Short QT syndrome
| Short QT syndrome | |
 | |
|---|---|
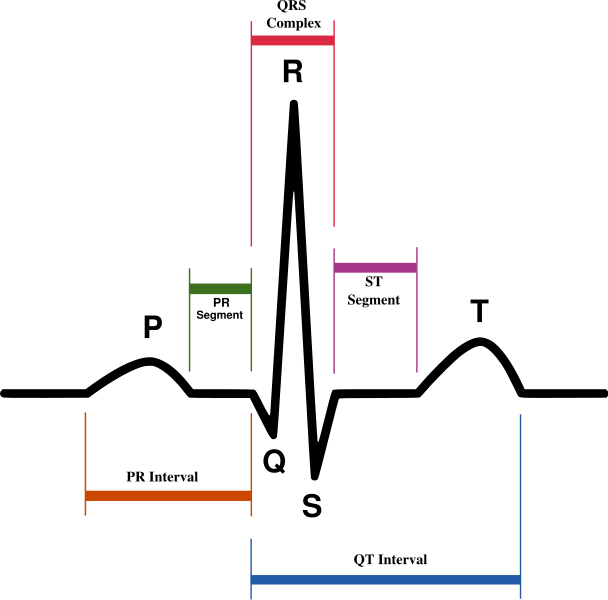
| Schematic representation of normal ECG trace (sinus rhythm), with waves, segments, and intervals labeled. | |
| DiseasesDB | 11105 |
|
WikiDoc Resources for Short QT syndrome |
|
Articles |
|---|
|
Most recent articles on Short QT syndrome Most cited articles on Short QT syndrome |
|
Media |
|
Powerpoint slides on Short QT syndrome |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Short QT syndrome |
|
Clinical Trials |
|
Ongoing Trials on Short QT syndrome at Clinical Trials.gov Trial results on Short QT syndrome Clinical Trials on Short QT syndrome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Short QT syndrome NICE Guidance on Short QT syndrome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Short QT syndrome Discussion groups on Short QT syndrome Patient Handouts on Short QT syndrome Directions to Hospitals Treating Short QT syndrome Risk calculators and risk factors for Short QT syndrome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Short QT syndrome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [3]
Synonyms and keywords: SQTS; short QT; short QTc; QT interval shortening
Overview
Short QT syndrome is a rare autosomal dominant inhereted disease of the electrical conduction system of the heart due to gain-of-function mutations in genes encoding for cardiac potassium channels KCNH2, KCNQ1 and KCNJ2. The QT interval is short (≤ 300 ms) and does not significantly change with heart rate, and there are tall and peaked T waves. The heart is structurally normal. It is associated with an increased risk of atrial fibrillation, syncope and sudden death.
Historical Perspective
The syndrome was first described by Dr. Prebe Bjerregaard MD, DMSc in 1999[1].
First Patient with Short QT Syndrome
Shalon Hill, a 17 year old white female, underwent laparoscopic cholecystectomy at Anderson Hospital, Maryville, Illinois in 1999 which was complicated by atrial fibrillation with a rapid ventricular response (RVR) at 150-200 beats/min along with acute pulmonary edema[1][2]. The atrial fbrillation with RVR was treated with DC cardioversion and she was discharged to home in normal sinus rhythm on digoxin. The atrial fibrillation recurred 6 weeks later and she was found at that time to have a short QT interval of 225 mseconds which was treated with prophylactic therapy with propafenone. She then remained asymptomatic for 6 months and the propafenone was discontinued. However, the atrial fibrillation recurred 2 months after the propafenone was discontinued, and it was therefore resumed. She remained asymptomatic on propafenone, but an AICD was implanted given reports from around the world of sudden cardiac death.
First Family with Short QT Syndrome
EKGs of the first patient's family members were analyzed. The QT interval of her 21 year old brother was 240 msec, the QT interval of her 84 year old maternal grandfather was 240 msec, and the QT interval of her 51 year old mother was 230 msec. The EKG of here father was normal[1].
He brother was asymptomatic, and on August 13, 2003 was found to have inducible ventricular fibrillation on programmed electrical stimulation. This was treated with implantation of an implantable cardioverter defibrillator. Subsequently he complained of occasional palpitations and paroxysmal atrial fibrillation with a rapid ventricular response was noted on interrogation of the ICD.
