Droperidol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Cases of QT prolongation and/or torsade de pointes have been reported in patients receiving Droperidol at doses at or below recommended doses. Some cases have occurred in patients with no known risk factors for QT prolongation and some cases have been fatal.
See full prescribing information for complete Boxed Warning.
: Due to its potential for serious proarrhythmic effects and death, Droperidol should be reserved for use in the treatment of patients who fail to show an acceptable response to other adequate treatments, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs (see Warnings, Adverse Reactions, Contraindications, and Precautions).
|
Overview
Droperidol is a general anesthetic that is FDA approved for the {{{indicationType}}} of prophylaxis use in nausea and vomiting, associated with surgical or diagnostic procedures;. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cardiovascular: hypotension, tachycardia, neurologic: somnolence, postoperative, psychiatric: anxiety, dysphoric mood, hyperactive behavior, restlessness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Chemotherapy-induced nausea and vomiting; prophylaxis: optimal dosing and timing not yet defined
- Nausea and vomiting, associated with surgical or diagnostic procedures; prophylaxis: initial maximum dose 2.5 mg IM/IV, may repeat 1.25 mg dose based on patient response, caution is advise
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Agitation - Psychotic disorder.
- Chemotherapy-induced nausea and vomiting; Prophylaxis.
- Headache, Benign.
- Migraine.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Droperidol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Nausea and vomiting, associated with surgical or diagnostic procedures; Prophylaxis: (2 to 12 years) initial maximum dose 0.1 mg/kg slow IV/IM, may repeat 0.1 mg/kg dose based on patient response, caution is advised
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Droperidol in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Droperidol in pediatric patients.
Contraindications
- Droperidol is contraindicated in patients with known or suspected QT prolongation (i.e., QTc interval greater than 440 msec for males or 450 msec for females). This would include patients with congenital long QT syndrome.
- Droperidol (droperidol) is contraindicated in patients with known hypersensitivity to the drug.
- Droperidol is not recommended for any use other than for the treatment of perioperative nausea and vomiting in patients for whom other treatments are ineffective or inappropriate (see Warnings).
Warnings
|
Cases of QT prolongation and/or torsade de pointes have been reported in patients receiving Droperidol at doses at or below recommended doses. Some cases have occurred in patients with no known risk factors for QT prolongation and some cases have been fatal.
See full prescribing information for complete Boxed Warning.
: Due to its potential for serious proarrhythmic effects and death, Droperidol should be reserved for use in the treatment of patients who fail to show an acceptable response to other adequate treatments, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs (see Warnings, Adverse Reactions, Contraindications, and Precautions).
|
- Droperidol should be administered with extreme caution in the presence of risk factors for development of prolonged QT syndrome, such as:
- 1) clinically significant bradycardia (less than 50 bpm)
- 2) any clinically significant cardiac disease
- 3) treatment with Class I and Class III antiarrhythmics
- 4) treatment with monoamine oxidase inhibitors (MAOI's)
- 5) concomitant treatment with other drug products known to prolong the QT interval (see Precautions, Drug Interactions), and
- 6) electrolyte imbalance, in particular hypokalemia and hypomagnesemia, or concomitant treatment with drugs (e.g., diuretics) that may cause electrolyte imbalance.
Effects on Cardiac Conduction
- A dose-dependent prolongation of the QT interval was observed within 10 minutes of droperidol administration in a study of 40 patients without known cardiac disease who underwent extracranial head and neck surgery. Significant QT prolongation was observed at all three dose levels evaluated, with 0.1, 0.175, and 0.25 mg/kg associated with prolongation of median QTc by 37, 44, and 59 msec, respectively.
- Cases of QT prolongation and serious arrhythmias (e.g. torsade de pointes, ventricular arrythmias, cardiac arrest, and death) have been observed during post-marketing treatment with Droperidol. Some cases have occurred in patients with no known risk factors and at doses at or below recommended doses. There has been at least one case of nonfatal torsade de pointes confirmed by rechallenge.
- Based on these reports, all patients should undergo a 12-lead ECG prior to administration of Droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, Droperidol should NOT be administered. For patients in whom the potential benefit of Droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2 to 3 hours after completing treatment to monitor for arrhythmias.
- Fluids And Other Countermeasures To Manage Hypotension Should Be Readily Available.
- As with other CNS depressant drugs, patients who have received Droperidol (droperidol) should have appropriate surveillance.
- It is recommended that opioids, when required, initially be used in reduced doses.
