Methysergide: Difference between revisions
No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | {{Drugbox | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| verifiedrevid = 462251649 | | verifiedrevid = 462251649 | ||
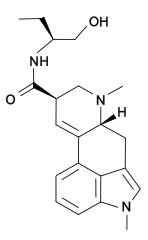
| IUPAC_name = (6a''R'',9''R'')-''N''-[(2''S'')-1-Hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-''fg'']quinoline-9-carboxamide | | IUPAC_name = (6a''R'',9''R'')-''N''-[(2''S'')-1-Hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-''fg'']quinoline-9-carboxamide | ||
| image = Methysergide. | | image = Methysergide chemical structure.png | ||
| width = 150 | | width = 150 | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = Deseril, Sansert | ||
| Drugs.com = {{drugs.com|CONS|methysergide}} | | Drugs.com = {{drugs.com|CONS|methysergide}} | ||
| MedlinePlus = a603022 | | MedlinePlus = a603022 | ||
| | | pregnancy_AU = C | ||
| pregnancy_US = X | |||
| legal_AU = S4 | |||
| legal_CA = Rx-only | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
<!--Identifiers--> | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| Line 128: | Line 26: | ||
| IUPHAR_ligand = 134 | | IUPHAR_ligand = 134 | ||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| DrugBank = DB00247 | | DrugBank = DB00247 | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ChemSpiderID = 9300 | | ChemSpiderID = 9300 | ||
| Line 137: | Line 35: | ||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ChEMBL = 1065 | | ChEMBL = 1065 | ||
<!--Chemical data--> | <!--Chemical data--> | ||
| C=21 | H=27 | N=3 | O=2 | | C=21 | H=27 | N=3 | O=2 | ||
| molecular_weight = 353.458 g/mol | | molecular_weight = 353.458 g/mol | ||
| smiles = O=C(N[C@@H](CC)CO)[C@@H]3/C=C2/c4cccc1c4c(cn1C)C[C@H]2N(C3)C | | smiles = O=C(N[C@@H](CC)CO)[C@@H]3/C=C2/c4cccc1c4c(cn1C)C[C@H]2N(C3)C | ||
| Line 147: | Line 46: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = KPJZHOPZRAFDTN-ZRGWGRIASA-N | | StdInChIKey = KPJZHOPZRAFDTN-ZRGWGRIASA-N | ||
}}__NOTOC__ | }} | ||
__NOTOC__ | |||
{{CMG}} | {{CMG}} | ||
==Overview== | ==Overview== | ||
'''Methysergide''' ('''1-[[methyl]]-''D''-[[lysergic acid]] [[butanol]][[amide]]''' or '''UML-491''') is a prescription drug formerly used for prophylaxis of [[cluster headaches]]/[[migraine headaches]], but is no longer recommended due to [[retroperitoneal fibrosis|retroperitoneal]]/retropulmonary fibrosis. | |||
'''Methysergide''' ('''1-[[methyl]]-''D''-[[lysergic acid]] [[butanol]][[amide]]''' or '''UML-491''') is a prescription drug formerly used for prophylaxis of [[cluster headaches]]/[[migraine headaches]], but is no longer recommended due to [[retroperitoneal fibrosis|retroperitoneal]]/retropulmonary fibrosis. | |||
==Medical uses== | ==Medical uses== | ||
Methysergide is used to treat headaches such as migraine and other recurrent throbbing headaches.<ref name="patient.co.uk">http://www.patient.co.uk/medicine/Methysergide.htm</ref> Methysergide is one of the most effective<ref name=Tam>{{cite journal |author=Joseph T, Tam SK, Kamat BR, Mangion JR |title=Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography |journal=Echocardiography |volume=20 |issue=3 |pages=283–7 |year=2003|pmid=12848667 |doi=10.1046/j.1540-8175.2003.03027.x}}</ref> medications for the prevention of migraine, but not for the treatment of an acute attack. | Methysergide is used to treat headaches such as migraine and other recurrent throbbing headaches.<ref name="patient.co.uk">http://www.patient.co.uk/medicine/Methysergide.htm</ref> Methysergide is one of the most effective<ref name=Tam>{{cite journal |author=Joseph T, Tam SK, Kamat BR, Mangion JR |title=Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography |journal=Echocardiography |volume=20 |issue=3 |pages=283–7 |year=2003|pmid=12848667 |doi=10.1046/j.1540-8175.2003.03027.x}}</ref> medications for the prevention of migraine, but not for the treatment of an acute attack. | ||
It is also used in [[carcinoid syndrome]] to treat severe [[diarrhea]].<ref name="patient.co.uk"/> It may also be used in the treatment of serotonin syndrome.<ref>{{cite journal|last=Sporer|first=KA|title=The Serotonin Syndrome Implicated Drugs, Pathophysiology and Management|journal=Drug Safety|date | It is also used in [[carcinoid syndrome]] to treat severe [[diarrhea]].<ref name="patient.co.uk"/> It may also be used in the treatment of serotonin syndrome.<ref>{{cite journal|last=Sporer|first=KA|title=The Serotonin Syndrome Implicated Drugs, Pathophysiology and Management|journal=Drug Safety|date=1995|volume=13|issue=2|pages=94-104|pmid=7576268|doi=10.2165/00002018-199513020-00004}}</ref> | ||
==Side effects== | ==Side effects== | ||
