Zopiclone
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral tablets 7.5 mg |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 52-59% bound to plasma protein |
| Metabolism | Various cytochrome P450 liver enzymes |
| Elimination half-life | ~6 hours ~9 hours for over 65 |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
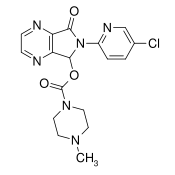
| Formula | C17H17ClN6O3 |
| Molar mass | 388.808 g/mol |
|
WikiDoc Resources for Zopiclone |
|
Articles |
|---|
|
Most recent articles on Zopiclone |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Zopiclone at Clinical Trials.gov Clinical Trials on Zopiclone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Zopiclone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Zopiclone Discussion groups on Zopiclone Directions to Hospitals Treating Zopiclone Risk calculators and risk factors for Zopiclone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Zopiclone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Zopiclone (pronounced Template:IPA2) sold as Imovane and Zimovane in Europe, and as the eszopiclone analogue Lunesta in North America, is a novel hypnotic agent used in the treatment of insomnia. Zopiclone is also available world wide under various other trade names. It was first developed by Sepracor and introduced in 1988 by Rhône-Poulenc S.A., now part of Sanofi-Aventis, the main worldwide manufacturer of the drug. Zopiclone is a controlled substance in the United States, Canada and some European countries and may be illegal to possess without a prescription.
While it acts on the BZ¹ receptor and is a short-acting hypnotic agent, it is not a benzodiazepine (with which it shares a number of characteristics and effects), but a cyclopyrrolone derivative, belonging to a novel chemical class which is structurally unrelated to existing hypnotics.
On April 4, 2005, the United States DEA listed zopiclone under Schedule IV, due to some evidence that the drug has addictive properties similar to benzodiazepines.
Zopiclone, as traditionally sold worldwide, is a racemic mixture of two stereoisomers, only one of which is active. In 2005, the pharmaceutical company Sepracor, of Marlborough, Massachusetts, began marketing the active stereoisomer eszopiclone under the name Lunesta in the United States. This had the consequence of placing what is a generic drug in most of the world under patent control in the United States, although in that country, it is expected to be available in generic form by the year 2010. It is already available off-patent in a number of European countries as well as Brazil. The eszopiclone/zopiclone difference is in the dosage—the strongest eszopiclone derivative dosage contains 3mg of the therapeutic stereoisomer, whereas, the highest zopiclone dosage (7.5mg) contains 3.75mg of the active stereoisomer. The two agents have not been studied via head-to-head clinical trials to determine if any clinical differences exist (e.g., efficacy, side-effects, developing dependence on the drug, and safety, etc.).
Zopiclone is known colloquially as a "Z drug", Other Z drugs include zaleplon (Sonata) and zolpidem (Ambien and AmbienCR) and were thought in initial studies to be less addictive and less habit forming than benzodiazepines. This appraisal has shifted somewhat in the last few years, as cases of addiction and habituation have been presented. It is recommended that zopiclone is taken on a "when required" basis, and daily or continuous use of the drug is not usually advised.
Adverse reactions
The side-effect most commonly seen in clinical trials is taste alteration or dysgeusia (bitter, metallic taste, which is usually fleeting in most users but can persist until the drug's half-life has expired). Palpitations may occur in the daytime after withdrawal from the drug after prolonged periods of use (>4 weeks).
More common reactions:
Gastrointestinal: bitter metallic taste, dry mouth. Nervous system: drowsiness, headaches, and fatigue. Unexpected mood changes have been noted, which if experienced should lead to the drug being withdrawn from the patient.
Less common reactions:
- Gastrointestinal: heartburn, constipation, diarrhoea, nausea, coated tongue, bad breath, anorexia or increased appetite, vomiting, epigastric pains, dyspepsia, dehydration, parageusia.
- Cardiovascular: palpitations in elderly patients.
- Skin: urticaria, tingling in the arms and legs.
- Miscellaneous: blurred vision, frequent micturition, mild to moderate increases in serum transaminases and/or alkaline phosphatase have been reported very rarely.
- Reproductive: impotence, ejaculation failure, anorgasmia in both women and men.
