Methysergide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Retroperitoneal Fibrosis, Pleuropulmonary Fibrosis and Fibrotic Thickening of Cardiac Valves May Occur in Patients Receiving Long-term Methysergide Maleate Therapy. Therefore, This Preparation Must Be Reserved for Prophylaxis in Patients Whose Vascular Headaches Are Frequent and/or Severe and Uncontrollable and Who Are Under Close Medical Supervision.
|
Overview
Methysergide is an antimigraine that is FDA approved for the prophylaxis of vascular headaches. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 4-8 mg daily.
- Tablets to be given with meals.
- There must be a medication-free interval of 3-4 weeks after every 6-month course of treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methysergide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methysergide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Methysergide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methysergide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methysergide in pediatric patients.
Contraindications
Hypersensitivity to the drug or to tartrazine (FD&C Yellow #5) or any other components of the formulation, pregnancy, lactation, peripheral vascular disease, severe arteriosclerosis, severe hypertension, coronary artery disease, phlebitis or cellulitis of the lower limbs, pulmonary disease, collagen diseases or fibrotic processes, impaired liver or renal function, valvular heart disease, debilitated states and serious infections.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Retroperitoneal Fibrosis, Pleuropulmonary Fibrosis and Fibrotic Thickening of Cardiac Valves May Occur in Patients Receiving Long-term Methysergide Maleate Therapy. Therefore, This Preparation Must Be Reserved for Prophylaxis in Patients Whose Vascular Headaches Are Frequent and/or Severe and Uncontrollable and Who Are Under Close Medical Supervision.
|
With long-term, uninterrupted administration, retroperitoneal fibrosis or related conditions — pleuropulmonary fibrosis and cardiovascular disorders with murmurs or vascular bruits have been reported. Patients must be warned to report immediately the following symptoms and to discontinue the drug: cold, numb, and painful hands and feet; leg cramps on walking; any type of girdle, flank, or chest pain, shortness of breath, or any associated symptomatology. Should any of these symptoms develop, methysergide should be discontinued. Continuous administration should not exceed 6 months. There must be a drug-free interval of 3-4 weeks after each 6-month course of treatment. The dosage should be reduced gradually during the last 2-3 weeks of each treatment course to avoid “headache rebound.”
The drug is not recommended for use in children.
Adverse Reactions
Clinical Trials Experience
ithin the recommended dose levels, the following side effects have been reported:
1) Fibrotic Complications Fibrotic changes have been observed in the retroperitoneal, pleuropulmonary, cardiac, and other tissues, either singly or, very rarely, in combination.
Retroperitoneal Fibrosis
This nonspecific fibrotic process is usually confined to the retroperitoneal connective tissue above the pelvic brim and may present clinically with one or more symptoms such as general malaise, fatigue, weight loss, backache, low grade fever (elevated sedimentation rate), urinary obstruction (girdle or flank pain, dysuria, polyuria, oliguria, elevated BUN), vascular insufficiency of the lower limbs (leg pain, Leriche syndrome, edema of legs, thrombophlebitis). The single most useful diagnostic procedure in suspected cases of retroperitoneal fibrosis is intravenous pyelography. Typical deviation and obstruction of one or both ureters may be observed.
Pleuropulmonary Complications
A similar nonspecific fibrotic process, limited to the pleural and immediately subjacent pulmonary tissues, usually presents clinically with dyspnea, tightness and pain in the chest, pleural friction rubs, and pleural effusion. These findings may be confirmed by chest X-ray.
Cardiac Complications
Nonrheumatic fibrotic thickenings of the aortic root and of the aortic and mitral valves usually present clinically with cardiac murmurs and dyspnea.
Other Fibrotic Complications
Several cases of fibrotic plaques, simulating Peyronie’s Disease have been described.
2) Cardiovascular Complications Encroachment of retroperitoneal fibrosis on the aorta, inferior vena cava and their common iliac branches may result in vascular insufficiency of the lower limbs, the presenting features of which are mentioned under Retroperitoneal Fibrosis.
