Lisinopril
{{DrugProjectFormSinglePage |authorTag=Ahmed Zaghw, M.D. [1], Amr Marawan, M.D. [2] |genericName=Lisinopril |aOrAn=an |drugClass=Angiotensin converting enzyme inhibitor, Thiazide diuretic |indication=hypertension, heart failure |hasBlackBoxWarning=Yes |adverseReactions=hypotension, syncope, hyperkalemia, dizziness, headache, renal function tests abnormal and cough |blackBoxWarningTitle=USE IN PREGNANCY |blackBoxWarningBody=* When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.
- When pregnancy is detected, enalapril maleate should be discontinued as soon as possible.
|fdaLIADAdult======Hypertension=====
- Dosing Information
- Initial dose (not receiving a diuretic): Lisinopril 10 mg PO qd should be used.
- Usual dosage range: 20-40 mg/day
- Initial dose (with receiving a diuretic): Lisinopril 5 mg PO qd should be used.
- The diuretic should be discontinued, if possible, for two to three days before beginning therapy with PRINIVIL to reduce the likelihood of hypotension.
- Maintenance dose: Lisinopril 20-40 mg PO qd on two divided doses, adjust dose based on response (MAX 80 mg/day)
- Dosing Information: Adjunct
- Initial dose : Lisinopril 5 mg PO qd
- When initiating treatment with lisinopril in patients with heart failure, the initial dose should be administered under medical observation, especially in those patients with low blood pressure (systolic blood pressure below 100 mmHg). The mean peak blood pressure lowering occurs six to eight hours after dosing. Observation should continue until blood pressure is stable. The concomitant diuretic dose should be reduced, if possible, to help minimize hypovolemia which may contribute to hypotension. (See WARNINGS and PRECAUTIONS, Drug Interactions.) The appearance of hypotension after the initial dose of PRINIVIL does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
- Maintenance dose: Lisinopril 5-20 mg PO qd
- Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia:
- In patients with heart failure who have hyponatremia (serum sodium less than 130 mEq/L) or moderate to severe renal impairment (creatinine clearance less than or equal to 30 mL/min or serum creatinine greater than 3 mg/dL), therapy with PRINIVIL should be initiated at a dose of 2.5 mg once a day under close medical supervision.
Acute myocardial infarction
- Dosing Information
- For hemodynamical stable patients within 24 hours of the onset of symptoms of acute myocardial infarction:
- Initial dose : 5 mg PO
- Followed by: 5 mg PO after 24 hours 10 mg after 48 hours
- Maintenance dose: 10 mg PO qd
- Dosing should continue for 6 weeks. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers. Patients with a low systolic blood pressure (less than or equal to 120 mmHg) when treatment is started or during the first 3 days after the infarct should be given a lower 2.5 mg oral dose of PRINIVIL (see WARNINGS). If hypotension occurs (systolic blood pressure less than or equal to 100 mmHg) a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure less than 90 mmHg for more than 1 hour) PRINIVIL should be withdrawn. For patients who develop symptoms of heart failure, see DOSAGE AND ADMINISTRATION, Heart Failure.
- Dosage Adjustment in Patients with Myocardial Infarction with Renal Impairment: In acute myocardial infarction, treatment with PRINIVIL should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosage adjustment in myocardial infarction patients with severe renal impairment has been performed.
Use in Elderly
- Dosing information
- In general, blood pressure response and adverse experiences were similar in younger and older patients given similar doses of PRINIVIL. Pharmacokinetic studies, however, indicate that maximum blood levels and area under the plasma concentration time curve (AUC) are doubled in older patients, so that dosage adjustments should be made with particular caution.
|offLabelAdultGuideSupport=There is limited information about Off-Label Guideline-Supported Use of Lisinopril in adult patients. |offLabelAdultNoGuideSupport======Diabetic nephropathy=====
- Dosing information
- Recommended dosage: 40 mg PO qd[1]
Diabetic retinopathy
- Dosing information
- Recommended dosage: 10 mg/day[2]
Erythrocytosis
- Dosing information
Hypertension - Transplantation
- Dosing information
- Initial dosage: 10 mg/day
- Maximum dosage: 40 mg/day[5]
Kidney disease, Nondiabetic
- Dosing information
- Monotherapy: 10 mg/day[6]
- Combination Therapy: Adding candesartan to ACE inhibitor therapy produced significant reductions in blood pressure and urinary protein excretion among normotensive patients with chronic renal disease and proteinuria, based on an open-label, controlled, crossover trial (n=60)[7]
Prophylaxis treatment of Migraine
- Dosing information
|fdaLIADPed=====Hypertension====
- Dosing Information for children 6 years or older
- Initial dose : Lisinopril 0.07 mg/kg po qd (up to 5 mg total) should be used.
- Maintenance dose: Lisinopril adjust based on response; doses above 0.61 mg/kg/day or 40 mg/day have not been studied.
|offLabelPedGuideSupport=There is limited information about Off-Label Guideline-Supported Use of Lisinopril in pediatric patients. |offLabelPedNoGuideSupport=There is limited information about Off-Label Non–Guideline-Supported Use of Lisinopril in pediatric patients. |contraindications=* History of hypersensitivity or angioedema related to previous treatment with an angiotensin converting enzyme inhibitor.
- Patients with hereditary or idiopathic angioedema.
|warnings====Anaphylactoid and Possibly Related Reactions===
Presumably because angiotensin converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including PRINIVIL) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis and/or larynx has been reported in patients treated with angiotensin converting enzyme inhibitors, including PRINIVIL. This may occur at any time during treatment. ACE inhibitors have been associated with a higher rate of angioedema in Black than in non-Black patients. In such cases PRINIVIL should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. Even in those instances where swelling of only the tongue is involved, without respiratory distress, patients may require prolonged observation since treatment with antihistamines and corticosteroids may not be sufficient. Very rarely, fatalities have been reported due to angioedema associated with laryngeal edema or tongue edema. Patients with involvement of the tongue, glottis or larynx are likely to experience airway obstruction, especially those with a history of airway surgery. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided. (See ADVERSE REACTIONS.) Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor (see also INDICATIONS AND USAGE and CONTRAINDICATIONS).