- Nervous system: agitation, anxiety, loss of memory including retrograde and anterograde amnesia, confusion, dizziness, weakness, somnolence, asthenia, moderate to severe euphoria and/or dysphoria, feeling of drunkenness, depression, coordination abnormality, hypotonia, speech disorder, hallucinations of various strengths, usually auditory and visual, behavioural disorders, aggression, tremor, rebound insomnia, nightmares, hypomania.
Pharmacokinetics
Zopiclone is a cyclopyrrolone hypnotic agent. It possesses a chiral centre and is commercially available as a racemic mixture. Methods involving high performance liquid chromatography (HPLC), gas chromatography, capillary electrophoresis (CE) and high performance thin layer chromatography have been developed for the quantification of zopiclone and its 2 main metabolites in biological samples. For the chiral determination of the enantiomers of zopiclone and its metabolites, HPLC and CE methods are available. After oral administration, zopiclone is rapidly absorbed, with a bioavailability of approximately 80%. The plasma protein binding of zopiclone has been reported to be between 45 and 80%. Zopiclone is rapidly and widely distributed to body tissues including the brain, and is excreted in urine, saliva and breast milk. Zopiclone is partly metabolised in the liver to form an inactive N-demethylated derivative and an active N-oxide metabolite. In addition, approximately 50% of the administered dose is decarboxylated and excreted via the lungs. Less than 7% of the administered dose is renally excreted as unchanged zopiclone. In urine, the N-demethyl and N-oxide metabolites account for 30% of the initial dose. The terminal elimination half-life (t1/2z) of zopiclone ranges from 3.5 to 6.5 hours. The pharmacokinetics of zopiclone in humans are stereoselective. After oral administration of the racemic mixture, Cmax (time to maximum plasma concentration), AUC (area under the plasma time-concentration curve) and t1/2z values are higher for the dextrorotatory enantiomer owing to the slower total clearance and smaller volume of distribution (corrected by the bioavailability), compared with the levorotatory enantiomer. In urine, the concentrations of the dextrorotatory enantiomers of the N-demethyl and N-oxide metabolites are higher than those of the respective antipodes. The pharmacokinetics of zopiclone are altered by aging and are influenced by renal and hepatic functions. Drug interactions have been observed with erythromycin, trimipramine and carbamazepine.
Pharmacology
The mechanism of action of zopiclone is similar to benzodiazepines, with similar effects on locomotor activity and on dopamine and serotonin turnover.[1][2] A meta-analysis of randomised controlled clinical trials which compared benzodiazepines to Zopiclone or other Z Drugs such as zolpidem, zaleplon has found that there are few clear and consistent differences between Zopiclone and the benzodiazepines in terms of sleep onset latency, total sleep duration, number of awakenings, quality of sleep, adverse events, tolerance, rebound insomnia and daytime alertness.[3] Zopiclone is in the cyclopyrrolone family of drugs. Other cyclopyrrolone drugs include suriclone. Zopiclone although molecularly different from benzodiazepines, shares an almost identical pharmacological profile as benzodiazepines including anxiolytic properties. Its mechanism of action is modulating benzodiazepine receptors.[4] In addition to zopiclone's benzodiazepine pharmacological properties it also has some barbiturate like properties.[5][6]
In EEG studies, zopiclone significantly increases the energy of the beta frequency band and shows characteristics of high-voltage slow waves, desynchronization of hippocampal theta waves and an increase in the energy of the delta frequency band. Zopiclone increases both stage 2 and slow wave sleep (SWS), while zolpidem, an omega1-selective compound, increases only SWS and causes no effect on stage 2 sleep. Zopiclone is less selective to the omega1 site and has higher affinity to the omega2 site than zaleplon. Zopiclone is therefore very similar pharmacologically to benzodiazepines.[7]
Dependence and withdrawal
Zopiclone was introduced and initially promoted as having less dependence and withdrawal than traditional benzodiazepine drugs. However zopiclone may have an even greater addictive potential than benzodiazepines.[8] Benzodiazepines act indiscriminately at α¹ α² α³ and α5 GABAa containing receptors. Zopiclone has high affinity for the alpha¹ subunit GABAa receptor and a low to intermediate action on α² and α³. receptors.[9] The alpha1 alpha2 and alpha3 GABAa receptors make up over 90% of all GABAa receptors in humans. The differences in receptor affinity and binding of zopiclone compared with benzodiazepines have led some to claims of a lower dependency risk with zopiclone.
