Ivabradine
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Protein binding | 70% |
| Metabolism | Hepatic (first-pass) >50%, CYP3A4-mediated |
| Elimination half-life | 2 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
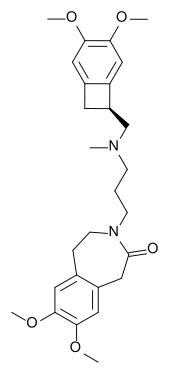
| Formula | C27H36N2O5 |
| Molar mass | 468.585 g/mol |
|
WikiDoc Resources for Ivabradine |
|
Articles |
|---|
|
Most recent articles on Ivabradine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ivabradine at Clinical Trials.gov Clinical Trials on Ivabradine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ivabradine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ivabradine Discussion groups on Ivabradine Patient Handouts on Ivabradine Directions to Hospitals Treating Ivabradine Risk calculators and risk factors for Ivabradine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ivabradine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ivabradine (INN) (Template:PronEng) is a novel medication used for the symptomatic management of stable angina pectoris. It is marketed under the trade name Procoralan (Servier Laboratories), and was also known as S-16257 during its development. Ivabradine acts by reducing the heart rate in a mechanism different from beta blockers and calcium channel blockers, two commonly prescribed antianginal drugs. It is classified as a cardiotonic agent. A study on the mortality and morbidity study regarding Ivabradine, named BEAUTIFUL has been conducted in 33 countries on 11.000 Coronary Artery Disease patients. The results have been announced in the European Society of Cardiology meeting in August 2008.
Mode of action
Ivabradine acts on the If (f is for "funny", so called because it had unusual properties compared with other current systems known at the time of its discovery) ion current, which is highly expressed in the sinoatrial node. If is a mixed Na+–K+ inward current activated by hyperpolarization and modulated by the autonomic nervous system. It is one of the most important ionic currents for regulating pacemaker activity in the sinoatrial (SA) node. Ivabradine selectively inhibits the pacemaker If current in a dose-dependent manner. Blocking this channel reduces cardiac pacemaker activity, slowing the heart rate and allowing more time for blood to flow to the myocardium.[1][2]
Uses
Ivabradine was approved by the European Medicines Agency in 2005. It is indicated for the symptomatic treatment of stable angina pectoris in patients with normal sinus rhythm, who have a contraindication to or intolerance to beta blockers. It has been shown to be non-inferior to the beta-blocker atenolol for this indication[3] and amlodipine.
Apart from angina, it is also being used off-label in the treatment of inappropriate sinus tachycardia.[4]
Dosage
A dose of 5 mg twice daily is recommended initially; after 1 month, it is recommended to increase to 7.5 mg twice daily to get the optimal efficacy linked to heart rate reduction. Given limited experience in the elderly, the manufacturer recommends a starting dose of 2.5 mg[5].
Adverse effects
14.5% of all patients taking ivabradine experience luminous phenomena (by patients described as sensations of enhanced brightness in a fully maintained visual field). This is probably due to blockage of I h ion channels in the retina which are very similar to cardiac If. These symptoms are mild, transient, fully reversible and non-severe. In clinical studies about 1% of all patients had to discontinue the drug because of these sensations, which occurred on average 40 days after commencement of the drug.[3]
Bradycardia (unusually slow heart rate) occurs at 2% and 5% for doses of 7.5 and 10 mg respectively (compared to 4.3% in atenonol)[3]. 2.6-4.8% reported headaches[3]. Other common adverse drug reactions (1–10% of patients) include first-degree AV block, ventricular extrasystoles, dizziness and/or blurred vision.[6]
Contraindications
Ivabradine is contraindicated in sick sinus syndrome, and cannot be used concominantly with inhibitors of CYP3A4 such as azole antifungals (such as ketoconazole), macrolide antibiotics, nefazodone and the anti-HIV drugs nelfinavir and ritonavir[5].
References
- ↑ Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP (1994). "Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49". Br. J. Pharmacol. 112 (1): 37–42. PMID 8032660.
- ↑ Sulfi S, Timmis AD (2006). "Ivabradine -- the first selective sinus node I(f) channel inhibitor in the treatment of stable angina". Int. J. Clin. Pract. 60 (2): 222–8. doi:10.1111/j.1742-1241.2006.00817.x. PMID 16451297.
- ↑ 3.0 3.1 3.2 3.3 Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K (2005). "Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina". Eur. Heart J. 26 (23): 2529–36. doi:10.1093/eurheartj/ehi586. PMID 16214830.
- ↑ Yusuf S, Camm AJ (2003). "Sinus tachyarrhythmias and the specific bradycardic agents: a marriage made in heaven?". J. Cardiovasc. Pharmacol. Ther. 8 (2): 89–105. PMID 12808482.
- ↑ 5.0 5.1 "Servier, procolaran product description for healthcare professionals". Retrieved 2007-10-14.
- ↑ Anonymous (2006). "New medicines: Procoralan". Pharmaceutical Journal. 276 (7386): 131.}
External links
- Official site
- Manufacturer´s web site
- Procoralan UK
- Źródło: "http://pl.wikipedia.org/wiki/Iwabradyna"
- Regarding the BEAUTIFUL Study: "http://www.medical-tribune.com.tr/node/262"
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Antianginals
- Cardiovascular Drugs
- Drug