It has a known [[adverse drug reaction|side effect]], [[retroperitoneal fibrosis]],<ref>[http://www.emedicine.com/radio/topic605.htm emedicine.com (2002)]</ref> which is severe, although uncommon. Other severe but uncommon side effects include pleural fibrosis, and subendocardial fibrosis. | It has a known [[adverse drug reaction|side effect]], [[retroperitoneal fibrosis]],<ref>[http://www.emedicine.com/radio/topic605.htm emedicine.com (2002)]</ref> which is severe, although uncommon. Other severe but uncommon side effects include pleural fibrosis, and subendocardial fibrosis. | ||
In addition, there is an increased risk of left-sided [[cardiac valve dysfunction]].<ref name=Tam/><ref name=mayo>[http://pph.poweradvocates.com/fen_phen_study_mayo.html 1997 Mayo Clinic study linking heart disease to Fen Phen] Valvular heart disease associated with fenfluramine-phentermine</ref> | In addition, there is an increased risk of left-sided [[cardiac valve dysfunction]].<ref name=Tam/><ref name=mayo>[http://pph.poweradvocates.com/fen_phen_study_mayo.html 1997 Mayo Clinic study linking heart disease to Fen Phen] Valvular heart disease associated with fenfluramine-phentermine</ref> | ||
== | ==Pharmacology== | ||
Methysergide interacts with [[5-HT receptor|serotonin (5-HT) receptors]]. Its [[therapeutic effect]] in migraine [[prophylaxis]] has been associated with its antagonism at the [[5-HT2B|5-HT<sub>2B</sub> receptor]].<ref name="pmid8743744">{{cite journal |author=Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H|title=Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache?|journal=Eur. J. Neurosci. |volume=8 |issue=5 |pages=959–67 |date=May 1996 |pmid=8743744 |doi= 10.1111/j.1460-9568.1996.tb01583.x|url=}}</ref> | Methysergide interacts with [[5-HT receptor|serotonin (5-HT) receptors]]. Its [[therapeutic effect]] in migraine [[prophylaxis]] has been associated with its antagonism at the [[5-HT2B|5-HT<sub>2B</sub> receptor]].<ref name="pmid8743744">{{cite journal |author=Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H|title=Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache?|journal=Eur. J. Neurosci. |volume=8 |issue=5 |pages=959–67 |date=May 1996 |pmid=8743744 |doi= 10.1111/j.1460-9568.1996.tb01583.x|url=}}</ref> | ||
Furthermore, it is an [[receptor antagonist|antagonist]] at the [[5-HT2C|5-HT<sub>2C</sub> receptor]], while at the [[5-HT1A|5-HT<sub>1A</sub> receptor]] it serves as a partial agonist.<ref name=Rang187>{{cite book|author=Rang, H. P. |title=Pharmacology |publisher=Churchill Livingstone |location=Edinburgh |year=2003 |pages=|isbn=0-443-07145-4 |oclc= |doi=}} Page 187</ref><ref name="pmid2933009">{{cite journal |author=Saxena PR, Lawang A |title=A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors |journal=Arch Int Pharmacodyn Ther |volume=277|issue=2 |pages=235–52 |date=October 1985 |pmid=2933009 |doi= |url=}}</ref><ref>http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9681</ref> It is known to have [[partial agonist]] effects on some of the other 5-HT receptors as well.<ref name="pmid510385">{{cite journal |author=Colpaert FC, Niemegeers CJ, Janssen PA |title=In vivo evidence of partial agonist activity exerted by purported 5-hydroxytryptamine antagonists |journal=Eur. J. Pharmacol. |volume=58 |issue=4 |pages=505–9 |date=October 1979 |pmid=510385 |doi= 10.1016/0014-2999(79)90326-1|url=}}</ref> Methysergide is metabolised into [[methylergometrine]] in humans, which is responsible for its psychedelic effects.<ref>{{cite journal|last=Bredberg|first=U.|author2=Eyjolfsdottir, G. S. |author3=Paalzow, L. |author4=Tfelt-Hansen, P. |author5=Tfelt-Hansen, V. |title=Pharmacokinetics of methysergide and its metabolite methylergometrine in man|journal=European Journal of Clinical Pharmacology|date=1 January 1986|volume=30|issue=1|pages=75–77|doi=10.1007/BF00614199|pmid=3709634}}</ref> | |||
==History== | |||
Methysergide was approved by the [[Food and Drug Administration (United States)|U.S. Food and Drug Administration]] (FDA) in 1962. | |||
[[Novartis]] withdrew it from the U.S. market after taking over [[Sandoz]], but currently lists it as a product.{{Citation needed|date=August 2013}} | |||
[[ | ==Synthesis== | ||
[[File:Methysergide synthesis.png|thumb|center|500px|Methysergide synthesis: [[Sandoz]] Ltd.]] | |||
==See also== | ==See also== | ||
*[[Triptans]] | *[[Triptans]] | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 18:41, 21 January 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Deseril, Sansert |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a603022 |
| Pregnancy category | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H27N3O2 |
| Molar mass | 353.458 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Methysergide (1-methyl-D-lysergic acid butanolamide or UML-491) is a prescription drug formerly used for prophylaxis of cluster headaches/migraine headaches, but is no longer recommended due to retroperitoneal/retropulmonary fibrosis.
