Nabilone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nabilone is a Cannabinoid and antihemetic that is FDA approved for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Common adverse reactions include hypotension, xerostomia, asthenia, ataxia, dyssomnia, headache, poor concentration, somnolence, vertigo, visual disturbance, dysphoric mood and euphoria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Nausea and Vomiting in Cancer Therapy
- The usual adult dosage is 1 or 2 mg 2 times a day. On the day of chemotherapy, the initial dose should be given 1 to 3 hours before the chemotherapeutic agent is administered. To minimize side effects, it is recommended that the lower starting dose be used and that the dose be increased as necessary. A dose of 1 or 2 mg the night before may be useful. The maximum recommended daily dose is 6 mg given in divided doses 3 times a day.
- Nabilone may be administered 2 or 3 times a day during the entire course of each cycle of chemotherapy and, if needed, for 48 hours after the last dose of each cycle of chemotherapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nabilone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nabilone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nabilone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nabilone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nabilone in pediatric patients.
Contraindications
Nabilone is contraindicated in any patient who has a history of hypersensitivity to any cannabinoid.
Warnings
- The effects of Nabilone may persist for a variable and unpredictable period of time following its oral administration. *Adverse psychiatric reactions can persist for 48 to 72 hours following cessation of treatment.
- Nabilone has the potential to affect the CNS, which might manifest itself in dizziness, drowsiness, euphoria “high”, ataxia, anxiety, disorientation, depression, hallucinations and psychosis.
- Nabilone can cause tachycardia and orthostatic hypotension.
- Because of individual variation in response and tolerance to the effects of Nabilone patients should remain under supervision of a responsible adult especially during initial use of Nabilone and during dose adjustments.
- Patients receiving treatment with Nabilone should be specifically warned not to drive, operate machinery, or engage in any hazardous activity while receiving Nabilone.
- Nabilone should not be taken with alcohol, sedatives, hypnotics, or other psychoactive substances because these substances can potentiate the central nervous system effects of nabilone.
Adverse Reactions
Clinical Trials Experience
Commonly Encountered Reactions
- During controlled clinical trials of Nabilone, virtually all patients experienced at least one adverse reaction. The most commonly encountered events were drowsiness, vertigo, dry mouth, euphoria (feeling “high”), ataxia, headache, and concentration difficulties.
Comparative Incidence of Reactions
- Accurate estimates of the incidence of adverse events associated with the use of any drug are difficult to obtain. Estimates are influenced by factors such as drug dose, detection technique, setting, and physician judgments, among others. Consequently, the tables presented below are presented solely to indicate the relative frequency of adverse events reported in representative controlled clinical studies conducted to evaluate the safety and efficacy of Nabilone under relatively similar conditions of use. The figures cited cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice, in which patient characteristics and other factors may differ from those that prevailed in the clinical trials. These incidence figures also cannot be compared with those obtained from other clinical studies involving related drug products because each group of drug trials is conducted under a different set of conditions. Finally, it is important to emphasize that these tabulations do not reflect the relative severity and/or clinical importance of the adverse events.