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid reactions during desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure: Sudden and potentially life-threatening anaphylactoid reactions have been reported in some patients dialyzed with high-flux membranes (e.g., AN69®) and treated concomitantly with an ACE inhibitor. In such patients, dialysis must be stopped immediately, and aggressive therapy for anaphylactoid reactions be initiated. Symptoms have not been relieved by antihistamines in these situations. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of antihypertensive agent. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension
Excessive hypotension is rare in patients with uncomplicated hypertension treated with PRINIVIL alone. Patients with heart failure given PRINIVIL commonly have some reduction in blood pressure with peak blood pressure reduction occurring 6 to 8 hours post dose, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed; caution should be observed when initiating therapy. (See DOSAGE AND ADMINISTRATION.) Patients at risk of excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include those with the following conditions or characteristics: heart failure with systolic blood pressure below 100 mmHg, hyponatremia, high-dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or increase salt intake cautiously before initiating therapy with PRINIVIL in patients at risk for excessive hypotension who are able to tolerate such adjustments. (See PRECAUTIONS, Drug Interactions, and ADVERSE REACTIONS.) Patients with acute myocardial infarction in the GISSI - 3 study had a higher (9.0 percent versus 3.7 percent) incidence of persistent hypotension (systolic blood pressure <90 mmHg for more than 1 hour) when treated with PRINIVIL. Treatment with PRINIVIL must not be initiated in acute myocardial infarction patients at risk of further serious hemodynamic deterioration after treatment with a vasodilator (e.g., systolic blood pressure of 100 mmHg or lower) or cardiogenic shock. In patients at risk of excessive hypotension, therapy should be started under very close medical supervision and such patients should be followed closely for the first two weeks of treatment and whenever the dose of PRINIVIL and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease, or in patients with acute myocardial infarction, in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident. If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses of PRINIVIL which usually can be given without difficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dose reduction or discontinuation of PRINIVIL or concomitant diuretic may be necessary.
Leukopenia/Neutropenia/Agranulocytosis
Another angiotensin converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients but more frequently in patients with renal impairment especially if they also have a collagen vascular disease. Available data from clinical trials of PRINIVIL are insufficient to show that PRINIVIL does not cause agranulocytosis at similar rates. Marketing experience has revealed rare cases of leukopenia/neutropenia and bone marrow depression in which a causal relationship to lisinopril cannot be excluded. Periodic monitoring of white blood cell counts in patients with collagen vascular disease and renal disease should be considered.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice or hepatitis and progresses to fulminant hepatic necrosis, and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
PRECAUTIONS
General
Aortic Stenosis/Hypertrophic Cardiomyopathy: As with all vasodilators, lisinopril should be given with caution to patients with obstruction in the outflow tract of the left ventricle. Impaired Renal Function: As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin converting enzyme inhibitors, including PRINIVIL, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death. In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur. Experience with another angiotensin converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of PRINIVIL and/or diuretic therapy. In such patients renal function should be monitored during the first few weeks of therapy. Some patients with hypertension or heart failure with no apparent pre-existing renal vascular disease have developed increases in blood urea nitrogen and serum creatinine, usually minor and transient, especially when PRINIVIL has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of the diuretic and/or PRINIVIL may be required. Patients with acute myocardial infarction in the GISSI - 3 study, treated with PRINIVIL, had a higher (2.4 percent versus 1.1 percent) incidence of renal dysfunction in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration). In acute myocardial infarction, treatment with PRINIVIL should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. If renal dysfunction develops during treatment with PRINIVIL (serum creatinine concentration exceeding 3 mg/dL or a doubling from the pre-treatment value) then the physician should consider withdrawal of PRINIVIL. Evaluation of patients with hypertension, heart failure, or myocardial infarction should always include assessment of renal function. (See DOSAGE AND ADMINISTRATION.)
Hyperkalemia: In clinical trials hyperkalemia(serum potassium greater than 5.7 mEq/L) occurred in approximately 2.2 percent of hypertensive patients and 4.8 percent of patients with heart failure. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was a cause of discontinuation of therapy in approximately 0.1 percent of hypertensive patients, 0.6 percent of patients with heart failure and 0.1 percent of patients with myocardial infarction. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes. Hyperkalemia can cause serious, sometimes fatal, arrhythmias. PRINIVIL should be used cautiously, if at all, with these agents and with frequent monitoring of serum potassium. (See Drug Interactions.)
Cough: Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough. Surgery/Anesthesia: In patients undergoing major surgery or during anesthesia with agents that produce hypotension, PRINIVIL may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion. |clinicalTrials=* Lisinopril have been found to be generally well tolerated in controlled clinical trials involving 1969 patients with hypertension or heart failure. For the most part, adverse experiences were mild and transient.
Hypertension
- In clinical trials in patients with hypertension treated with lisinopril, discontinuation of therapy due to clinical adverse experiences occurred in 5.7% of patients. The overall frequency of adverse experiences could not be related to total daily dosage within the recommended therapeutic dosage range.
- For adverse experiences occurring in greater than 1% of patients with hypertension treated with lisinopril or lisinopril plus hydrochlorothiazide in controlled clinical trials, and more frequently with lisinopril and/or lisinopril plus hydrochlorothiazide than placebo, comparative incidence data are listed in the table below.