Her mother is a 51 year old healthy white female with a history of 3 episodes of sustained palpitations and paroxysmal atrial fibrillation. She has remained asymptomatic on propafenone since April, 2003. Programmed electrical stimulation on September 29, 2003 induced both atrial and ventricular fibrillation and an AICD was implanted.
Her maternal grandfather was an 84 year old white male who had chronic atrial fibrillation, coronary artery disease and hypertension who died following an embolic stroke.
Classification
- Short QT syndrome type 1 (SQT1): This variant is due to a gain-of-function mutation of the rapid component of the delayed rectifier potassium current HERG (KCNH2) channel(IKr)[3]. The variant is a result of missense mutations which increase IKr. It is associated with sudden death and sudden infant death syndrome.
- Short QT syndrome type 2 (SQT2): Caused by a mutation in the KCNQ1 gene[4]. In the first patient, a g919c substitution in the KCNQ1 gene encoding for the K+ channel KvLQT1 was identified. The mutation led to a gain of function in in the KvLQT1 (I(Ks)) channel. This variant is associated with ventricular fibrillation.
- Short QT syndrome type 3 (SQT3): This variant results from a G514A substitution in the KCNJ2 gene ( a change from aspartic acid to asparagine at position 172 (D172N))[5]. This causes a defect in the gene coding for the inwardly rectifying Kir2.1 (I(K1)) channel. The ECG shows asymmetrical T waves. These patients have an increased risk for re-entry arrhythmias.
- Short QT syndrome type 3 (SQT4): A loss of function mutation in the CACNA1C gene alters the encoding for the α1- and β2b-subunits of the L-type calcium channel. The phenotype is similar to Brugada syndrome combined with a short QT interval. There is an increased risk of sudden cardiac death.
- Short QT syndrome type 3 (SQT5): A loss of function mutation in the CACNB2B gene alters the encoding for the α1- and β2b-subunits of the L-type calcium channel. The phenotype is similar to Brugada syndrome combined with a short QT interval. There is an increased risk of sudden cardiac death.
Pathophysiology
It has been hypothesized that short QT syndrome is due to increased activity of outward potassium currents in phase 2 and 3 of the cardiac action potential. This would cause a shortening of the plateau phase of the action potential (phase 2), causing a shortening of the overall action potential, leading to an overall shortening of refractory periods and the QT interval. In the families afflicted by short QT syndrome, two different missense mutations have been described in the human ether-a-go-go gene (HERG). These mutations result in expression of the same amino acid change in the cardiac IKr ion channel. This mutated IKr has increased activity compared to the normal ion channel, and would theoretically explain the above hypothesis.
Genetics
In the families afflicted by short QT syndrome, mutations have been described in three genes, KvLQT1, the human ether-a-go-go gene (HERG), and KCNJ2. Mutations in the KCNH2, KCNJ2, and KCNQ1 genes cause short QT syndrome. These genes provide instructions for making proteins that act as channels across the cell membrane. These channels transport positively charged atoms (ions) of potassium into and out of cells. In cardiac muscle, these ion channels play critical roles in maintaining the heart's normal rhythm. Mutations in the KCNH2, KCNJ2, or KCNQ1 gene increase the activity of the channels, which changes the flow of potassium ions between cells. This disruption in ion transport alters the way the heart beats, leading to the abnormal heart rhythm characteristic of short QT syndrome. Short QT syndrome appears to have an autosomal dominant pattern of inheritance.
Due to the autosomal dominant inheritance pattern, individuals may have family members with a history of unexplained or sudden death at a young age (even in infancy), palpitations, or atrial fibrillation.
Causes
The causes of shortening of the QT interval can be divided into primary causes (Short QT syndrome types 1-5) and secondary causes such as drugs and electrolyte disturbances.
Common Causes
Causes in Alphabetical Order
- Acidosis
- Altered autonomic tone
- Digoxin
- Hypercalcaemia
- Hyperkalemia
- Hyperthermia
- Lanatoside C
- Rufinamide
- Short QT syndrome type 1
- Short QT syndrome type 2
- Short QT syndrome type 3
- Short QT syndrome type 4
- Short QT syndrome type 5
Differentiating Short QT Syndrome from other Disorders
In contrast to Long QT Syndrome (LQTS), there is often no specific trigger (such as a loud noise or exercise) for an episode of arrhythmia.
Epidemiology and Demographics
Since the syndrome was first described in 2000, < 30 cases have been identified.
Age
The median age of presentation is 30 years, but ranges from just weeks to the sixth decade of life.