The following tables list in order of decreasing frequency the adverse reactions encountered by a substantial proportion of patients treated with Nabilone participating in representative controlled clinical trials.
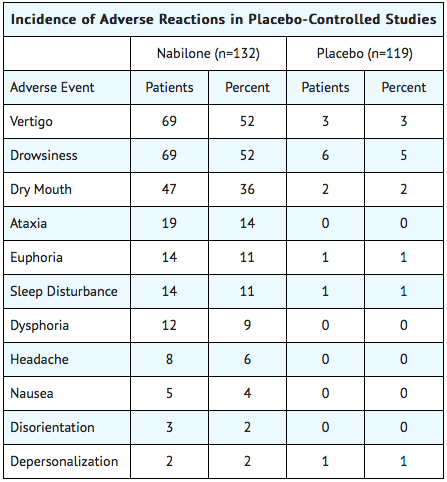
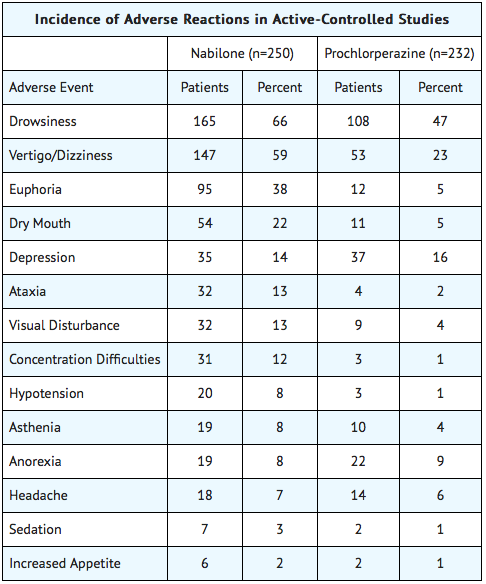
Adverse Reactions by Body System
Blood and Hematopoietic
Cardiovascular
- Orthostatic hypotension
- Hypotension
- Tachycardia
- Syncope
- Palpitation
- Flushing
- Hypertension
- Arrhythmia
- Cerebral vascular accident
Eye and Ear
- Vision disturbance
- Ear tightness
- Eye irritation
- Eye dryness
- Equilibrium dysfunction
- Tinnitus
- Eye disorder
- Amblyopia
- Eye swelling
- Eyelid diseases
- Pupil dilation
- Photophobia
- Visual field defect
Gastrointestinal
- Dry mouth
- Nausea
- Anorexia
- Vomiting
- Diarrhea
- Abdominal pain
- Constipation
- Aphthous ulcer
- Mouth irritation
- Gastritis
- Dyspepsia
Genitourinary
Infection
Metabolic and Endocrine
Musculoskeletal
Nervous System
- Drowsiness
- Vertigo
- Ataxia
- Decreased concentration
- Sedation
- Hallucinations
- Paresthesia
- Tremor
- Memory disturbance
- Perception disturbance
- Convulsions
- Dystonia
- Numbness
- Akathisia
Psychiatric
- Euphoria (feeling “high”)
- Sleep disturbance
- Depression
- Confusion
- Disorientation
- Anxiety
- Depersonalization syndrome
- Speech disorder
- Abnormal dreams
- Insomnia
- Mood swings
- Inebriated feeling
- Toxic psychosis
- Paranoia
- Apathy
- Thought disorder
- Withdrawal
- Panic disorder
- Phobic neurosis
- Emotional disorder
- Hyperactivity
Respiratory
- Dyspnea
- Pharyngitis
- Nasal congestion
- Sinus headache
- Thick tongue
- Dry throat
- Dry nose
- Wheezing
- Nosebleed
- Cough
- Voice change
- Chest pain
Skin and Appendages
Miscellaneous and Ill-Defined Conditions
- Headache
- Fatigue
- Lightheadedness
- Coordination disturbance
- Asthenia
- Dysphoria
- Dizziness
- Taste change
- Excessive appetite
- Chills
- Excessive sweating
- Nervousness
- Malaise
- Postural dizziness
- Twitch
- Irritability
- Fever
- Inhibited walking
- Unconsciousness
- Hypotonia
- Impaired urination
Postmarketing Experience
Nabilone has been marketed internationally since 1982. The following adverse reactions listed in order of decreasing frequency by body system have been reported since Nabilone has been marketed. All events are listed regardless of causality assessment.
Blood and Hematopoietic
Cardiovascular
Eye and Ear
Gastrointestinal
Nervous System
- Hallucinations
- CNS depression
- CNS stimulation
- Ataxia
- Stupor
- Vertigo
- Convulsion
- Circumoral paresthesia
Psychiatric
- Somnolence
- Confusion
- Euphoria
- Depression
- Dysphoria
- Depersonalization
- Anxiety
- Psychosis
- Emotional lability
Miscellaneous and Ill-Defined Conditions
Drug Interactions
- Potential interactions between Nabilone 2 mg, and diazepam 5 mg; sodium secobarbital 100 mg; alcohol 45 mL (absolute laboratory alcohol); or codeine 65 mg, were evaluated in 15 subjects. Only a single combination was utilized at any one time. The subjects were evaluated according to physiologic (i.e., heart rate and blood pressure), psychometric, psychomotor, and subjective parameters. In this study, as expected, the depressant effects of the combinations were additive. Psychomotor function was particularly impaired with concurrent use of diazepam. Caution must thus be used when administering nabilone in combination with any CNS depressant.
- Nabilone is purportedly highly bound to plasma proteins, and therefore, might displace other protein-bound drugs. Therefore, practitioners should monitor patients for a change in dosage requirements when administering nabilone to patients receiving other highly protein-bound drugs. Published reports of drug-drug interactions involving cannabinoids are summarized in the following table.