Heart Failure
- In patients with heart failure treated with lisinopril for up to four years, discontinuation of therapy due to clinical adverse experiences occurred in 11.0% of patients. In controlled studies in patients with heart failure, therapy was discontinued in 8.1% of patients treated with lisinopril for 12 weeks, compared to 7.7% of patients treated with placebo for 12 weeks.
- The following table lists those adverse experiences which occurred in greater than 1% of patients with heart failure treated with lisinopril or placebo for up to 12 weeks in controlled clinical trials, and more frequently on lisinopril than placebo.
- Also observed at >1% with lisinopril but more frequent or as frequent on placebo than lisinopril in controlled trials were asthenia, angina pectoris, nausea, dyspnea, cough, and pruritus.
- Worsening of heart failure, anorexia, increased salivation, muscle cramps, back pain, myalgia, depression, chest sound abnormalities, and pulmonary edema were also seen in controlled clinical trials, but were more common on placebo than lisinopril.
Acute Myocardial Infarction
- In the GISSI-3 trial, in patients treated with lisinopril for six weeks following acute myocardial infarction, discontinuation of therapy occurred in 17.6% of patients.
- Patients treated with lisinopril had a significantly higher incidence of hypotension and renal dysfunction compared with patients not taking lisinopril.
- In the GISSI-3 trial, hypotension (9.7%), renal dysfunction (2.0%), cough (0.5%), post infarction angina (0.3%), skin rash and generalized edema (0.01%), and angioedema (0.01%) resulted in withdrawal of treatment. In elderly patients treated with lisinopril, discontinuation due to renal dysfunction was 4.2%.
- Other clinical adverse experiences occurring in 0.3% to 1.0% of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and rarer, serious, possibly drug-related events reported in uncontrolled studies or marketing experience are listed below, and within each category are in order of decreasing severity.
Body as a Whole
- Anaphylactoid reactions , syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise.
Cardiovascular
- Cardiac arrest; myocardial infarction or cerebrovascular accident possibly secondary to excessive hypotension in high risk patients; pulmonary embolism and infarction, arrhythmias (including ventricular tachycardia, atrial tachycardia, atrial fibrillation, bradycardia and premature ventricular contractions), palpitations, transient ischemic attacks, paroxysmal nocturnal dyspnea, orthostatic hypotension, decreased blood pressure, peripheral edema, vasculitis.
Digestive
- Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) , vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic
- Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia, and thrombocytopenia.
Endocrine
Metabolic
- Weight loss, dehydration, fluid overload, gout, weight gain.
Musculoskeletal
- Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric
- Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability and nervousness.
Respiratory System
- Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin
- Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, pemphigus, herpes zoster, erythema multiforme, urticaria, pruritus, alopecia, flushing, diaphoresis, photosensitivity.
Special Senses
- Blurred vision, taste alteration, anosmia, tinnitus, conjunctivitis, dry eyes, tearing.
Urogenital
- Renal failure, oliguria, renal dysfunction (see [[Lisinopril#[[Lisinopril#Warnings|Warnings and Warnings and Precautions]]|[[Lisinopril#Warnings|Warnings and Warnings and Precautions]] and Warnings and Precautions]] and Dosage and Administration, flank pain, gynecomastia, impotence.
Miscellaneous
- A symptom complex has been reported which may include some or all of the following: a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia/myositis, fever, serositis, vasculitis, leukocytosis, eosinophilia, photosensitivity, rash and other dermatologic manifestations.
Angioedema
- Angioedema has been reported in patients receiving enalaprilat, with an incidence higher in black than in non-black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with enalaprilat should be discontinued and appropriate therapy instituted immediately (see [[Lisinopril#[[Lisinopril#Warnings|Warnings and Warnings and Precautions]]|[[Lisinopril#Warnings|Warnings and Warnings and Precautions]] and Warnings and Precautions]]).
Hypotension
- In the hypertensive patients, hypotension occurred in 0.9 percent and syncope occurred in 0.5 percent of patients following the initial dose or during extended therapy. Hypotension or syncope was a cause for discontinuation of therapy in 0.1 percent of hypertensive patients. In heart failure patients, hypotension occurred in 6.7 percent and syncope occurred in 2.2 percent of patients. Hypotension or syncope was a cause for discontinuation of therapy in 1.9 percent of patients with heart failure (see [[Lisinopril#Warnings|Warnings and Warnings and Precautions]]).
Fetal/Neonatal Morbidity and Mortality
- See [[Lisinopril#Warnings|Warnings and Warnings and Precautions]], Fetal/Neonatal Morbidity and Mortality.
Cough
Pediatric Patients
- The adverse experience profile for pediatric patients appears to be similar to that seen in adult patients.
Clinical Laboratory Test Findings
Serum Electrolytes
Creatinine, Blood Urea Nitrogen
- In controlled clinical trials minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 0.2 percent of patients with essential hypertension treated with enalaprilat alone. Increases are more likely to occur in patients receiving concomitant diuretics or in patients with renal artery stenosis (see Warnings and Precautions). In patients with heart failure who were also receiving diuretics with or without digitalis, increases in blood urea nitrogen or serum creatinine, usually reversible upon discontinuation of enalaprilat and/or other concomitant diuretic therapy, were observed in about 11 percent of patients. Increases in blood urea nitrogen or creatinine were a cause for discontinuation in 1.2 percent of patients.
Hematology
- Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.3 g percent and 1.0 vol percent, respectively) occur frequently in either hypertension or congestive heart failure patients treated with enalaprilat but are rarely of clinical importance unless another cause of anemia coexists. In clinical trials, less than 0.1 percent of patients discontinued therapy due to anemia. Hemolytic anemia, including cases of hemolysis in patients with G-6-PD deficiency, has been reported; a causal relationship to enalapril cannot be excluded.