Intrinsic vasoconstriction of large and small arteries, involving one or more vessels or merely a segment of a vessel, may occur at any stage of therapy. Depending on the vessel involved, this complication may present with chest pain, abdominal pain, or cold, numb, painful extremities with or without paresthesias and diminished or absent pulses. Progression to ischemic tissue damage has rarely been reported. Prompt withdrawal of the drug at the first signs of impaired circulation is recommended (see WARNINGS) to obviate such effects.
Postural hypotension and tachycardia have also been observed.
3) Gastrointestinal Symptoms Nausea, vomiting, diarrhea, heartburn, abdominal pain. These effects tend to appear early and can frequently be obviated by gradual introduction of the medication and by administration of the drug with meals. Constipation and elevation of gastric HCl have also been reported.
4) CNS Symptoms Seizure, insomnia, drowsiness, mild euphoria, dizziness, ataxia, lightheadedness, hyperesthesia, unworldly feelings (described variously as “dissociation”, “hallucinatory experiences”, etc.). Some of these symptoms may be associated with vascular headaches, per se, and may, therefore, be unrelated to the drug.
5) Dermatological Manifestations Facial flush, telangiectasia, and nonspecific rashes have rarely been reported. Increased hair loss may occur, but in many instances the tendency has abated despite continued therapy.
6) Edema Peripheral edema, and, more rarely, localized brawny edema may occur.
Dependent edema has responded to lowered doses, salt restriction, or diuretics.
7) Weight Gain Weight gain may be a reason to caution patients regarding their caloric intake.
8) Hematological Manifestations Neutropenia, eosinophilia, and thrombocytopenia.
9) Miscellaneous Weakness, arthralgia, myalgia, fever, and mydriasis.
Postmarketing Experience
There is limited information regarding Methysergide Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Methysergide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Methysergide in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methysergide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methysergide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Methysergide in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Methysergide in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Methysergide in geriatric settings.
Gender
There is no FDA guidance on the use of Methysergide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methysergide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methysergide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methysergide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methysergide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methysergide in patients who are immunocompromised.
Administration and Monitoring
Administration
Methysergide maleate tablets, USP
- Tablets 2 mg
- Bright yellow, coated tablets with “SANDOZ” imprinted on one side, “78-58” imprinted on the other side, in black.
- Bottle of 100……………………………………………….……………..NDC 0078-0058-05
Monitoring
There is limited information regarding Methysergide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Methysergide and IV administrations.
Overdosage
Few cases of acute Sansert® (methysergide maleate) intoxication have been reported. The possible symptom complex is therefore not fully known. The following symptoms are based on these few case reports. Euphoria, hyperactivity, tachycardia, dilated pupils, and dizziness have been reported in a child with a dose of 20-24 mg of Sansert® (methysergide maleate). In adults, peripheral vasospasm, with diminished or absent pulses, coldness, mottling and cyanosis, has been observed at a dose of 200 mg. Ischemic tissue damage has not been reported in acute overdosage with Sansert® (methysergide maleate).
Treatment consists of removal of the offending drug by induction of emesis, or gastric lavage in the case of very recent intake, repeat dose administration of activated charcoal and catharsis. There is no evidence that forced diuresis accelerates the elimination of Sansert® (methysergide maleate). However, I.V. fluids may be given as a general supportive measure.
Treatment of peripheral vasospasm should consist of warmth, but not heat, and protection of the ischemic limbs. In reported cases of Sansert® (methysergide maleate) overdosage, the use of vasodilators has not been necessary. However, if vasospasm is persistent, severe, or if there is evidence of impending ischemic tissue damage, these agents may be beneficial. Careful nursing care is recommended in order to prevent tissue damage.
Up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Regional Poison Control Centers are listed in the Physicians’ Desk Reference®.*
Pharmacology
There is limited information regarding Methysergide Pharmacology in the drug label.
Mechanism of Action
methysergide maleate) has been shown, in vitro and in vivo, to inhibit or block the effects of serotonin, a substance which may be involved in the mechanism of vascular headaches. Serotonin has been variously described as a central neurohumoral agent or chemical mediator, as a “headache substance” acting directly or indirectly to lower pain threshold (others in this category include tyramine; polypeptides, such as bradykinin; histamine; and acetylcholine), as an intrinsic “motor hormone” of the gastrointestinal tract, and as a “hormone” involved in connective tissue reparative processes. Suggestions have been made by investigators as to the mechanism whereby methysergide produces its clinical effects, but this has not been finally established.
