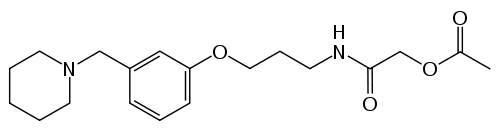
Roxatidine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 5–7% |
| Metabolism | Hepatic deacetylation Minor involvement of CYP2D6 and CYP2A6 |
| Elimination half-life | 5–7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H28N2O4 |
| Molar mass | 348.437 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Roxatidine |
|
Articles |
|---|
|
Most recent articles on Roxatidine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Roxatidine at Clinical Trials.gov Clinical Trials on Roxatidine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Roxatidine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Roxatidine Discussion groups on Roxatidine Patient Handouts on Roxatidine Directions to Hospitals Treating Roxatidine Risk calculators and risk factors for Roxatidine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Roxatidine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Roxatidine acetate is a specific and competitive histamine H2 receptor antagonist drug that is used to treat gastric ulcers, Zollinger–Ellison syndrome, erosive esophagitis, gastro-oesophageal reflux disease, and gastritis.[1][2]
Pharmacodynamic studies showed that 150 mg of roxatidine acetate were optimal in suppressing gastric acid secretion, and that a single bedtime dose of 150 mg was more effective than a dose of 75 mg twice daily in terms of inhibiting nocturnal acid secretion.[1]
It is available in countries including China, Japan, Korea, Germany, Italy, the Netherlands, Greece and South Africa.[2]
References
- ↑ 1.0 1.1 Murdoch D, McTavish D (1991). "Roxatidine acetate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic potential in peptic ulcer disease and related disorders". Drugs. 42 (2): 240–260. doi:10.2165/00003495-199142020-00006. PMID 1717223.
- ↑ 2.0 2.1 BioSpectrum Bureau 1 November 2012 Sinhuan's generic heart drug gets production approval
- Pages with script errors
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- H2 receptor antagonists
- Piperidines
- Phenol ethers
- Drug