Liver Function Tests
- Elevations of liver enzymes and/or serum bilirubin have occurred (see [[Lisinopril#Warnings|Warnings and Warnings and Precautions]], Hepatic Failure).
|postmarketing=Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
|drugInteractions=====Hypotension - Patient on Diuretic Therapy====
- Patients on diuretics and especially those in whom diuretic therapywas recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose until blood pressure has stabilized. When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed. Studies with ACE inhibitors in combination with diuretics indicate that the dose of the ACE inhibitor can be reduced when it is given with a diuretic.
Indomethacin
- In a study in 36 patients with mild to moderate hypertension where the antihypertensive effects of lisinopril alone were compared to lisinopril given concomitantly with indomethacin, the use of indomethacin was associated with a reduced effect, although the difference between the two regimens was not significant.
Other Agents
- Lisinopril have been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. This included post myocardial infarction patients who were receiving intravenous or transdermal nitroglycerin. No clinically important pharmacokinetic interactions occurred when lisinopril were used concomitantly with propranolol or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium
- Lisinopril attenuate potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium. Potassium sparing agents should generally not be used in patients with heart failure who are receiving lisinopril.
Lithium
- Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril are administered concomitantly with lithium.
|FDAPregCat=C |useInPregnancyFDA=Pregnancy Categories C (first trimester) and D (second and third trimesters). |useInPed=* The usual recommended starting dose is 0.07 mg/kg once daily (up to 5 mg total). Dosage should be adjusted according to blood pressure response. Doses above 0.61 mg/kg (or in excess of 40 mg) have not been studied in pediatric patients.
- Lisinopril is not recommended in pediatric patients < 6 years or in pediatric patients with glomerular filtration rate < 30 mL/ min/1.73m2.
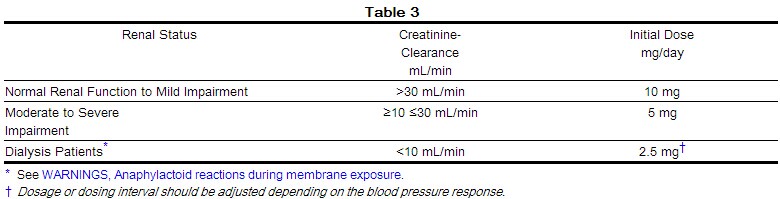
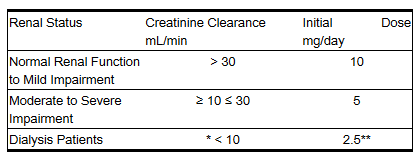
|useInGeri=* In general, blood pressure response and adverse experiences were similar in younger and older patients given similar doses of lisinopril tablets. Pharmacokinetic studies, however, indicate that maximum blood levels and area under the plasma concentration time curve (AUC) are doubled in older patients, so that dosage adjustments should be made with particular caution. |useInRenalImpair=====Dosage Adjustment in Renal Impairment====
- The usual dose of lisinopril tablets (10 mg) is recommended for patients with creatinine clearance >30 mL/min (serum creatinine of up to approximately 3 mg/dL). For patients with creatinine clearance ≥10 mL/min ≤30 mL/min (serum creatinine ≥3 mg/dL), the first dose is 5 mg once daily. For patients with creatinine clearance <10 mL/min (usually on hemodialysis) the recommended initial dose is 2.5 mg. The dosage may be titrated upward until blood pressure is controlled or to a maximum of 40 mg daily.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
- In patients with heart failure who have hyponatremia (serum sodium <130 mEq/L) or moderate to severe renal impairment (creatinine clearance ≤30 mL/min or serum creatinine >3 mg/dL), therapy with lisinopril tablets should be initiated at a dose of 2.5 mg once a day under close medical supervision.
Dosage Adjustment in Patients With Myocardial Infarction with Renal Impairment
- In acute myocardial infarction, treatment with lisinopril tablets should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosing adjustments in myocardial infarction patients with severe renal impairment has been performed.
|othersTitle=Usage in other medical conditions |useInOthers=====Heart Failure====
- Lisinopril tablets are indicated as adjunctive therapy with diuretics and (usually) digitalis. The recommended starting dose is 5 mg once a day. When initiating treatment with lisinopril in patients with heart failure, the initial dose should be administered under medical observation, especially in those patients with low blood pressure (systolic blood pressure below 100 mmHg). The mean peak blood pressure lowering occurs six to eight hours after dosing. Observation should continue until blood pressure is stable. The concomitant diuretic dose should be reduced, if possible, to help minimize hypovolemia which may contribute to hypotension. The appearance of hypotension after the initial dose of lisinopril tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension. The usual effective dosage range is 5 to 20 mg per day administered as a single daily dose.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
- In patients with heart failure who have hyponatremia (serum sodium <130 mEq/L) or moderate to severe renal impairment (creatinine clearance ≤30 mL/min or serum creatinine >3 mg/dL), therapy with lisinopril tablets should be initiated at a dose of 2.5 mg once a day under close medical supervision.
Acute Myocardial Infarction
- In hemodynamically stable patients within 24 hours of the onset of symptoms of acute myocardial infarction, the first dose of lisinopril tablets is 5 mg given orally, followed by 5 mg after 24 hours, 10 mg after 48 hours and then 10 mg of lisinopril tablets once daily. Dosing should continue for six weeks. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin, and beta-blockers. Patients with a low systolic blood pressure (≤120 mmHg) when treatment is started or during the first 3 days after the infarct should be given a lower 2.5 mg oral dose of lisinopril tablets. If hypotension occurs (systolic blood pressure ≤100 mmHg) a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure ≤90 mmHg for more than 1 hour) lisinopril tablets should be withdrawn. For patients who develop symptoms of heart failure.
