rate. From the first week of treatment, onset of sleep and the quality of sleep were significantly improved without daytime clumsiness as assessed by patients.[1][7]
Agomelatine appears to cause fewer sexual side effects and discontinuation effects than paroxetine.[1] It appears better tolerated than the SSRIs.[10]
- Hyperhidrosis (excess sweating that is not proportionate to the ambient temperature)
- Abdominal pain
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Back pain
- Fatigue
- Increased ALAT and ASAT (liver enzymes)
- Headache
- Dizziness
- Somnolence
- Insomnia
- Migraine
- Anxiety
- Paraesthesia (abnormal sensations [e.g. itching, burning, tingling, etc.] due to malfunctioning of the peripheral nerves)
- Blurred vision
- Eczema
- Pruritus (itching)
- Urticaria
- Agitation
- Irritability
- Restlessness
- Aggression
- Nightmares
- Abnormal dreams
Note the following two side effects are often due to an underlying disease, namely bipolar disorder.
- Mania
- Hypomania
- Suicidal ideation
- Suicidal behaviour
- Hallucinations
- Hepatitis
- Increased GGT and/or alkaline phosphatase
- Liver failure
- Jaundice
- Erythematous rash
- Face oedema and angioedema
- Weight gain or loss, which tends to be less significant than with SSRIs[32]
Contraindications
Agomelatine is contraindicated in patients with kidney or liver impairment.[7] According to information disclosed by Les Laboratoires Servier on October 10, 2012, guidelines for the follow-up of patients treated with Valdoxan have been modified in concert with the European Medicines Agency. As some patients may experience increased levels of liver enzymes in their blood during treatment with Valdoxan, doctors have to run laboratory tests to check that the liver is working properly at the initiation of the treatment and then periodically during treatment, and subsequently decided whether pursue the treatment or not.[33] No relevant modification in agomelatine pharmacokinetic parameters in patients with severe renal impairment has been observed. However, only limited clinical data on its use in depressed patients with severe or moderate renal impairment with major depressive episodes is available. Therefore, caution should be exercised when prescribing agomelatine to these patients.[7]
Interactions
Agomelatine is a substrate of CYP1A2, CYP2C9 and CYP2C19 and hence CYP1A2, CYP2C9 and CYP2C19 inhibitors (e.g. the SSRI antidepressant, fluvoxamine) reduce its clearance and can hence lead to an increase in agomelatine exposure.[1][30] There is also the potential for agomelatine to interact with alcohol to increase the risk of hepatotoxicity (liver toxicity).[1][30]
Overdose
Agomelatine is expected to be relatively safe in overdose.[34]
Dependence and withdrawal
No dosage tapering is needed on treatment discontinuation.[7] Agomelatine has no abuse potential as measured in healthy volunteer studies.[1][7]
Structure
The chemical structure of agomelatine is very similar to that of melatonin. Where melatonin has an NH group, agomelatine has an HC=CH group. Thus melatonin contains an indole part, whereas agomelatine has a naphthalene bioisostere instead.[35]
Mechanism of action
Agomelatine is a melatonin receptor agonist (MT1 (Ki=0.1nM) and MT2 (Ki=0.12nM)) and a 5-HT2C (Ki=631nM) receptor antagonist.[36] Binding studies indicate that it has no effect on monoamine uptake and no affinity for adrenergic, histaminergic, cholinergic, dopaminergic and benzodiazepine receptors, nor other serotonergic receptors.[7]
Agomelatine resynchronises circadian rhythms in animal models of delayed sleep phase syndrome.[37] By antagonizing 5-HT2C receptors, it disinhibits/increases noradrenaline and dopamine release specifically in the frontal cortex. Therefore, it is sometimes classified as a norepinephrine–dopamine disinhibitor. It has no influence on the extracellular levels of serotonin. Agomelatine has shown an antidepressant-like effect in animal models of depression (learned helplessness test, despair test, chronic mild stress) as well as in models with circadian rhythm desynchronisation and in models related to stress and anxiety. In humans, agomelatine has positive phase shifting properties; it induces a phase advance of sleep, body temperature decline and melatonin onset.[7]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "VALDOXAN® Product Information" (PDF). TGA eBusiness Services. Servier Laboratories Pty Ltd. 2013-09-23. Retrieved 2013-10-14.
- ↑ 2.0 2.1 "Questions and Answers on Recommendation for Refusal of Marketing Authorisation" (PDF). European Medicines Agency. 18 November 2006. Retrieved 6 July 2009.
- ↑ "CHMP Assessment Report for Valdoxan" (PDF). European Medicines Agency. 20 November 2008. Retrieved 6 July 2009.
- ↑ Bentham, Clara (2006-03-29). "Servier and Novartis sign licensing agreement for agomelatine, a novel treatment for depression". Servier UK. Archived from the original on 16 April 2009. Retrieved 2009-05-15.
