Sandbox m ex
| Cervical intraepithelial neoplasia | |
| ICD-10 | D06, N87 |
|---|---|
| ICD-9 | 233.1, 622.10 |
| MedlinePlus | 001491 |
| MeSH | D018290 |
Pap Smear
Cervical dysplasia that is seen on a Pap smear is called squamous intraepithelial lesion (SIL). These changes may be graded as:
- Low-grade (LSIL)
- High-grade (HSIL)
- Possibly cancerous (malignant)
If a Pap smear shows abnormal cells or cervical dysplasia, further testing or monitoring will be recommended:
- Follow-up Pap smears may be recommended for mild cases
- Colposcopy-directed biopsy can confirm the condition
- Cone biopsy may be done after colposcopy
Dysplasia that is seen on a biopsy of the cervix is called cervical intraepithelial neoplasia (CIN). It is grouped into three categories:
- CIN I -- mild dysplasia
- CIN II -- moderate to marked dysplasia
- CIN III -- severe dysplasia to carcinoma in situ
Some strains of human papillomavirus (HPV) are known to cause cervical cancer. An HPV DNA test can identify the high-risk types of HPV linked to such cancer. This may be done:
- As a screening test for women over age 30
- For women of any age who have a slightly abnormal Pap test result
HPV subtypes 16 and 18 deregulate the genes E6 and E7 which code for proteins that inhibit p53 and Retinoblastoma protein (Rb), which are two important tumor suppressor genes in humans. The p53 gene product is involved in regulation of apoptosis (cell suicide), and Rb is responsible for halting the cell cycle at the G1-phase. When Rb function is impaired, the cell is allowed to progress to S-phase and complete mitosis, resulting in proliferation and hence neoplastic transformation.
Hysterectomy
Microinvasive cancer (stage IA) is usually treated by hysterectomy (removal of the whole uterus including part of the vagina). For stage IA2, the lymph nodes are removed as well. An alternative for patients who desire to remain fertile is a local surgical procedure such as a loop electrical excision procedure (LEEP) or cone biopsy.
Trachelectomy
- If a cone biopsy does not produce clear margins,[1] one more possible treatment option for patients who want to preserve their fertility is a trachelectomy.[2]
- This attempts to surgically remove the cancer while preserving the ovaries and uterus, providing for a more conservative operation than a hysterectomy.
- It is a viable option for those in stage I cervical cancer which has not spread; however, it is not yet considered a standard of care,[3] as few doctors are skilled in this procedure.
- Even the most experienced surgeon cannot promise that a trachelectomy can be performed until after surgical microscopic examination, as the extent of the spread of cancer is unknown.
- If the surgeon is not able to microscopically confirm clear margins of cervical tissue once the patient is under general anesthesia in the operating room, a hysterectomy may still be needed. This can only be done during the same operation if the patient has given prior consent.
- Due to the possible risk of cancer spread to the lymph nodes in stage 1b cancers and some stage 1a cancers, the surgeon may also need to remove some lymph nodes from around the womb for pathologic evaluation.
Radical Trachelectomy
A radical trachelectomy can be performed abdominally[4] or vaginally[5] and there are conflicting opinions as to which is better.[6] A radical abdominal trachelectomy with lymphadenectomy usually only requires a two to three day hospital stay, and most women recover very quickly (approximately six weeks). Complications are uncommon, although women who are able to conceive after surgery are susceptible to preterm labor and possible late miscarriage.[7] It is generally recommended to wait at least one year before attempting to become pregnant after surgery.[8] Recurrence in the residual cervix is very rare if the cancer has been cleared with the trachelectomy.[9] Yet, it is recommended for patients to practice vigilant prevention and follow up care including pap screenings/colposcopy, with biopsies of the remaining lower uterine segment as needed (every 3-4 months for at least 5 years) to monitor for any recurrence in addition to minimizing any new exposures to HPV through safe sex practices until one is actively trying to conceive.
Radical Hysterectomy
Early stages (IB1 and IIA less than 4 cm) can be treated with radical hysterectomy with removal of the lymph nodes or radiation therapy. Radiation therapy is given as external beam radiotherapy to the pelvis and brachytherapy (internal radiation). Patients treated with surgery who have high risk features found on pathologic examination are given radiation therapy with or without chemotherapy in order to reduce the risk of relapse.