Natural History, Complications, Prognosis
Short QT syndrome is associated with an increased risk of atrial fibrillation, syncope and sudden death due to ventricular fibrillation.
Screening
A young patient with lone atrial fibrillation should be assessed for short QT syndrome.
Diagnosis
Secondary causes of a short QT interval such as drugs and electrolyte disturbances should be ruled out before embarking on an evaluation as to whether the patient has one of the short QT syndrome variants.
Diagnostic Criteria
Recent diagnostic criteria have been published out of the Arrhythmia Research Laboratory at the University of Ottawa Heart Institute from Drs. Michael H Gollob and Jason D Roberts.[6]
The Short QT Syndrome diagnostic criteria is based on a point system as follows:
- QTc in milliseconds
- <370 = 1 point
- <350 = 2 points
- <330 = 3 points
- J point - T peak interval in milliseconds
- <120 = 1 point
- Clinical History
- Sudden cardiac arrest = 2 points
- Polymorphic VT or VF = 2 points
- Unexplained syncope = 1 point
- Atrial fibrillation = 1 point
- Family History
- 1st or 2nd degree relative with SQTS = 2 points
- 1st or 2nd degree relative with sudden death = 1 point
- Sudden infant death syndrome = 1 point
- Genotype
- Genotype positive = 2 points
- Mutation of undetermined significance in a culprit gene = 1 point
The points are summed and interpreted as follows:
- > or equal to 4 points: High-probability of SQTS
- 3 Points: Intermediate probability of SQTS
- 2 points or less: Low probability of SQTS
Symptoms
Sudden Death
Sudden death may be the first presentation of the disease.
- The most common symptom of short QT syndrome is cardiac arrest (34%)[7].
- The first symptom of short QT syndrome is most often cardiac arrest (28%)[7]
Syncope
- Syncope is the first symptom in 24% of patients and is most likely due to self-terminating ventricular fibrillation.
Palpitations
- Palpitations are present in 31% of patients.
Atrial Fibrillation
- Atrial fibrillation is present in up to 80% of patients with short QT syndrome.
Triggers
In contrast to Long QT Syndrome (LQTS), there is often no specific trigger (such as a loud noise or exercise) for an episode of arrhythmia.
Screening
Short QT syndrome should be excluded in patients without structural heart disease presenting with sudden cardiac death.
Electrocardiogam
The diagnosis of short QT syndrome on the EKG is based upon three criteria:
Duration of the QT Interval
While the QT interval is generally short, the QT interval alone cannot be used to distinguish the patient with short QT syndrome from a normal patient (similar to long QT syndrome).
SQTS 1,2,3
The QTc is < 300-320 msec.[3][4][5]
SQTS 4,5
The QTc is just under 360 msec
As recently reviewed by Viskin (12), males with QTc <330 ms and females with QTc <340 ms should be diagnosed with SQTS even if they are asymptomatic since this values are very rare in healthy population. In addition, QTc intervals shorter than 360 and 370 ms (males and females respectively) should only be considered diagnostic of SQTS when supported by symptoms or family history because they overlap with healthy population.
typically short and ≤ 300-320 ms
Variability of the QT Interval with Heart Rate
The short QT interval does not vary significantly with the heart rate.
Morphology of the T Wave
Tall, peaked T waves in the right precordial leads may also be noted.
Rhythm
70% of patients with short QT have a history of either paroxysmal atrial fibrillation or permanent atrial fibrillation, and atrial fibrillation is the first sign of short QT syndrome in 50% of patients. In young patients with lone atrial fibrillation, the patient should be screened for short QT syndrome.
Electrophysiologic Studies
In the electrophysiology lab, individuals with short QT syndrome are noted to have short refractory periods (ranging from 120 to 180 ms), both in the atria as well as in the ventricles. Ventricular fibrillation is induced on programmed stimulation in 90% of patients with short QT syndrome. Despite the high rate of VF inducibility, the risk of sudden death in an individual patient is difficult to predict given the genetic and clinical heterogeneity of short QT syndrome and the limited number of patients with short follow-up to date.
Genetic Testing
Mutations in the KCNH2, KCNJ2, and KCNQ1 genes cause short QT syndrome. These genes provide instructions for making proteins that act as channels across the cell membrane. These channels transport positively charged atoms (ions) of potassium into and out of cells. In cardiac muscle, these ion channels play critical roles in maintaining the heart's normal rhythm. Mutations in the KCNH2, KCNJ2, or KCNQ1 gene increase the activity of the channels, which changes the flow of potassium ions between cells. This disruption in ion transport alters the way the heart beats, leading to the abnormal heart rhythm characteristic of short QT syndrome. Short QT syndrome appears to have an autosomal dominant pattern of inheritance.