- ↑ "Clinical trials for agomelatine". ClinicalTrials.gov. National Institutes of Health. Retrieved 6 July 2009.
- ↑ Novartis drops future blockbuster agomelatine. Scrip Intelligence, Oct 25 2011 (retrieved Oct 30, 2011).
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 "Summary of Product Characteristics" (PDF). European Medicine Agency. Retrieved 2013-10-14.
- ↑ Montgomery, SA; Kasper, S (September 2007). "Severe depression and antidepressants: focus on a pooled analysis of placebo-controlled studies on agomelatine". Int Clin Psychopharmacol. 22 (5): 283–91. doi:10.1097/YIC.0b013e3280c56b13. PMID 17690597.
- ↑ Kasper S, Hajak G, Wulff K, Hoogendijk WJ, Montejo AL, Smeraldi E, Rybakowski JK, Quera-Salva MA, Wirz-Justice AM, Picarel-Blanchot F, Baylé FJ (February 2010). "Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline". J Clin Psychiatry. 71 (2): 109–20. doi:10.4088/JCP.09m05347blu. PMID 20193645.
- ↑ 10.0 10.1 Demyttenaere, K; Corruble, E; Hale, A; Quera-Salva, MA; Picarel-Blanchot, F; Kasper, S (June 2013). "A pooled analysis of six month comparative efficacy and tolerability in four randomized clinical trials: agomelatine versus escitalopram, fluoxetine, and sertraline" (PDF). CNS Spectrums. 18 (3): 163–170. doi:10.1017/S1092852913000060. PMID 23472671.
- ↑ Singh, SP; Sing, V; Kar, N (April 2012). "Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal". International Journal of Neuropsychopharmacology. 15 (3): 417–428. doi:10.1017/S1461145711001301. PMID 21859514.
- ↑ Template:Cite doi
- ↑ Avedisova, A; Marachev, M (2013). "2639 – The effectiveness of agomelatine (valdoxan) in the treatment of atypical depression". European Psychiatry. 28 (Suppl. 1): 1. doi:10.1016/S0924-9338(13)77272-9. ISSN 0924-9338.
- ↑ Heun, R; Coral, RM; Ahokas, A; Nicolini, H; Teixeira, JM, Dehelean, P (2013). "1643 – Efficacy of agomelatine in more anxious elderly depressed patients. A randomized, double-blind study vs placebo". European Psychiatry. 28 (Suppl. 1): 1. doi:10.1016/S0924-9338(13)76634-3. ISSN 0924-9338.
- ↑ Stein, DJ; Picarel-Blanchot, F; Kennedy, SH (March 2013). "Efficacy of the novel antidepressant agomelatine for anxiety symptoms in major depression". Human Psychopharmacology: Clinical and Experimental. 28 (2): 151–159. doi:10.1002/hup.2294. PMID 23532747.
- ↑ Lemoine, P; Guilleminault, C; Alvarez, E (November 2007). "Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine". J Clin Psychiatry. 68 (11): 1723–32. doi:10.4088/JCP.v68n1112. PMID 18052566.
- ↑ "Valdoxan: A New Approach to The Treatment of Depression". Medical News Today. MediLexicon International Ltd. 2005-04-05. Archived from the original on 15 April 2009. Retrieved 14 May 2009.
- ↑ Millan MJ, Brocco M, Gobert A, Dekeyne A (February 2005). "Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade". Psychopharmacology (Berl.). 177 (4): 448–58. doi:10.1007/s00213-004-1962-z. PMID 15289999.
- ↑ PMID 23755745 (PMID 23755745)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Fornaro, M (February 2011). "Switching from serotonin reuptake inhibitors to agomelatine in patients with refractory obsessive-compulsive disorder: a 3 month follow-up case series". Annals of General Psychiatry. 10 (1): 5. doi:10.1186/1744-859X-10-5. PMC 3058071. PMID 21356085.
- ↑ De Berardis, D (2012). "Agomelatine augmentation of escitalopram therapy in treatment-resistant obsessive-compulsive disorder: a case report" (PDF). Case Reports in Psychiatry. 2012: 1. doi:10.1155/2012/642752. PMC 3474211. PMID 23094178. 642752.
- ↑ De Berardis, D; Serroni, N; Campanella, D; Olivieri, L; Moschetta, FS; Conti, CM; Conti, P; Di Giannantonio, M (April 2012). "A case of obsessive-compulsive disorder successfully treated with agomelatine monotherapy". Journal of Clinical Psychopharmacology. 32 (2): 289–290. doi:10.1097/JCP.0b013e318249298c. PMID 22388158.
- ↑ Bhutada, P; Dixit, P; Thakur, K; Deshmukh, P; Kaulaskar, S (July 2013). "Effects of agomelatine in a murine model of obsessive–compulsive disorder: Interaction with meta-chlorophenylpiperazine, bicuculline, and diazepam". The Kaohsiung Journal of Medical Sciences. 29 (7): 362–367. doi:10.1016/j.kjms.2012.11.003. PMID 23768699.