Structure
is a partially synthetic compound structurally related to lysergic acid butanolamide, well-known as methylergonovine in obstetrical practice as an oxytocic agent.
Chemically, methysergide maleate is designated as ergoline-8-carboxamide, 9,10-didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethyl-, (8ß)-, (Z)-2-butenedioate (1:1) (salt).
Its structural formula is:
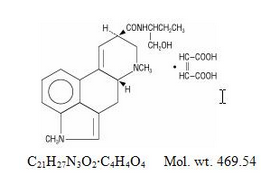
Methylation in the number 1 position of the ring structure enormously enhances the antagonism to serotonin which is present to a much lesser degree in the partially methylated compound (methylergonovine maleate) as well as profoundly altering other pharmacologic properties.
Pharmacodynamics
There is limited information regarding Methysergide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Methysergide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Methysergide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Methysergide Clinical Studies in the drug label.
How Supplied
Methysergide maleate tablets, USP
- Tablets 2 mg
- Bright yellow, coated tablets with “SANDOZ” imprinted on one side, “78-58” imprinted on the other side, in black.
- Bottle of 100……………………………………………….……………..NDC 0078-0058-05
Storage
- Below 86°F (30°C); tight container.
Images
Drug Images
{{#ask: Page Name::Methysergide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
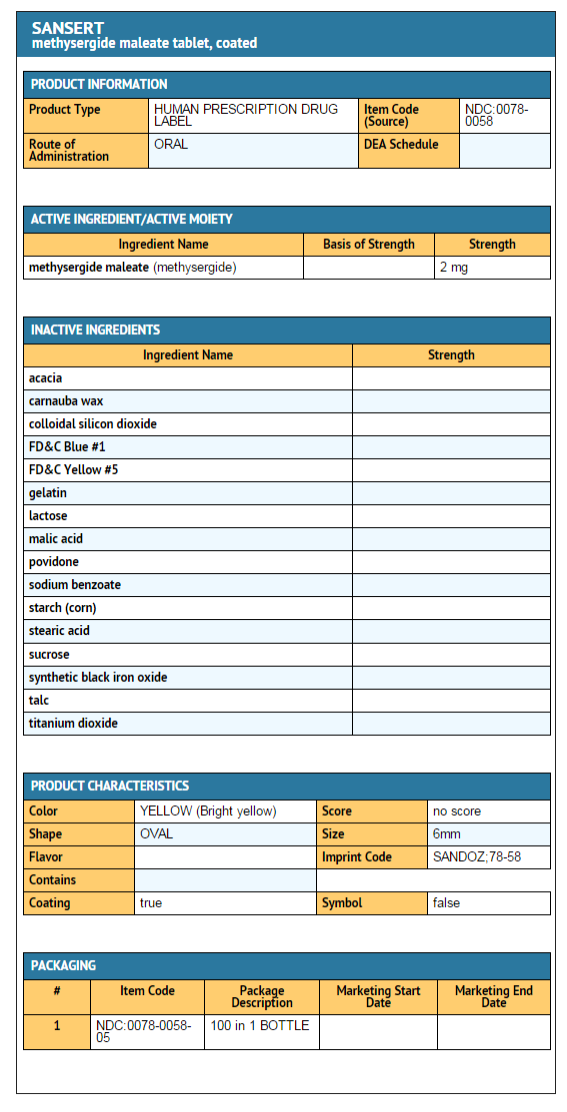
{{#ask: Label Page::Methysergide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Sansert® (methysergide maleate) is intended for use as a preventive agent in the treatment of vascular headaches. It should not be used for acute migraine attacks. If, after a 3-week trial period, Sansert® (methysergide maleate) has not been effective in decreasing the frequency or intensity of headaches, it is unlikely that longer administration of Sansert® (methysergide maleate) will be beneficial.
Patients should be advised to report the following symptoms immediately and to discontinue the drug: cold, numb, and painful hands and feet; leg cramps on walking; any type of girdle, flank, or chest pain; shortness of breath; or any associated symptomatology. There must be a drug-free interval of 3-4 weeks after each 6-month course of treatment.
Sansert® (methysergide maleate) should be taken with meals. Weight gain may necessitate modification of diet.