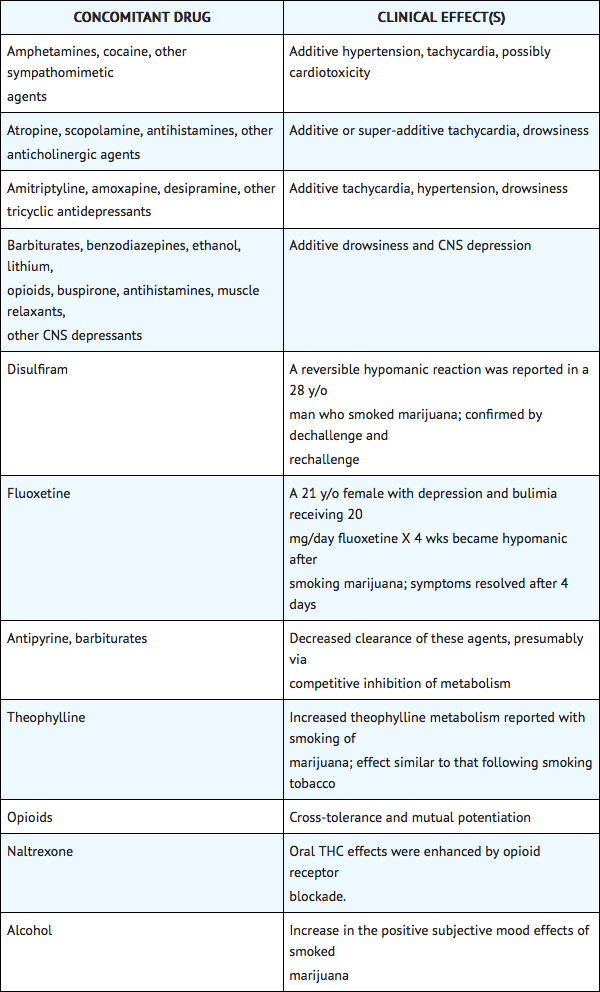
Use in Specific Populations
Pregnancy
- Teratology studies conducted in pregnant rats at doses up to 12 mg/kg/day (about 16 times the human dose on a body surface area basis) and in pregnant rabbits at doses up to 3.3 mg/kg/day (about 9 times the human dose on a body surface area basis) did not disclose any evidence for a teratogenic potential of nabilone. However, there was dose related developmental toxicity in both species as evidenced by increases in embryo lethality, fetal resorptions, decreased fetal weights and pregnancy disruptions. In rats, postnatal developmental toxicity was also observed. There are no adequate and well-controlled studies in pregnant women. Because animal studies cannot rule out the possibility of harm, Nabilone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nabilone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nabilone during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in breast milk. Because many drugs including some cannabinoids are excreted in breast milk it is not recommended that Nabilone be given to nursing mothers.
Pediatric Use
- Safety and effectiveness have not been established in patients younger than 18 years of age. Caution is recommended in prescribing Nabilone to children because of psychoactive effects.
Geriatic Use
- Clinical studies of Nabilone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Nabilone should be used with caution in elderly patients aged 65 and over because they are generally more sensitive to the psychoactive effects of drugs and Nabilone can elevate supine and standing heart rates and cause postural hypotension.
Gender
There is no FDA guidance on the use of Nabilone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nabilone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nabilone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nabilone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nabilone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nabilone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Nabilone Administration in the drug label.
Monitoring
There is limited information regarding Nabilone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Nabilone and IV administrations.
Overdosage
Signs and Symptoms
- Signs and symptoms of overdosage are an extension of the psychotomimetic and physiologic effects of Nabilone.
Treatment
- To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
- Overdosage may be considered to have occurred, even at prescribed dosages, if disturbing psychiatric symptoms are present. In these cases, the patient should be observed in a quiet environment and supportive measures, including reassurance, should be used. Subsequent doses should be withheld until patients have returned to their baseline mental status; routine dosing may then be resumed if clinically indicated. In such instances, a lower initiating dose is suggested. In controlled clinical trials, alterations in mental status related to the use of Nabilone resolved within 72 hours without specific medical therapy. In overdose settings, attention should be paid to vital signs, since both hypertension and hypotension have been known to occur; tachycardia and orthostatic hypotension were most commonly reported.
- No cases of overdosage with more than 10 mg/day of nabilone were reported during clinical trials. Signs and symptoms that would be expected to occur in large overdose situations are psychotic episodes, including hallucinations, anxiety reactions, respiratory depression, and coma. If psychotic episodes occur, the patient should be managed conservatively, if possible. For moderate psychotic episodes and anxiety reactions, verbal support and comforting may be sufficient. In more severe cases, antipsychotic drugs may be useful; however, the utility of antipsychotic drugs in cannabinoid psychosis has not been systematically evaluated. Support for their use is drawn from limited experience using antipsychotic agents to manage cannabis overdoses. Because of the potential for drug-drug interactions (e.g., additive CNS depressant effects due to nabilone and chlorpromazine), such patients should be closely monitored.
- Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, as well as other laboratory values and physical assessments. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
- The use of forced diuresis, peritoneal dialysis, hemodialysis, charcoal hemoperfusion, or cholestyramine has not been reported. In the presence of normal renal function, most of a dose of nabilone is eliminated through the biliary system. Treatment for respiratory depression and comatose state consists in symptomatic and supportive therapy. Particular attention should be paid to the occurrence of hypothermia. If the patient becomes hypotensive, consider fluids, inotropes, and/or vasopressors. The estimated oral median lethal dose in female mice is between 1,000 and 2,000 mg/kg; in the female rat, it is greater than 2,000 mg/kg,
Pharmacology
| |
Nabilone
| |
| Systematic (IUPAC) name | |
| (6aR,10aR)-rel-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)- | |
| Identifiers | |
| CAS number | |
| ATC code | A04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 372.541 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 20% after first-pass by the liver |
| Protein binding | similar to THC (+/-97%) |
| Metabolism | ? |
| Half life | 2 hours, with metabolites around 35 hours. |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral form (PO)- capsule |
Mechanism of Action
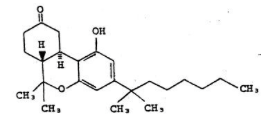
- Chemically, nabilone is similar to the active ingredient found in naturally occurring Cannabis sativa L. [Marijuana; delta-9-tetrahydrocannabinol (delta-9-THC)]. Nabilone is (±)-trans-3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6-6-dimethyl-9H-dibenzo[b,d]pyran-9-one and has the empirical formula C24H36O3. It has a molecular weight of 372.55. The structural formula is as follows:

Structure
There is limited information regarding Nabilone Structure in the drug label.
Pharmacodynamics
Nabilone (nabilone) is an orally active synthetic cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS). It has been suggested that the antiemetic effect of nabilone is caused by interaction with the cannabinoid receptor system, i.e., the CB (1) receptor, which has been discovered in neural tissues.
Nontherapeutic Effects
- Nabilone, a synthetic cannabinoid, has the potential to be abused and to produce psychological dependence. Nabilone has complex effects on the central nervous system. Its effects on the mental state (i.e., "inner mental life") are similar to those of cannabis. Subjects given Nabilone may experience changes in mood (euphoria, detachment, depression, anxiety, panic, paranoia), decrements in cognitive performance and memory, a decreased ability to control drives and impulses, and alterations in the experience of reality (e.g., distortions in the perception of objects and the sense of time, hallucinations). These phenomena appear to be more common when larger doses of Nabilone are administered; however, a full-blown picture of psychosis (psychotic organic brain syndrome) may occur in patients receiving doses within the lower portion of the therapeutic range.
- Data on the chronic use of Nabilone are not available; experience with cannabis suggests that chronic use of cannabinoids may be associated with a variety of untoward effects on motivation, cognition, judgment, as well as other mental status changes. Whether these phenomena reflect the underlying character of individuals chronically abusing cannabis or are a result of the use of cannabis is not known.
- The simultaneous use of Nabilone and alcohol or barbiturates may produce additive depressive effects on central nervous system function. Possible changes in mood and other adverse behavioral effects may occur in patients receiving Nabilone. Patients should remain under supervision of a responsible adult while using Nabilone. Nabilone has central nervous system activity. It produces relaxation, drowsiness, and euphoria in the recommended dosage range. Tolerance to these effects develops rapidly and is readily reversible.
- In addition to effects on the mental state, Nabilone has several systemic actions; most prominent are dry mouth and hypotension. Nabilone has been observed to elevate supine and standing heart rates and to cause supine and orthostatic hypotension. In clinical studies, oral administration of 2 mg of Nabilone did produce some decrease in airway resistance in normal controls but had no effect in patients with asthma. No other nontherapeutic effects of clinical significance due to Nabilone have been reported.
Pharmacokinetics
Absorption and Distribution
- Nabilone (nabilone) appears to be completely absorbed from the human gastrointestinal tract when administered orally. Following oral administration of a 2 mg dose of radiolabeled nabilone, peak plasma concentrations of approximately 2 ng/mL nabilone and 10 ng equivalents/mL total radioactivity are achieved within 2.0 hours. The plasma half-life (T1/2) values for nabilone and total radioactivity of identified and unidentified metabolites are about 2 and 35 hours, respectively. The initial rapid disappearance of radioactivity represents uptake and distribution of nabilone into tissue and the slower phase elimination by metabolism and excretion. The apparent volume of distribution of nabilone is about 12.5 L/kg. Nabilone exhibits dose linearity within its therapeutic range. Clinical data suggests that the intake of food does not significantly affect either the rate or extent of absorption.