- Precancerous changes of the cervix and early stage cervical cancer,physical examination findings can be normal. Special tests and tools are needed to spot such conditions:
- A Pap smear is used for screening precancers and cervical cancer, but final diagnosis cannot be made.
- The human papillomavirus (HPV) DNA test may be done along with a pap test. Or it may be used after a woman has had an abnormal Pap test result. It may also be used as a main test.
- If abnormal changes are found, the cervix is usually examined using colposcope.cervical biopsy can be taken form tissue and examined.
Radiation and Chemotherapy
Larger early stage tumors (IB2 and IIA more than 4 cm) may be treated with radiation therapy and cisplatin-based chemotherapy, hysterectomy (which then usually requires adjuvant radiation therapy), or cisplatin chemotherapy followed by hysterectomy.
Advanced stage tumors (IIB-IVA) are treated with radiation therapy and cisplatin-based chemotherapy.
On June 15, 2006, the US Food and Drug Administration approved the use of a combination of two chemotherapy drugs, hycamtin and cisplatin for women with late-stage (IVB) cervical cancer treatment.[10] Combination treatment has significant risk of neutropenia, anemia, and thrombocytopenia side effects. Hycamtin is manufactured by GlaxoSmithKline.
Contraindicated medications
Invasive cervical carcinoma is considered a relative contraindication to the use of the following medications:
According to the International Federation of Gynecology and Obstetrics, survival improves when radiotherapy is combined with cisplatin-based chemotherapy.[11]
As the cancer metastasizes to other parts of the body, prognosis drops dramatically because treatment of local lesions is generally more effective than whole body treatments such as chemotherapy. Regular screening has meant that pre cancerous changes and early stage cervical cancers have been detected and treated early. Figures suggest that cervical screening is saving 5,000 lives each year in the UK by preventing cervical cancer.[12] Interval evaluation of the patient after therapy is imperative. Recurrent cervical cancer detected at its earliest stages might be successfully treated with surgery, radiation, chemotherapy, or a combination of the three. Thirty-five percent of patients with invasive cervical cancer have persistent or recurrent disease after treatment.[13]In screening a general or low-risk population, most Pap results are normal.
In the United States, about 2–3 million abnormal Pap smear results are found each year.[14] Most abnormal results are mildly abnormal (ASC-US (typically 2–5% of Pap results) or low-grade squamous intraepithelial lesion (LSIL) (about 2% of results)), indicating HPV infection.[citation needed] Although most low-grade cervical dysplasias spontaneously regress without ever leading to cervical cancer, dysplasia can serve as an indication that increased vigilance is needed.
In a typical scenario, about 0.5% of Pap results are high-grade SIL (HSIL), and less than 0.5% of results indicate cancer; 0.2 to 0.8% of results indicate Atypical Glandular Cells of Undetermined Significance (AGC-NOS).
As liquid based preparations (LBPs) become a common medium for testing, atypical result rates have increased. The median rate for all preparations with low-grade squamous intraepithelial lesions using LBPs was 2.9% compared with a 2003 median rate of 2.1%. Rates for high-grade squamous intraepithelial lesions (median, 0.5%) and atypical squamous cells have changed little.[15]
It's important for you to take care of yourself by eating well and staying as active as you can during the sometimes rough treatment of cervical cancer. You need the right amount of calories to maintain a good weight. You also need enough protein to keep up your strength. Eating well may help you feel better and have more energy. However, you may not feel like eating during or soon after treatment. You may be uncomfortable or tired. You may find that foods don't taste as good as they used to. In addition, the side effects of treatment (such as poor appetite, nausea, vomiting, or mouth sores) can make it hard to eat well. Your doctor, a registered dietitian, or another health care provider can suggest ways to cope with these problems. Research shows that people with cancer feel better when they stay active. Walking, yoga, swimming, and other activities can keep you strong and increase your energy. Exercise may reduce nausea and pain and make treatment easier to handle. It also can help relieve stress. Whatever physical activity you choose, be sure to talk to your doctor before you start.
You'll need regular checkups after treatment for cervical cancer. Checkups help ensure that any changes in your health are noted and treated if needed. If you have any health problems between checkups, you should contact your doctor. Your doctor will check for the return of cancer. Even when the cancer seems to have been completely removed or destroyed, the disease sometimes returns because undetected cancer cells remained somewhere in the body after treatment. Checkups may include a physical exam, Pap tests, and chest x-rays.