Precautions with Alcohol
Alcohol-Methysergide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Methysergide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Methysergide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a603022 |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H27N3O2 |
| Molar mass | 353.458 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Synonyms / Brand Names: Sansert®
Overview
Methysergide (1-methyl-D-lysergic acid butanolamide or UML-491) is a prescription drug formerly used for prophylaxis of cluster headaches/migraine headaches, but is no longer recommended due to retroperitoneal/retropulmonary fibrosis. It was sold under the brand names Sansert and Deseril in 2 mg dosages.
Category
Antimigraine Drugs
FDA Package Insert
Sansert (methysergide maleate) Tablet, Coated
Indications and Usage | Dosage and Administration | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | How Supplied/Storage and Handling | Patient Counseling Information
 |
Medical uses
Methysergide is used to treat headaches such as migraine and other recurrent throbbing headaches.[1] Methysergide is one of the most effective[2] medications for the prevention of migraine, but not for the treatment of an acute attack.
It is also used in carcinoid syndrome to treat severe diarrhea.[1] It may also be used in the treatment of serotonin syndrome.[3]
Side effects
It has a known side effect, retroperitoneal fibrosis,[4] which is severe, although uncommon. Other severe but uncommon side effects include pleural fibrosis, and subendocardial fibrosis.
In addition, there is an increased risk of left-sided cardiac valve dysfunction.[2][5]
Mechanism of Action
Methysergide interacts with serotonin (5-HT) receptors. Its therapeutic effect in migraine prophylaxis has been associated with its antagonism at the 5-HT2B receptor.[6]
Furthermore, it is an antagonist at the 5-HT2C receptor, while at the 5-HT1A receptor it serves as a partial agonist.[7][8][9] It is known to have partial agonisteffects on some of the other 5-HT receptors as well.[10] Methysergide is metabolised intomethylergometrine in humans, which is responsible for its psychedelic effects.[11]
Historical Perspective
Methysergide was approved by the U.S. Food and Drug Administration (FDA) in 1962.
Novartis withdrew it from the U.S. market after taking over Sandoz, but currently lists it as a product.
See also
References
- ↑ 1.0 1.1 http://www.patient.co.uk/medicine/Methysergide.htm
- ↑ 2.0 2.1 Joseph T, Tam SK, Kamat BR, Mangion JR (2003). "Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography". Echocardiography. 20 (3): 283–7. doi:10.1046/j.1540-8175.2003.03027.x. PMID 12848667.
- ↑ Sporer, KA (1995). "The Serotonin Syndrome Implicated Drugs, Pathophysiology and Management". Drug Safety. 13 (2): 94–104. doi:10.2165/00002018-199513020-00004. PMID 7576268.
- ↑ emedicine.com (2002)
- ↑ 1997 Mayo Clinic study linking heart disease to Fen Phen Valvular heart disease associated with fenfluramine-phentermine
- ↑ Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H (May 1996). "Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache?". Eur. J. Neurosci. 8 (5): 959–67. doi:10.1111/j.1460-9568.1996.tb01583.x. PMID 8743744.
- ↑ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 187
- ↑ Saxena PR, Lawang A (October 1985). "A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors". Arch Int Pharmacodyn Ther. 277 (2): 235–52. PMID 2933009.
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9681
- ↑ Colpaert FC, Niemegeers CJ, Janssen PA (October 1979). "In vivo evidence of partial agonist activity exerted by purported 5-hydroxytryptamine antagonists". Eur. J. Pharmacol. 58 (4): 505–9. doi:10.1016/0014-2999(79)90326-1. PMID 510385.
- ↑ Bredberg, U. (1 January 1986). "Pharmacokinetics of methysergide and its metabolite methylergometrine in man". European Journal of Clinical Pharmacology. 30 (1): 75–77. doi:10.1007/BF00614199. PMID 3709634. Unknown parameter
|coauthors=ignored (help)
External links
- Novartis Sansert site.
- Novartis Sansert product description.
- Migraines.org More detailed information on methysergide.
- neurologychannel.com, general information on migraines.
- History of methysergide in migraine.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Date and year
- Pages with citations using unsupported parameters
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Antimigraine drugs
- Lysergamides