Dosage Adjustment in Patients With Myocardial Infarction with Renal Impairment
- In acute myocardial infarction, treatment with lisinopril tablets should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosing adjustments in myocardial infarction patients with severe renal impairment has been performed.
|administration=Oral |monitoring=FDA Package Insert for Lisinopril contains no information regarding Drug Monitoring. |IVCompat=FDA Package Insert for Lisinopril contains no information regarding IV compatibility. |overdose=Following a single oral dose of 20 g/kg, no lethality occurred in rats and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution. Lisinopril can be removed by hemodialysis. (See Warnings and Precautions, Anaphylactoid reactions during membrane exposure.) |drugBox={{Infobox drug2 | Verifiedfields = changed | Watchedfields = changed | caption = Chemical structure of lisinopril | verifiedrevid = 432833853 | IUPAC_name = N2-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline | image = Lisinopril Structural Formulae V.2.svg
| tradename = Prinivil, Tensopril, Zestril, Hipril | ASHP = a692051 | Drugs.com = Monograph | eMedicine = lisinopril | MedlinePlus = a692051 | pregnancy_category = C (1st trimester) / D (2nd and 3rd trimester)[10] | legal_status = Rx-only | routes_of_administration = Oral
| bioavailability = approx. 25%, but wide range between individuals (6 to 60%) | protein_bound = 0 | metabolism = None | elimination_half-life = 12 hours | excretion = Eliminated unchanged in urine
| CAS_number = 83915-83-7
| ChemSpiderID_Ref =
| ChemSpiderID = 4514933
| ATC_prefix = C09
| ATC_suffix = AA03
| ATC_supplemental =
| ChEBI_Ref =
| ChEBI = 43755
| PubChem = 5362119
| DrugBank_Ref =
| DrugBank = APRD00560
| KEGG_Ref =
| KEGG = D00362
| ChEMBL_Ref =
| ChEMBL = 1237
|synonyms = (2S)-1-[(2S)-6-amino-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic acid
| UNII_Ref =
| UNII = 7Q3P4BS2FD
| PDB_ligand = LPR
| C=21 | H=31 | N=3 | O=5
| molecular_weight = 405.488 g/mol
| smiles = O=C(O)[C@H]2N(C(=O)[C@@H](N[C@H](C(=O)O)CCc1ccccc1)CCCCN)CCC2
| InChIKey = RLAWWYSOJDYHDC-BZSNNMDCBR
| InChI = 1/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1
| StdInChI_Ref =
| StdInChI = 1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1
| StdInChIKey_Ref =
| StdInChIKey = RLAWWYSOJDYHDC-BZSNNMDCSA-N
}}
|mechAction=* Lisinopril inhibits angiotensin converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex.
- The beneficial effects of isinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L. In the same study, patients treated with lisinopril and hydrochlorothiazide for up to 24 weeks had a mean decrease in serum potassium of 0.1 mEq/L; approximately 4% of patients had increases greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity. ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of lisinopril remains to be elucidated. While the mechanism through which lisinopril lower blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, lisinopril are antihypertensive even in patients with low-renin hypertension.
- Although lisinopril were antihypertensive in all races studied, black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to monotherapy than nonblack patients.
- Concomitant administration of lisinopril and hydrochlorothiazide further reduced blood pressure in black and non-black patients and any racial differences in blood pressure response were no longer evident.
|structure=PRINIVIL® (Lisinopril), a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5•2H2O and its structural formula is:
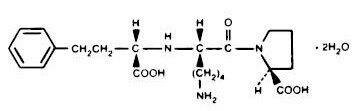
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.52. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
PRINIVIL is supplied as 5 mg, 10 mg, and 20 mg tablets for oral administration. In addition to the active ingredient lisinopril, each tablet contains the following inactive ingredients: calcium phosphate, mannitol, magnesium stearate, and starch. The 10 mg and 20 mg tablets also contain iron oxide.
|PD=* Administration of lisinopril to patients with hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is usually not observed although it can occur and should be anticipated in volume and/or salt-depleted patients. When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
- In most patients studied, onset of antihypertensive activity was seen at one hour after oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by 6 hours. Although an antihypertensive effect was observed 24 hours after dosing with recommended single daily doses, the effect was more consistent and the mean effect was considerably larger in some studies with doses of 20 mg or more than with lower doses. However, at all doses studied, the mean antihypertensive effect was substantially smaller 24 hours after dosing than it was 6 hours after dosing.
- In some patients achievement of optimal blood pressure reduction may require two to four weeks of therapy. The antihypertensive effects of lisinopril are maintained during long term therapy. Abrupt withdrawal of lisinopril has not been associated with a rapid increase in blood pressure, or a significant increase in blood pressure compared to pretreatment levels.
- Two dose-response studies utilizing a once daily regimen were conducted in 438 mild to moderate hypertensive patients not on a diuretic. Blood pressure was measured 24 hours after dosing. An antihypertensive effect of lisinopril was seen with 5 mg in some patients. However, in both studies blood pressure reduction occurred sooner and was greater in patients treated with 10, 20 or 80 mg of lisinopril. In controlled clinical studies, lisinopril 20-80 mg have been compared in patients with mild to moderate hypertension to hydrochlorothiazide 12.5-50 mg and with atenolol 50-200 mg; and in patients with moderate to severe hypertension to metoprolol 100-200 mg. It was superior to hydrochlorothiazide in effects on systolic and diastolic pressure in a population that was 3/4 caucasian. Lisinopril were approximately equivalent to atenolol and metoprolol in effects on diastolic blood pressure, and had somewhat greater effects on systolic blood pressure.
- Lisinopril had similar effectiveness and adverse effects in younger and older (>65 years) patients. They were less effective in blacks than in caucasians.
- In hemodynamic studies in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with little or no change in cardiac output and in heart rate. In a study in nine hypertensive patients, following administration of lisinopril, there was an increase in mean renal blood flow that was not significant. Data from several small studies are inconsistent with respect to the effect of lisinopril on glomerular filtration rate in hypertensive patients with normal renal function, but suggest that changes, if any, are not large.