- As with other neuroleptic agents, very rare reports of neuroleptic malignant syndrome (altered consciousness, muscle rigidity and autonomic instability) have occurred in patients who have received Droperidol (droperidol). Since it may be difficult to distinguish neuroleptic malignant syndrome from malignant hyperpyrexia in the perioperative period, prompt treatment with dantrolene should be considered if increases in temperature, heart rate or carbon dioxide production occur.
Adverse Reactions
Clinical Trials Experience
QT interval prolongation, torsade de pointes, cardiac arrest, and ventricular tachycardia have been reported in patients treated with Droperidol. Some of these cases were associated with death. Some cases occurred in patients with no known risk factors, and some were associated with droperidol doses at or below recommended doses.
- Physicians should be alert to palpitations, syncope, or other symptoms suggestive of episodes of irregular cardiac rhythm in patients taking Droperidol and promptly evaluate such cases (see Warnings, Effects on Cardiac Conduction).
- The most common somatic adverse reactions reported to occur with Droperidol (droperidol) are mild to moderate hypotension and tachycardia, but these effects usually subside without treatment. If hypotension occurs and is severe or persists, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy.
- The most common behavioral adverse effects of Droperidol (droperidol) include dysphoria, postoperative drowsiness, restlessness, hyperactivity and anxiety, which can either be the result of an inadequate dosage (lack of adequate treatment effect) or of an adverse drug reaction (part of the symptom complex of akathisia).
- Care should be taken to search for extrapyramidal signs and symptoms (dystonia, akathisia, oculogyric crisis) to differentiate these different clinical conditions. When extrapyramidal symptoms are the cause, they can usually be controlled with anticholinergic agents.
- Postoperative hallucinatory episodes (sometimes associated with transient periods of mental depression) have also been reported.
- Other less common reported adverse reactions include anaphylaxis, dizziness, chills and/or shivering, laryn-gospasm, and bronchospasm.
- Elevated blood pressure, with or without pre-existing hypertension, has been reported following administration of Droperidol combined with Sublimaze (fentanyl citrate) or other parenteral analgesics. This might be due to unexplained alterations in sympathetic activity following large doses: however, it is also frequently attributed to anesthetic or surgical stimulation during light anesthesia.
Postmarketing Experience
There is limited information regarding Droperidol Postmarketing Experience in the drug label.
Drug Interactions
Potentially Arrhythmogenic Agents: Any drug known to have the potential to prolong the QT interval should not be used together with Droperidol. Possible pharmacodynamic interactions can occur between Droperidol and potentially arrhythmogenic agents such as class I or III antiarrhythmics, antihistamines that prolong the QT interval, antimalarials, calcium channel blockers, neuroleptics that prolong the QT interval, and antidepressants.
- Caution should be used when patients are taking concomitant drugs known to induce hypokalemia or Hypomagnesemia as they may precipitate QT prolongation and interact with Droperidol. These would include diuretics, laxatives and supraphysiological use of steroid hormones with mineralocorticoid potential.
- CNS Depressant Drugs: Other CNS depressant drugs (e.g., barbiturates, tranquilizers, opioids and general anesthetics) have additive or potentiating effects with Droperidol. Following the administration of Droperidol, the dose of other CNS depressant drugs should be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
- No carcinogenicity studies have been carried out with Droperidol. The micronucleus test in female rats revealed no mutagenic effects in single oral doses as high as 160 mg/kg. An oral study in rats (Segment I) revealed no impairment of fertility in either male or females at 0.63. 2.5 and 10 mg/kg doses (approximately 2.9 and 36 times maximum recommended human iv/im dosage).
Pregnancy: Category C
- Droperidol administered intravenously has been shown to cause a slight increase in mortality of the newborn rat at 4.4 times the upper human dose. At 44 times the upper human dose, mortality rate was comparable to that for control animals. Following intramuscular administration, increased mortality of the offspring at 1.8 times the upper human dose is attributed to CNS depression in the dams who neglected to remove placentae from their offspring. Droperidol has not been shown to be teratogenic in animals. There are no adequate and well-controlled studies in pregnant women. Droperidol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
- There are insufficient data to support the use of Droperidol in labor and delivery. Therefore, such use is not recommended.
Nursing Mothers
- It is not known whether Droperidol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Droperidol is administered to a nursing mother.
Pediatric Use
- The safety of Droperidol in children younger than two years of age has not been established.