Metabolism
- Metabolism of nabilone is extensive and several metabolites have been identified. Precise information concerning the metabolites that may accumulate is not available. The relative activities of the metabolites and the parent drug have not been established. There are at least two metabolic pathways involved in the biotransformation of nabilone. A minor pathway is initiated by the stereospecific enzymatic reduction of the 9-keto moiety of nabilone to produce the isomeric carbinol metabolite. The peak concentrations of nabilone and its carbinol metabolites are comparable, but their combined exposures in plasma do not account for more than 20% of that of total radioactivity. Secondly, a metabolite of nabilone in feces has been identified as a diol formed by reduction of the 9-keto group plus oxidation at the penultimate carbon of the dimethylheptyl side chain. In addition, there is evidence of extensive metabolism of Nabilone by multiple P450 enzyme isoforms. In vitro P450 inhibition studies using human liver microsomes showed that nabilone did not significantly inhibit CYP1A2, CYP2A6, CYP2C19, CYP2D6, and CYP3A4 (using midazolam and nifedipine as substrates). Nabilone had a weak inhibitory effect on CYP2E1 and CYP3A4 (testosterone IC50 > 50 µM) and had a moderate inhibitory effect on CYP2C8 and CYP2C9 (IC50 > 10 µM). However, in clinical use, the very low nabilone plasma concentration is unlikely to interfere with the P450-mediated degradation of co-administered drugs. Chronic oral administration of 1 mg t.i.d. for 14 days to 3 subjects gave no indication there was any significant accumulation of nabilone. Available evidence suggests that one or more of the metabolites has a terminal elimination half-life that exceeds that of nabilone. Consequently, in repeated use, the metabolites may accumulate at concentrations in excess of the parent drug.
Elimination
- The route and rate of the elimination of nabilone and its metabolites are similar to those observed with other cannabinoids, including delta-9-THC (dronabinol). When nabilone is administered intravenously, the drug and its metabolites are eliminated mainly in the feces (approximately 67%) and to a lesser extent in the urine (approximately 22%) within 7 days. Of the 67% recovered from the feces, 5% corresponded to the parent compound and 16% to its carbinol metabolite. Following oral administration about 60% of nabilone and its metabolites were recovered in the feces and about 24% in urine. Therefore, it appears that the major excretory pathway is the biliary system. The effects of age, gender, hepatic dysfunction, and renal insufficiency on the metabolism and elimination of nabilone have not been determined.
Nonclinical Toxicology
There is limited information regarding Nabilone Nonclinical Toxicology in the drug label.
Clinical Studies
- Nabilone was evaluated for its effectiveness and safety in the treatment of nausea and vomiting induced by cancer chemotherapy in patients receiving a wide variety of chemotherapy regimens, including low-dose cisplatin (20 mg/m2) in both placebo-controlled and active controlled (prochlorperazine) trials. During Nabilone treatment patients reported a higher incidence of adverse effects. The most frequent were drowsiness, vertigo, dry mouth and euphoria. However, most of the adverse effects occurring with Nabilone were of mild to moderate severity.
How Supplied
- Nabilone® capsules (blue and white): 1 mg (bottles of 50 capsules) NDC 0037-1221-50. Capsules are imprinted with MEDA on the blue cap and a four-digit code (1221) on the white body.
Storage
- Store at controlled room temperature, 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
Images
Drug Images
{{#ask: Page Name::Nabilone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
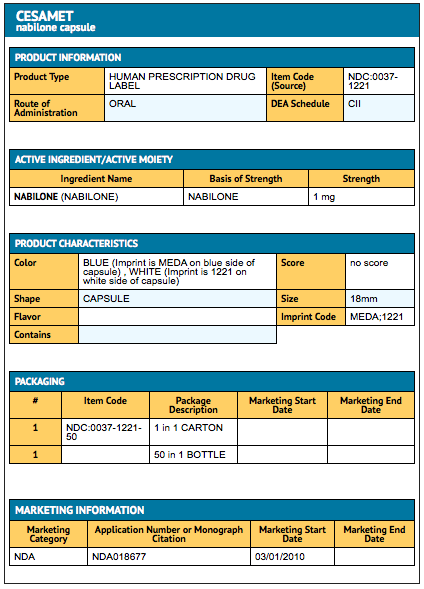
{{#ask: Label Page::Nabilone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Nabilone Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Nabilone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Nabilone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nabilone |Label Name=Nabiline Package.png
}}