- In patients with renovascular hypertension lisinopril have been shown to be well tolerated and effective in controlling blood pressure.
|PK=* Following oral administration of lisinopril, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25%, with large intersubject variability (6%-60%) at all doses tested (5-80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. \
- The absolute bioavailability of lisinopril is reduced to 16% in patients with stable NYHA Class II-IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers. Upon multiple dosing, lisinopril exhibits an effective half-life of accumulation of 12 hours.
- Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below 30 mL/min. Above this glomerular filtration rate, the elimination half-life is little changed. With greater impairment, however, peak and trough lisinopril levels increase, time to peak concentration increases and time to attain steady state is prolonged. Older patients, on average, have (approximately doubled) higher blood levels and the area under the plasma concentration time curve (AUC) than younger patients. Lisinopril can be removed by hemodialysis.
- Studies in rats indicate that lisinopril crosses the blood-brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
|nonClinToxic====Carcinogenesis, Mutagenesis, Impairment of Fertility===
- There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg/kg/day (about 56 or 9 times* the maximum recommended daily human dose,
based on body weight and body surface area, respectively).
- There was no evidence of carcinogenicity when lisinopril was administered for 92 weeks to (male and female) mice at doses up to 135 mg/kg/day (about 84 times* the maximum recommended daily human dose). This dose was 6.8 times the maximum human dose based on body surface area in mice. *Calculations assume a human weight of 50 kg and human body surface area of 1.62 m2. Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
|clinicalStudies====Pediatric Patients===
In a clinical study involving 115 hypertensive pediatric patients 6 to 16 years of age, patients who weighed <50 kg received either 0.625, 2.5, or 20 mg of lisinopril daily and patients who weighed ≥50 kg received either 1.25, 5, or 40 mg of lisinopril daily. At the end of 2 weeks, lisinopril administered once daily lowered trough blood pressure in a dose-dependent manner with consistent antihypertensive efficacy demonstrated at doses >1.25 mg (0.02 mg/kg). This effect was confirmed in a withdrawal phase, where the diastolic pressure rose by about 9 mmHg more in patients randomized to placebo than it did in patients who were randomized to remain on the middle and high doses of lisinopril. The dose-dependent antihypertensive effect of lisinopril was consistent across several demographic subgroups: age, Tanner stage, gender, race. In this study, lisinopril was generally well-tolerated. In the above pediatric studies, lisinopril was given either as tablets or in a suspension for those children and infants who were unable to swallow tablets or who required a lower dose than is available in tablet form (see DOSAGE AND ADMINISTRATION, Preparation of Suspension).
During baseline-controlled clinical trials, in patients receiving digitalis and diuretics, single doses of PRINIVIL resulted in decreases in pulmonary capillary wedge pressure, systemic vascular resistance and blood pressure accompanied by an increase in cardiac output and no change in heart rate. In two placebo-controlled, 12-week clinical studies using doses of PRINIVIL up to 20 mg, PRINIVIL as adjunctive therapy to digitalis and diuretics improved the following signs and symptoms due to congestive heart failure: edema, rales, paroxysmal nocturnal dyspnea and jugular venous distention. In one of the studies beneficial response was also noted for: orthopnea, presence of third heart sound and the number of patients classified as NYHA Class III and IV. Exercise tolerance was also improved in this study. The effect of lisinopril on mortality in patients with heart failure has not been evaluated. The once daily dosing for the treatment of congestive heart failure was the only dosage regimen used during clinical trial development and was determined by the measurement of hemodynamic responses.
Acute Myocardial Infarction
The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI - 3) study was a multicenter, controlled, randomized, unblinded clinical trial conducted in 19,394 patients with acute myocardial infarction admitted to a coronary care unit. It was designed to examine the effects of short-term (6 week) treatment with lisinopril, nitrates, their combination, or no therapy on short-term (6 week) mortality and on long-term death and markedly impaired cardiac function. Patients presenting within 24 hours of the onset of symptoms who were hemodynamically stable were randomized, in a 2 x 2 factorial design, to six weeks of either PRINIVIL alone (n=4841), nitrates alone (n=4869), PRINIVIL plus nitrates (n=4841), or open control (n=4843). All patients received routine therapies, including thrombolytics (72%), aspirin (84%), and a beta-blocker (31%), as appropriate, normally utilized in acute myocardial infarction (MI) patients.
The protocol excluded patients with hypotension (systolic blood pressure ≤100 mmHg), severe heart failure, cardiogenic shock and renal dysfunction (serum creatinine >2 mg/dL and/or proteinuria >500 mg/24 h). Doses of PRINIVIL were adjusted as necessary according to protocol. (See DOSAGE AND ADMINISTRATION.)
Study treatment was withdrawn at six weeks except where clinical conditions indicated continuation of treatment. The primary outcomes of the trial were the overall mortality at six weeks and a combined endpoint at six months after the myocardial infarction, consisting of the number of patients who died, had late (day 4) clinical congestive heart failure, or had extensive left ventricular damage defined as ejection fraction ≤35%, or an akinetic-dyskinetic [A-D] score ≥45%. Patients receiving PRINIVIL (n=9646) alone or with nitrates, had an 11 percent lower risk of death (2p [two-tailed]=0.04) compared to patients receiving no PRINIVIL (n=9672) (6.4 percent versus 7.2 percent, respectively) at six weeks. Although patients randomized to receive PRINIVIL for up to six weeks also fared numerically better on the combined end-point at 6 months, the open nature of the assessment of heart failure, substantial loss to follow-up echocardiography, and substantial excess use of lisinopril between 6 weeks and 6 months in the group randomized to 6 weeks of lisinopril, preclude any conclusion about this endpoint.