It is often located in the chest area. Most malignant teratomas can spread throughout the body, and have spread by the time of diagnosis.
A number of other cancers are often associated with these tumors, including:
- Acute myelogenous leukemia (AML)
- Embryonal rhabdomyosarcoma
- Malignant histiocytosis
- Myelodysplasia (MDS)
- Small cell undifferentiated carcinoma
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [11]
|
Teratoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox m ex On the Web |
|
American Roentgen Ray Society Images of Sandbox m ex |
| Sandbox m ex |
Overview
DiseasesDB = 3604 |
DiseasesDB_mult = Template:DiseasesDB2 Template:DiseasesDB2 | ICD10 = | ICD9 = | ICDO = 9080 | OMIM = | MedlinePlus = | MeshID = D013724 |
Pathology classification of individual teratomas
Teratomas commonly are classified using the Gonzalez-Crussi grading system: 0 or mature (benign); 1 or immature, probably benign; 2 or immature, possibly malignant (cancerous); and 3 or frankly malignant. See also cancer staging. Teratomas are also classified by their content: a solid teratoma contains only tissues (perhaps including more complex structures); a cystic teratoma contain only pockets of fluid or semi-fluid such as cerebrospinal fluid, sebum, or fat; a mixed teratoma contains both solid and cystic parts. Cystic teratomas usually are grade 0 and, conversely, grade 0 teratomas usually are cystic.
Grade 0, 1 and 2 pure teratomas have the potential to become malignant (grade 3), and malignant pure teratomas have the potential to metastasize. These rare forms of teratoma with malignant transformation may contain elements of somatic (non germ cell) malignancy such as leukemia, carcinoma or sarcoma.[16] A teratoma may contain elements of other germ cell tumors, in which case it is not a pure teratoma but rather is a mixed germ cell tumor and is malignant. In infants and young children, these elements usually are endodermal sinus tumor, followed by choriocarcinoma. Finally, a teratoma can be pure and not malignant yet highly aggressive: this is exemplified by growing teratoma syndrome, in which chemotherapy eliminates the malignant elements of a mixed tumor, leaving pure teratoma which paradoxically begins to grow very rapidly.
"Benign" teratoma may prove to be malignant
A "benign" grade 0 (mature) teratoma nonetheless has a non-zero risk of malignancy. Recurrence with malignant endodermal sinus tumor has been reported in cases of formerly benign mature teratoma,[17] even in fetiform teratoma and fetus in fetu.[18][19] A grade 1 immature teratoma that appears to be benign (e.g., because AFP is not elevated) has a much higher risk of malignancy, and requires adequate follow-up.[20][21][22][23]
Teratoma with malignant transformation
A teratoma with malignant transformation or TMT is a very rare form of teratoma that may contain elements of somatic (non germ cell) malignant tumors such as leukemia, carcinoma or sarcoma.[16] Of 641 children with pure teratoma, 9 developed TMT[24]: 5 carcinoma, 2 glioma, and 2 embryonal (here, these last are classified among germ cell tumors).
Extraspinal ependymoma
Extraspinal ependymoma, usually considered to be a glioma (a type of non-germ cell tumor), may be an unusual form of mature teratoma.[25]
References
- ↑ [1]
- ↑ [2]
- ↑ [3]
- ↑ [4]
- ↑ [5]
- ↑ [6]
- ↑ [7]
- ↑ [8]
- ↑ [9]
- ↑ [10]
- ↑ Committee on Practice Bulletins-Gynecology (2002). "ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas, number 35, May 2002". Obstetrics and gynecology. 99 (5 Pt 1): 855–67. PMID 11978302.
- ↑ "Cervical cancer statistics and prognosis". Cancer Research UK. Retrieved 2007-03-24.
- ↑ "Cervical Cancer". Cervical Cancer: Pathology, Symptoms and Signs, Diagnosis, Prognosis and Treatment. Armenian Health Network, Health.am.
- ↑ "Pap Smear". Retrieved 2008-12-27.