Publications in the British Medical Journal have cast some doubt on the claim that zopiclone has a low dependence potential. Physical dependence and recreational abuse and withdrawal syndromes similar to those seen in benzodiazepine withdrawal has been described. Withdrawal symptoms included anxiety, tachycardia, tremor, sweats, flushes, palpitations, derealisation, and further insomnia.[10]
High dose misuse of zopiclone and increasing popularity amongst drug abusers has also been described with zopiclone[11] Due to the risk of tolerance and physical dependence zopiclone is only recommended for the short term (2–4 weeks) relief of insomnia, or alternatively, long term infrequent use. Long-term zopiclone users who have become physically dependent are usually recommended to cross over to an equivalent dose of diazepam (Valium®) which has a longer half life and reduce their dosage over a period of months to avoid severe or unpleasant withdrawal symptoms. According to the World Health Organisation Zopiclone although molecularly is not a benzodiazepine binds with high affinity to benzodiazepine receptors and stated that Zopiclone is cross tolerant with benzodiazepines and one can substitute one for the other. In the review of Zopiclone by the World Health Organisation they found that the appearance of withdrawal symptoms usually occurred either when the drug was misused in excessive doses or when use of zopiclone was prolonged. The withdrawal symptoms from Zopiclone reported included anxiety, tachycardia, tremor, sweating, rebound insomnia, derealisation, convulsions, palpitations and flushes.[12]
The risk of dependency on zopiclone when used for less than 4 weeks or used occasionally is very low.
Alcohol has cross tolerance with GABAa receptor positive modulators such as the benzodiazepines and the nonbenzodiazepine drugs. For this reason alcoholics or recovering alcoholics may be at increased risk of physical dependency on zopiclone. Also, alcoholics and drug abusers may be at increased risk of abusing and or becoming psychologically dependent on zopiclone. Zopiclone should be avoided in those with a history of Alcoholism, drug misuse (illicit or prescription misuse), or in those with history of physical dependency or psychological dependency on sedative-hypnotic drugs.
Special precautions
Zopiclone induces impairment of motor function.[13] Driving or operating machinery should be avoided after taking zopiclone.
Zopiclone increases sway in older people. Falls are a significant cause of death in older people.
Abuse
Zopiclone and other sedative hypnotic drugs are detected frequently in cases of people suspected of driving under the influence of drugs. Other drugs including the benzodiazepines and zolpidem are also found in high numbers of suspected drugged drivers. Many drivers have blood levels far exceeding the therapeutic dose range suggesting a high degree of abuse potential for benzodiazepines, zolpidem and zopiclone.[14]
Zopiclone has cross tolerance with barbiturates and is able to suppress barbiturate withdrawal signs. Zopiclone is frequently self administered intravenously in studies on monkeys suggesting a high risk of abuse potential.[15]
Overdose
Overdose of zopiclone may present with excessive sedation, depressed respiratory function which may progress to coma and possibly death. Zopiclone combined with alcohol, opiates or other CNS depressants may be even more likely to lead to fatal overdoses. Zopiclone overdosage can be treated with the benzodiazepine receptor antagonist flumazenil which displaces zopiclone from its binding site the benzodiazepine receptor and therefore rapidly reverses the effects of zopiclone.[16]
Death certificates show that fatal zopiclone overdoses are on the rise.[17]
Availability
Zopiclone is also sold under a wide variety of other brand names world wide including:
- Insomnium - Argentina
- Sedolox and Somnal - Austria
- Rhovane - Canada
- Alpaz, Losopil, Nuctane, Zetix, Zometic and Zonix - Chile
- Imovane, Imoclone and Imozop - Denmark
- Zopinox, Imovane - Finland