Patients with acute myocardial infarction, treated with PRINIVIL had a higher (9.0 percent versus 3.7 percent, respectively) incidence of persistent hypotension (systolic blood pressure <90 mmHg for more than 1 hour) and renal dysfunction (2.4 percent versus 1.1 percent) in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration). (See ADVERSE REACTIONS, ACUTE myocardial infarction.) |howSupplied=====2.5 mg Tablets==== 2.5 mg Tablets (NDC 0310-0135) white, round, biconvex, uncoated tablets identified as “ZESTRIL 2 1/2” on one side and “135” on the other side are supplied in bottles of 100 tablets.
5 mg Tablets
5 mg Tablets (NDC 0310-0130) pink, capsule-shaped, biconvex, bisected, uncoated tablets, identified “ZESTRIL” on one side and “130” on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
10 mg Tablets
10 mg Tablets (NDC 0310-0131) pink, round, biconvex, uncoated tablets identified “ZESTRIL 10” debossed on one side, and “131” debossed on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
20 mg Tablets
20 mg Tablets (NDC 0310-0132) red, round, biconvex, uncoated tablets identified “ZESTRIL 20” debossed on one side, and “132” debossed on the other side are supplied in bottles of 100 tablets and unit dose packages of 100 tablets.
30 mg Tablets
30 mg Tablets (NDC 0310-0133) red, round, biconvex, uncoated tablets identified “ZESTRIL 30” debossed on one side, and “133” debossed on the other side are supplied in bottles of 100 tablets.
40 mg Tablets
40 mg Tablets (NDC 0310-0134) yellow, round, biconvex, uncoated tablets identified “ZESTRIL 40” debossed on one side, and “134” debossed on the other side are supplied in bottles of 100 tablets. |storage=Store at controlled room temperature, 15-30°C (59-86°F), and protect from moisture. Dispense in a tight container, if product package is subdivided. |fdaPatientInfo=* Angioedema: Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin converting enzyme inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
- Symptomatic Hypotension: Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual syncope occurs, the patients should be told to discontinue the drug until they have consulted with the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with their physician.
- Hyperkalemia: Patients should be told not to use salt substitutes containing potassium without consulting their physician.
- Hypoglycemia: Diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor should be told to closely monitor for hypoglycemia, especially during the first month of combined use. (See Drug Interactions.)
- Leukopenia/Neutropenia: Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of leukopenia/neutropenia.
- Pregnancy: Female patients of childbearing age should be told about the consequences of exposure to PRINIVIL during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
|alcohol=Alcohol-Lisinopril tablet interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |lookAlike=lisinopril - Lipitor Zestril - Zegerid Zestril - Zetia Zestril - ZyPREXA[11] |[[Lisinopril#Warnings|Warnings and Warnings and Precautions]]=====Anaphylactoid and Possibly Related Reactions====
- Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including Lisinopril) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema
- Angioedema of the face, extremities, lips, tongue, glottis and/or larynx has been reported in patients treated with angiotensin converting enzyme inhibitors, including Lisinopril. This may occur at any time during treatment. In such cases Lisinopril should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. In instances where swelling has been confined to the face and lips the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms. Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided.
Intestinal Angioedema
- Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid reactions during desensitization
- Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure
- Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension
- Excessive hypotension is rare in uncomplicated hypertensive patients treated with Lisinopril alone. Patients with heart failure given Lisinopril commonly have some reduction in blood pressure, especially with the first dose, but discontinuation of therapy for continuing symptomatic hypotension usually is not necessary when dosing instructions are followed; caution should be observed when initiating therapy. Patients at risk for excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include those with the following conditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or increase salt intake cautiously before initiating therapy with Lisinopril in patients at risk for excessive hypotension who are able to tolerate such adjustments. In patients at risk for excessive hypotension, therapy should be started under very close medical supervision and such patients should be followed closely for the first two weeks of treatment and whenever the dose of enalapril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease, in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
- If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses of Lisinopril, which usually can be given without difficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dose reduction or discontinuation of Lisinopril or concomitant diuretic may be necessary.
Neutropenia/Agranulocytosis
- Another angiotensin converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients but more frequently in patients with renal impairment especially if they also have a collagen vascular disease. Available data from clinical trials of enalapril are insufficient to show that enalapril does not cause agranulocytosis at similar rates. Marketing experience has revealed cases of neutropenia or agranulocytosis in which a causal relationship to enalapril cannot be excluded. Periodic monitoring of white blood cell counts in patients with collagen vascular disease and renal disease should be considered.
Hepatic Failure
- Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis, and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Fetal/Neonatal Morbidity and Mortality
- ACE inhibitors can cause fetal and neonatal morbidity and death when administered to pregnant women. Several dozen cases have been reported in the world literature. When pregnancy is detected, ACE inhibitors should be discontinued as soon as possible.
- In a published restrospective epidemiological study, infants whose mothers had taken an ACE inhibitor during their first trimester of pregnancy appeared to have an increased risk of major congenital malformations compared with infants whose mothers had not undergone first trimester exposure to ACE inhibitor drugs. The number of cases of birth defects is small and the findings of this study have not yet been repeated.
- The use of ACE inhibitors during the second and third trimesters of pregnancy has been associated with fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death. Oligohydramnios has also been reported, presumably resulting from decreased fetal renal function; oligohydramnios in this setting has been associated with fetal limb contractures, craniofacial deformation, and hypoplastic lung development. Prematurity, intrauterine growth retardation, and patent ductus arteriosus have also been reported, although it is not clear whether these occurrences were due to the ACE-inhibitor exposure.
- These adverse effects do not appear to have resulted from intrauterine ACE-inhibitor exposure that has been limited to the first trimester. Mothers whose embryos and fetuses are exposed to ACE inhibitors only during the first trimester should be so informed. Nonetheless, when patients become pregnant, physicians should make every effort to discontinue the use of Lisinopril as soon as possible.