Centers Performing Genetic Testing for Short QT Syndrome
Treatment
Device Based Therapy
An implantable cardioverter-defibrillator (ICD) is indicated in[8]:
- Symptomatic patients
- Patients with a family history of sudden cardiac death
Pharmacologic Therapy
Short QT Syndrome 1 (SQT1)
Patients with Short QT Syndrome 1 (SQT1) have a mutation in KCNH2 (HERG). Class IC and III antiarrhythmic drugs do not produce any significant QT interval prolongation [9][10] . In four out of four patients, Quinidine prolonged the QT interval from 263 +/- 12 msec to 362 +/-25 msec, most likely due to its effects on prolonging the action potential and by virtue of its action on the IK channels. Although Quinidine was successful in preventing the inducibility of ventricular fibrillation in 4 out of 4 patients, it is unclear if the prolongation of the QT interval by quinidine would reduce the risk of sudden cardiac death. Although pharmacotherapy can be used to suppress the occurrence of atrial fibrillation in patients with SQT1, AICD implantation is the mainstay of therapy, and pharmacotherapy to prevent sudden death should is only indicated if AICD implantation is not possible.
References
- ↑ 1.0 1.1 1.2 Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman BR, Bjerregaard P (2000). "Idiopathic short QT interval: a new clinical syndrome?". Cardiology. 94 (2): 99–102. doi:47299 Check
|doi=value (help). PMID 11173780. Retrieved 2012-09-03. - ↑ http://www.shortqtsyndrome.org/short_qt_history.htm
- ↑ 3.0 3.1 Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C (2004). "Sudden death associated with short-QT syndrome linked to mutations in HERG". Circulation. 109 (1): 30–5. doi:10.1161/01.CIR.0000109482.92774.3A. PMID 14676148. Retrieved 2012-09-02. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Bellocq C, van Ginneken AC, Bezzina CR, Alders M, Escande D, Mannens MM, Baró I, Wilde AA (2004). "Mutation in the KCNQ1 gene leading to the short QT-interval syndrome". Circulation. 109 (20): 2394–7. doi:10.1161/01.CIR.0000130409.72142.FE. PMID 15159330. Retrieved 2012-09-02. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J (2005). "A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene". Circulation Research. 96 (7): 800–7. doi:10.1161/01.RES.0000162101.76263.8c. PMID 15761194. Retrieved 2012-09-02. Unknown parameter
|month=ignored (help) - ↑ Gollob M, Redpath C, Roberts J. (2011). "The Short QT syndrome: Proposed Diagnostic Criteria". J Am Coll Cardiol. 57 (7): 802–812. doi:10.1016/j.jacc.2010.09.048. PMID 21310316.
- ↑ 7.0 7.1 Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haïssaguerre M, Schimpf R, Borggrefe M, Wolpert C (2007). "Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death". Circulation. 115 (4): 442–9. doi:10.1161/CIRCULATIONAHA.106.668392. PMC 1952683. PMID 17224476. Retrieved 2012-09-02. Unknown parameter
|month=ignored (help) - ↑ Borggrefe M. FESC, Wolpert C, Veltmann C, Giustetto C, Gaita F, Schimpf R. Short QT Syndrome : A new primary electrical disease, ESC E journal, Vol 3 N°34, 10 May 2005. [1]
- ↑ Gaita F, Giustetto C, Bianchi F, Schimpf R, Haissaguerre M, Calo L, Brugada R, Antzelevitch C, Borggrefe M, Wolpert C. (2004). "Short QT syndrome: pharmacological treatment". J Am Coll Cardiol. 43 (8): 1494–1499. doi:10.1016/j.jacc.2004.02.034. PMID 15093889.
- ↑ Wolpert C, Schimpf R, Giustetto C, Antzelevitch C, Cordeiro J, Dumaine R, Brugada R, Hong K, Bauersfeld U, Gaita F, Borggrefe M (2005). "Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG". Journal of Cardiovascular Electrophysiology. 16 (1): 54–8. doi:10.1046/j.1540-8167.2005.04470.x. PMC 1474841. PMID 15673388. Retrieved 2012-09-03. Unknown parameter
|month=ignored (help)