- ↑ Eversole, GM; Moriarty, AT; Schwartz, MR; Clayton, AC; Souers, R; Fatheree, LA; Chmara, BA; Tench, WD; Henry, MR (2010). "Practices of participants in the college of american pathologists interlaboratory comparison program in cervicovaginal cytology, 2006". Archives of pathology & laboratory medicine. 134 (3): 331–5. doi:10.1043/1543-2165-134.3.331. PMID 20196659.
- ↑ 16.0 16.1 Harms D, Zahn S, Göbel U, Schneider DT (2006). "Pathology and molecular biology of teratomas in childhood and adolescence". Klinische Pädiatrie. 218 (6): 296–302. doi:10.1055/s-2006-942271. PMID 17080330.
- ↑ Ohno Y, Kanematsu T (1998). "An endodermal sinus tumor arising from a mature cystic teratoma in the retroperitoneum in a child: is a mature teratoma a premalignant condition?". Hum. Pathol. 29 (10): 1167–9. PMID 9781660.
- ↑ Chen YH, Chang CH, Chen KC, Diau GY, Loh IW, Chu CC (2007). "Malignant transformation of a well-organized sacrococcygeal fetiform teratoma in a newborn male". J. Formos. Med. Assoc. 106 (5): 400–2. PMID 17561476. (publisher offers free full text PDF to registered users)
- ↑ Hopkins KL, Dickson PK, Ball TI, Ricketts RR, O'Shea PA, Abramowsky CR (1997). "Fetus-in-fetu with malignant recurrence". J. Pediatr. Surg. 32 (10): 1476–9. PMID 9349774.
- ↑ Muscatello L, Giudice M, Feltri M (2005). "Malignant cervical teratoma: report of a case in a newborn". European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 262 (11): 899–904. doi:10.1007/s00405-005-0917-2. PMID 15895292.
- ↑ Ukiyama E, Endo M, Yoshida F, Tezuka T, Kudo K, Sato S, Akatsuka S, Hata J (2005). "Recurrent yolk sac tumor following resection of a neonatal immature gastric teratoma". Pediatr. Surg. Int. 21 (7): 585–8. doi:10.1007/s00383-005-1404-y. PMID 15928937.
- ↑ Bilik R, Shandling B, Pope M, Thorner P, Weitzman S, Ein SH (1993). "Malignant benign neonatal sacrococcygeal teratoma". J. Pediatr. Surg. 28 (9): 1158–60. PMID 7508500.
- ↑ Hawkins E, Issacs H, Cushing B, Rogers P (1993). "Occult malignancy in neonatal sacrococcygeal teratomas. A report from a Combined Pediatric Oncology Group and Children's Cancer Group study". The American journal of pediatric hematology/oncology. 15 (4): 406–9. PMID 7692755.
- ↑ Biskup W, Calaminus G, Schneider DT, Leuschner I, Göbel U (2006). "Teratoma with malignant transformation: experiences of the cooperative GPOH protocols MAKEI 83/86/89/96". Klinische Pädiatrie. 218 (6): 303–8. doi:10.1055/s-2006-942272. PMID 17080331.
- ↑ Aktuğ T, Hakgüder G, Sarioğlu S, Akgür FM, Olguner M, Pabuçcuoğlu U. (2000) Sacrococcygeal extraspinal ependymomas: the role of coccygectomy. J Pediatr Surg. 35(3):515-518. PubMed
|
WikiDoc Resources for Sandbox m ex |
|
Articles |
|---|
|
Most recent articles on Sandbox m ex Most cited articles on Sandbox m ex |
|
Media |
|
Powerpoint slides on Sandbox m ex |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sandbox m ex at Clinical Trials.gov Clinical Trials on Sandbox m ex at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sandbox m ex
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sandbox m ex Discussion groups on Sandbox m ex Patient Handouts on Sandbox m ex Directions to Hospitals Treating Sandbox m ex Risk calculators and risk factors for Sandbox m ex
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sandbox m ex |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
See Ben Underwood for a case who compensated for the resulting blindness by developing human echolocation.