- Rarely (probably less often than once in every thousand pregnancies), no alternative to ACE inhibitors will be found. In these rare cases, the mothers should be apprised of the potential hazards to their fetuses, and serial ultrasound examinations should be performed to assess the intraamniotic environment.
- If oligohydramnios is observed, Lisinopril should be discontinued unless it is considered lifesaving for the mother. Contraction stress testing (CST), a non-stress test (NST), or biophysical profiling (BPP) may be appropriate, depending upon the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
- Infants with histories of in utero exposure to ACE inhibitors should be closely observed for hypotension, oliguria, and hyperkalemia. If oliguria occurs, attention should be directed toward support of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required as means of reversing hypotension and/or substituting for disordered renal function. Enalapril, which crosses the placenta, has been removed from neonatal circulation by peritoneal dialysis with some clinical benefit, and theoretically may be removed by exchange transfusion, although there is no experience with the latter procedure.
- No teratogenic effects of enalapril were seen in studies of pregnant rats and rabbits. On a body surface area basis, the doses used were 57 times and 12 times, respectively, the maximum recommended human daily dose (MRHDD).
Aortic Stenosis/Hypertrophic Cardiomyopathy
As with all vasodilators, lisinopril should be given with caution to patients with obstruction in the outflow tract of the left ventricle.
Impaired Renal Function
- As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be
anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin converting enzyme inhibitors, including Lisinopril, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death.
- In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur.
Experience with another angiotensin-converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of Lisinopril and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy. Some patients with hypertension or heart failure with no apparent pre-existing renal vascular disease have developed increases in blood urea nitrogen and serum creatinine, usually minor and transient, especially when Lisinoprilhas been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of the diuretic and/or Lisinopril may be required.
- Patients with acute myocardial infarction in the GISSI-3 trial treated with Lisinoprilhad a higher (2.4% versus 1.1%) incidence of renal
dysfunction in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration). In acute myocardial infarction, treatment with Lisinopril should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. If renal dysfunction develops during treatment with Lisinopril (serum creatinine concentration exceeding 3 mg/dL or a doubling from the pre-treatment value) then the physician should consider withdrawal of Lisinopril.
- Evaluation of patients with hypertension, heart failure, or myocardial infarction should always include assessment of renal function.
Hyperkalemia
- In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in approximately 2.2% of hypertensive
patients and 4.8% of patients with heart failure. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was a cause of discontinuation of therapy in approximately 0.1% of hypertensive patients, 0.6% of patients with heart failure and 0.1% of patients with myocardial infarction. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes, which should be used cautiously, if at all, with lisinopril.
Cough
- Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been
reported with all ACE inhibitors, almost always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/Anesthesia
- In patients undergoing major surgery or during anesthesia with agents that produce hypotension, lisinopril may block
angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Angioedema
- Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin-converting enzyme
inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Symptomatic Hypotension
- Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual
syncope occurs, the patient should be told to discontinue the drug until they have consulted with the prescribing physician.
- All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of
reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with their physician.
Hyperkalemia
- Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Leukopenia/Neutropenia
- Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a
sign of leukopenia/neutropenia.
Pregnancy
- Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to ACE
inhibitors, and they should also be told that these consequences do not appear to have resulted from intrauterine ACE inhibitor exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible. }} {{#subobject:
|Page Name=Lisinopril |Pill Name=No_image.jpg |Drug Name=Lisinopril |Pill Ingred=starch, corn / calcium phosphate, dibasic, dihydrate / magnesium stearate / mannitol / talc|+sep=; |Pill Imprint=4209;V |Pill Dosage=2.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=7.00 |Pill Scoring=1 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=0603-4209-21
}}
{{#subobject:
|Page Name=Lisinopril
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_03.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_04.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_02.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_panel_01.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_03.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_02.jpg
}}
{{#subobject:
|Label Page=Lisinopril |Label Name=Lisinopril_label_01.jpg
}}
- ↑ Schjoedt KJ, Astrup AS, Persson F, Frandsen E, Boomsma F, Rossing K et al. (2009) Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy: a randomised crossover trial. Diabetologia 52 (1):46-9. DOI:10.1007/s00125-008-1184-8 PMID: 18974967
- ↑ Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A et al. (1998) Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 351 (9095):28-31. PMID: 9433426
- ↑ Midtvedt K, Stokke ES, Hartmann A (1996) Successful long-term treatment of post-transplant erythrocytosis with losartan. Nephrol Dial Transplant 11 (12):2495-7. PMID: 9017632
- ↑ Pisani F, Tisone G, Alciati E, Vennarecci G, Pieragostini E, Casciani CU (1994) Role of ACE inhibitors in the treatment of erythrocytosis in patients with renal allograft. Transplant Proc 26 (5):2602-3. PMID: 7940809
- ↑ Brozena SC, Johnson MR, Ventura H, Hobbs R, Miller L, Olivari MT et al. (1996) Effectiveness and safety of diltiazem or lisinopril in treatment of hypertension after heart transplantation. Results of a prospective, randomized multicenter trail. J Am Coll Cardiol 27 (7):1707-12. PMID: 8636558
- ↑ Shiigai T, Shichiri M (2001) Late escape from the antiproteinuric effect of ace inhibitors in nondiabetic renal disease. Am J Kidney Dis 37 (3):477-83. PMID: 11228170
- ↑ Kincaid-Smith P, Fairley K, Packham D (2002) Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant 17 (4):597-601. PMID: 11917051
- ↑ Schrader H, Stovner LJ, Helde G, Sand T, Bovim G (2001) Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 322 (7277):19-22. PMID: 11141144
- ↑ Bender WI (1995) ACE inhibitors for prophylaxis of migraine headaches. Headache 35 (8):470-1. PMID: 7591740
- ↑ "Lisinopril". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ↑ "https://www.ismp.org". External link in
|title=(help)