Medical uses
Methysergide is used to treat headaches such as migraine and other recurrent throbbing headaches.[1] Methysergide is one of the most effective[2] medications for the prevention of migraine, but not for the treatment of an acute attack.
It is also used in carcinoid syndrome to treat severe diarrhea.[1] It may also be used in the treatment of serotonin syndrome.[3]
Side effects
It has a known side effect, retroperitoneal fibrosis,[4] which is severe, although uncommon. Other severe but uncommon side effects include pleural fibrosis, and subendocardial fibrosis.
In addition, there is an increased risk of left-sided cardiac valve dysfunction.[2][5]
Pharmacology
Methysergide interacts with serotonin (5-HT) receptors. Its therapeutic effect in migraine prophylaxis has been associated with its antagonism at the 5-HT2B receptor.[6] Furthermore, it is an antagonist at the 5-HT2C receptor, while at the 5-HT1A receptor it serves as a partial agonist.[7][8][9] It is known to have partial agonist effects on some of the other 5-HT receptors as well.[10] Methysergide is metabolised into methylergometrine in humans, which is responsible for its psychedelic effects.[11]
History
Methysergide was approved by the U.S. Food and Drug Administration (FDA) in 1962.
Novartis withdrew it from the U.S. market after taking over Sandoz, but currently lists it as a product.[citation needed]
Synthesis
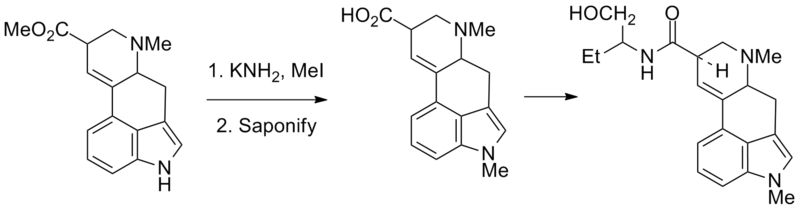
See also
References
- ↑ 1.0 1.1 http://www.patient.co.uk/medicine/Methysergide.htm
- ↑ 2.0 2.1 Joseph T, Tam SK, Kamat BR, Mangion JR (2003). "Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography". Echocardiography. 20 (3): 283–7. doi:10.1046/j.1540-8175.2003.03027.x. PMID 12848667.
- ↑ Sporer, KA (1995). "The Serotonin Syndrome Implicated Drugs, Pathophysiology and Management". Drug Safety. 13 (2): 94–104. doi:10.2165/00002018-199513020-00004. PMID 7576268.
- ↑ emedicine.com (2002)
- ↑ 1997 Mayo Clinic study linking heart disease to Fen Phen Valvular heart disease associated with fenfluramine-phentermine
- ↑ Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H (May 1996). "Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache?". Eur. J. Neurosci. 8 (5): 959–67. doi:10.1111/j.1460-9568.1996.tb01583.x. PMID 8743744.
- ↑ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 187
- ↑ Saxena PR, Lawang A (October 1985). "A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors". Arch Int Pharmacodyn Ther. 277 (2): 235–52. PMID 2933009.
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9681
- ↑ Colpaert FC, Niemegeers CJ, Janssen PA (October 1979). "In vivo evidence of partial agonist activity exerted by purported 5-hydroxytryptamine antagonists". Eur. J. Pharmacol. 58 (4): 505–9. doi:10.1016/0014-2999(79)90326-1. PMID 510385.
- ↑ Bredberg, U.; Eyjolfsdottir, G. S.; Paalzow, L.; Tfelt-Hansen, P.; Tfelt-Hansen, V. (1 January 1986). "Pharmacokinetics of methysergide and its metabolite methylergometrine in man". European Journal of Clinical Pharmacology. 30 (1): 75–77. doi:10.1007/BF00614199. PMID 3709634.
External links
- Novartis Sansert site.
- Novartis Sansert product description.
- Migraines.org More detailed information on methysergide.
- neurologychannel.com, general information on migraines.
- History of methysergide in migraine.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from August 2013
- Articles with invalid date parameter in template
- Antimigraine drugs
- Lysergamides