Use in Specific Populations
Pregnancy
- Droperidol administered intravenously has been shown to cause a slight increase in mortality of the newborn rat at 4.4 times the upper human dose. At 44 times the upper human dose, mortality rate was comparable to that for control animals. Following intramuscular administration, increased mortality of the offspring at 1.8 times the upper human dose is attributed to CNS depression in the dams who neglected to remove placentae from their offspring. Droperidol has not been shown to be teratogenic in animals. There are no adequate and well-controlled studies in pregnant women. Droperidol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Droperidol in women who are pregnant.
Labor and Delivery
- There are insufficient data to support the use of Droperidol in labor and delivery. Therefore, such use is not recommended.
Nursing Mothers
- It is not known whether Droperidol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Droperidol is administered to a nursing mother.
Pediatric Use
- The safety of Droperidol in children younger than two years of age has not been established.
Geriatic Use
There is no FDA guidance on the use of Droperidol in geriatric settings.
Gender
There is no FDA guidance on the use of Droperidol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Droperidol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Droperidol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Droperidol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Droperidol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Droperidol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Droperidol Administration in the drug label.
Monitoring
There is limited information regarding Droperidol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Droperidol and IV administrations.
Overdosage
Manifestations
- The manifestations of Droperidol (droperidol) overdosage are an extension of its pharmacologic actions and may include QT prolongation and serious arrhythmias (e.g., torsade de pointes) (see Box Warning, Warnings, and Precautions).
Treatment
- In the presence of hypoventilation or apnea, oxygen should be administered and respiration should be assisted or controlled as indicated. A patent airway must be maintained; an oropharyngeal airway or endotracheal tube might be indicated. The patient should be carefully observed for 24 hours; body warmth and adequate fluid intake should be maintained. If hypotension occurs and is severe or persists, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy (see Precautions).
- If significant extrapyramidal reactions occur in the context of an overdose, an anticholinergic should be administered.
- The intravenous Median Lethal Dose of Droperidol is 20 to 43 mg/kg in mice; 30 mg/kg in rats; 25 mg/kg in dogs and 11 to 13 mg/kg in rabbits. The intramuscular Median Lethal Dose of Droperidol is 195 mg/kg in mice; 104 to 110 mg/kg in rats; 97 mg/kg in rabbits and 200 mg/kg in guinea pigs.
Pharmacology
| |
| |
Droperidol
| |
| Systematic (IUPAC) name | |
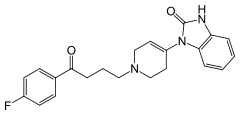
| 1-{1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,5,6-tetrahydropyridin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 379.428 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Half life | 2.3 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C (US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous, Intramuscular |
Mechanism of Action
- Droperidol (droperidol) produces marked tranquilization and sedation. It allays apprehension and provides a state of mental detachment and indifference while maintaining a state of reflex alertness.
- Droperidol produces an antiemetic effect as evidenced by the antagonism of apomorphine in dogs. It lowers the incidence of nausea and vomiting during surgical procedures and provides antiemetic protection in the postoperative period.
- Droperidol potentiates other CNS depressants. It produces mild alpha-adrenergic blockade, peripheral vascular dilatation and reduction of the pressor effect of epinephrine. It can produce hypotension and decreased peripheral vascular resistance and may decrease pulmonary arterial pressure (particularly if it is abnormally high). It may reduce the incidence of epinephrine-induced arrhythmias, but it does not prevent other cardiac arrhythmias.
- The onset of action of single intramuscular and intravenous doses is from three to ten minutes following administration, although the peak effect may not be apparent for up to thirty minutes. The duration of the tranquilizing and sedative effects generally is two to four hours, although alteration of alertness may persist for as long as twelve hours.
Structure
Droperidol contains droperidol, a neuroleptic (tranquilizer) agent. Droperidol® (droperidol) Injection is available in ampules and vials. Each milliliter contains 2.5 mg of droperidol in an aqueous solution adjusted to pH 3.4 ± 0.4 with lactic acid. Droperidol is chemically identified as l-(l-[3-(p-fluorobenzoyl) propyl]-l,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone with a molecular weight of 379.43. The structural formula of droperidol is:

- Molecular formula: C22H22FN3O2, partition coefficient in n-octanol: water: 3.46, pKa: 7.46
- INAPSINE is a sterile, non-pyrogenic, aqueous solution for intravenous or intramuscular injection.
Pharmacodynamics
There is limited information regarding Droperidol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Droperidol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Droperidol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Droperidol Clinical Studies in the drug label.
How Supplied
There is limited information regarding Droperidol How Supplied in the drug label.
Storage
There is limited information regarding Droperidol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Droperidol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Droperidol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Droperidol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Droperidol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Droperidol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Droperidol Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Droperidol |Label Name=Droperidol label.png
}}