- Optidorm, Ximovan, Zodurat, Zop, Zopi-Puren, Zopicalm and Zopiclodura - Germany
- Somnosan - Germany, Portugal
- Amvey, Eurovan, Zolief and Zomni - Hong Kong
- Zopicon - India
- Zileze, Zimoclone, Zopitan and Zorclone - Republic of Ireland
- Zimovane - Republic of Ireland, United Kingdom
- Nocturno - Israel
- Imovane (Имован) - Argentina, Australia, Belgium, Brazil, Canada, Chile, Czech Republic, Denmark, Finland, France, Greece, Hong Kong, Hungary, Iceland, Israel, Italy, Malaysia, Mexico, Netherlands, Norway, New Zealand, Russia, South Africa, Singapore, Sweden, Switzerland, Turkey, Venezuela
- Relaxon (Релаксон) and Somnol (Сомнол) - Russia
- Alchera, Z-Dorm, Zopimed and Zopivane - South Africa
- Datolan, Limovan, Siaten and Zopicalma - Spain
- Zopiklon - Denmark, Norway, Sweden
- Amoban - Japan
- Hypnor - Egypt
References
- ↑ Liu HJ (1985). "Pharmacologic studies of the central action of zopiclone: effects on locomotor activity and brain monoamines in rats". Int J Clin Pharmacol Ther Toxicol. 23 (3): 121–8. PMID 2581904. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Sato K (1985). "Pharmacologic studies of central actions of zopiclone: influence on brain monoamines in rats under stressful condition". Int J Clin Pharmacol Ther Toxicol. 23 (4): 204–10. PMID 2860074. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Dündar, Y (2004). "Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis". Hum Psychopharmacol. 19 (5): 305–22. PMID 15252823. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Blanchard JC (1983). "Suriclone: a new cyclopyrrolone derivative recognizing receptors labeled by benzodiazepines in rat hippocampus and cerebellum". J Neurochem. 40 (3): 601–7. PMID 6298365. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Julou L (1983). "Pharmacological studies on zopiclone". Pharmacology. 27 (2): 46–58. PMID 6142468. Unknown parameter
|coauthors=ignored (help) - ↑ Blanchard JC (1983). "Brain receptors and zopiclone". Pharmacology. 27 (2): 59–69. PMID 6322210. Unknown parameter
|coauthors=ignored (help) - ↑ Noguchi H (2004). "Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats" (pdf). J Pharmacol Sci. 94 (3 pages = 246-51). PMID 15037809. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Bramness JG (1998). "[Adverse effects of zopiclone]". Tidsskrift for den Norske laegeforening. 118 (13): 2029–32. PMID 9656789. Unknown parameter
|coauthors=ignored (help) - ↑ Molecular actions of (S)-desmethylzopiclone (SEP-174559), an anxiolytic metabolite of zopiclone
- ↑ Physical dependence on zopiclone: case reports
- ↑ Prescribing this drug to addicts may give rise to iatrogenic drug misuse
- ↑ World Health Organisation - Assessment of Zopiclone
- ↑ Yasui M (2005). "[Pharmacological profiles of benzodiazepinergic hypnotics and correlations with receptor subtypes]". 25 (3): 143–51. PMID 16045197. Text "Nihon Shinkei Seishin Yakurigaku Zasshi. " ignored (help); Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Jones AW (2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Ther Drug Monit. 29 (2): 248–60. PMID 17417081. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Yanagita T. (1982). "Dependence potential of zopiclone studied in monkeys". International pharmacopsychiatry. 17 (2): 216–27. PMID 6892368.
- ↑ Cienki, JJ (2005). "Zopiclone overdose responsive to flumazenil". Clin Toxicol (Phila). 43 (5): 385–6. PMID 16235515. Unknown parameter
|coauthors=ignored (help) - ↑ Carlsten, A (2003). "The role of benzodiazepines in elderly suicides". Scand J Public Health. 31 (3): 224–8. PMID 12850977. Unknown parameter
|coauthors=ignored (help)
External links
- Detailed pharmacological information
- Scheduling recommendation (PDF file)
- Details on scheduling
- Erowid zopiclone vault
- Support for Zopiclone dependency/addiction
de:Zopiclon no:Zopiklon fi:Tsopikloni sv:Zopiklon Template:Jb1
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Missing pipe
- Pages with citations using unnamed parameters
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Cyclopyrrolones
- Hypnotics
- Sedatives