- ↑ Crippa, JA; Hallak, JE; Zuardi, AW; Chagas, MH; Quevedo, J; Nardi, AE (October 2010). "Agomelatine in the treatment of social anxiety disorder". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 34 (7): 1357–1358. doi:10.1016/j.pnpbp.2010.07.007. PMID 20637821.
- ↑ Pjrek, E; Winkler, D; Konstantinidis, A; Willeit, M; Praschak-Rieder, N; Kasper, S (March 2007). "Agomelatine in the treatment of seasonal affective disorder". Psychopharmacology. 190 (4): 575–579. doi:10.1007/s00213-006-0645-3. PMID 17171557.
- ↑ Fornaro, M; McCarthy, MJ; De Berardis, D; De Pasquale, C; Tabaton, M; Martino, M; Colicchio, S; Cattaneo, CI; D'Angelo, E; Fornaro, P (2013). "Adjunctive agomelatine therapy in the treatment of acute bipolar II depression: a preliminary open label study". Neuropsychiatric Disease and Treatment. 9: 243–251. doi:10.2147/NDT.S41557. PMC 3575211. PMID 23430979.
- ↑ Calabrese, JR; Guelfi, JD; Perdrizet-Chevallier, C (September 2007). "Agomelatine adjunctive therapy for acute bipolar depression: preliminary open data". Bipolar Disorders. 9 (6): 628–635. doi:10.1111/j.1399-5618.2007.00507.x. PMID 17845278.
- ↑ Aguiar, CC; Almeida, AB; Araújo, PV; Vasconcelos, GS; Chaves, EM; do Vale, OC; Macêdo, DS; de Sousa, FC; Viana, GS; Vasconcelos, SM (July 2012). "Anticonvulsant effects of agomelatine in mice". Epilepsy & Behavior. 24 (3): 324–328. doi:10.1016/j.yebeh.2012.04.134. PMID 22658946.
- ↑ Niederhofer, H (May 2012). "Treating ADHD with Agomelatine". Journal of Attention Disorders. 16 (4): 346–348. doi:10.1177/1087054711417400. PMID 22491963.
- ↑ 30.0 30.1 30.2 30.3 30.4 Australian Medicines Handbook 2013. Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 9780980579093.
- ↑ 31.0 31.1 31.2 Joint Formulary Committee and Royal Pharmaceutical Society of Great Britain (2013). British National Formulary (BNF) 65. London, UK: Pharmaceutical Press. p. 253. ISBN 978-0857110848.
- ↑ Kennedy, SH; Rizvi, SJ (June 2010). "Agomelatine in the Treatment of Major Depressive Disorder Potential for Clinical Effectiveness". CNS Drugs. 24 (6): 479–499. doi:10.2165/11534420-000000000-00000. PMID 20192279.
- ↑ http://www.servier.com/content/information-about-valdoxan-patients.
- ↑ Template:Cite isbn
- ↑ B. Tinant, J.-P. Declercq, J. H. Poupaert, S. Yous, D. Lesieur (1994). "N-[2-(7-Methoxy-1-naphthyl)ethyl]acetamide, a potent melatonin analog". Acta Cryst. C. 50 (6): 907–910. doi:10.1107/S0108270193012922.
- ↑ Dridi, D; Zouiten, A; Mansour, HB (June 2013). "Depression: chronophysiology and chronotherapy". Biological Rhythm Research. 45: 1–15. doi:10.1080/09291016.2013.797657.
- ↑ Le Strat, Y; Philip Gorwood (27 August 2008). "Agomelatine, an innovative pharmacological response to unmet needs". J Psychopharmacol. SagePub. 22 (7): suppl 4–8. doi:10.1177/0269881108092593. PMID 18753276. Retrieved 2010-10-15.
External links
- Official product site
- Manufacturer web site
- Agomelatine Psychonauts Google Group
- Novartis pipeline
- Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors
- The Novel Melatonin Agonist Agomelatine (S20098) Is an Antagonist at 5-Hydroxytryptamine2C Receptors, Blockade of Which Enhances the Activity of Frontocortical Dopaminergic and Adrenergic Pathways
- Agomelatine treatment has promising results in transgenic murine model
- Clinical trial data in the United Kingdom via the NHS' National electronic Library of Medicines (NeLM)
- Clinical trial data in the United States via ClinicalTrials.gov
Template:Antidepressants Template:Hypnotics Template:Insomnia pharmacotherapies
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with incomplete PMID references
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed DrugBank identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Wikipedia articles needing clarification from July 2014
- Articles with invalid date parameter in template
- Pages with broken file links
- Antidepressants
- Melatonin receptor agonists
- 5-HT2C antagonists
- Acetamides
- Naphthol ethers
- Laboratoires Servier