In October 2007, researchers have identified the specific cell that causes retinoblastoma.[12]old daughter had the condition. Fisher missed Game 1 and half of Game 2 of the 2007 NBA playoff series versus the Golden State Warriors to be with his daughter for her surgery in New York City
Retinoblastoma is a cancer of the retina. Development of this tumor is initiated by mutations[1] that inactivate both copies of the RB1 gene, which codes for the retinoblastoma protein.[2]
It occurs mostly in children younger than 5 years and accounts for about 3% of the cancers occurring in children younger than 15 years. Adult cases have also been clinically recorded.[3] The estimated annual incidence is approximately 4 per million children.[4] It begins with white blotches in one or both eyes (leukocoria) which can be seen in photographs (this is distinct from the red-eye effect which is normal); or when light reflects off the eye, as when watching television
The term uterine cancer may refer to one of several different types of cancer which occur in the uterus. These include:
- Endometrial carcinomas originate from cells in the glands of the endometrium (uterine lining). These include the common and readily treatable well-differentiated endometrioid adenocarcinoma, as well as the more aggressive uterine papillary serous carcinoma and uterine clear-cell carcinoma.
- Endometrial stromal sarcomas originate from the connective tissues of the endometrium, and are far less common than endometrial carcinomas
- Malignant mixed müllerian tumors are rare endometrial tumors which show both glandular (carcinomatous) and stromal (sarcomatous) differentiation - their true cell of origin is unknown.
- Cervical cancer arises from the transitional zone of the cervix, the lower portion of the uterus which lies at the upper aspect of the vagina
- Sarcomas of the myometrium, or muscular layer of the uterus, are most commonly leiomyosarcomas. Uterine fibroids are non-cancerous smooth muscle tumors which are vastly more common than sarcomas.
A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI).
Treatment
There is no specific treatment for infectious mononucleosis, other than treating the symptoms. No antiviral drugs or vaccines are available. Some physicians have prescribed a 5-day course of steroids to control the swelling of the throat and tonsils. The use of steroids has also been reported to decrease the overall length and severity of illness, but these reports have not been published.
Prevention & Treatment
- Epstein-Barr Virus (EBV) [5]
- There is no vaccine to protect against EBV infection. You can help protect yourself by not kissing or sharing drinks, food, or personal items, like toothbrushes, with people who have EBV infection.
- There is no specific treatment for EBV. However, some things can be done to help relieve symptoms, including
- Drinking fluids to stay hydrated
- Getting plenty of rest
- Taking over-the-counter medications for pain and fever
Gas gangrene
Preferred regimen: Penicillin G 3-4 million units IV q4h AND (Clindamycin 900 mg IV q8h OR Tetracycline 500 mg IV q6h)[78]
Antibiotics are used if an infection is suspected. Fluoroquinolones are an option, although resistance of N. gonorrhoeae may limit their use. A cephalosporin (such as ceftriaxone) combined with doxycycline is an alternative. Azithromycin can be used for susceptible strains. In children, quinolones and doxycycline are best avoided. Since bacteria that cause urinary tract infections are often the cause of epididymitis in children, co-trimoxazole or suited penicillins (for example, cephalexin) can be used. If there is a sexually transmitted disease, the partner should also be treated.
Household remedies such as elevation of the scrotum and cold compresses applied regularly to the scrotum may relieve the pain. Painkillers or anti-inflammatory drugs are often necessary. Hospitalisation is indicated for severe cases, and check-ups can ensure the infection has cleared up.
- Doxycycline is the drug of choice.
- For people allergic to drugs of the tetracycline class, rifampicin is an alternative.
- Early clinical experience suggested that chloramphenicol may also be effective, however in vitro susceptibility testing revealed resistance.
- Preferred regimen: Doxycycline 100 mg IV q12h for 7-14 days
- Alternative regimen (1): Chloramphenicol
- Alternative regimen (2): Rifampin
- Pediatric regimen: Doxycycline 2.2 mg/kg PO bid (Children under 45 kg (100 lbs)) for 7-14 days
- Note: Patients should be treated for at least 3 days after the fever subsides and until there is evidence of clinical improvement
Febrile Neutropenia
Exception may be made for neutropenic patients in which delayed treatment could lead to serious complications.
After samples for cultures are obtained, febrile neutropenia should be aggressively treated with broad-spectrum antipseudomonal antibiotics. The antimicrobial regimen should be modified in accordance with the culture results.[6][7][8]
HIV/AIDS patients
HIV-infected persons with pyrexia and hypoxia, should be placed on therapy for Pneumocystis jirovecii infection and adjusted once the diagnosis is confirmed.[8]
Giant Cell Arteritis
Empiric corticosteroids may be considered in patients with suspected giant cell arteritis to prevent vascular complications such as stroke and blindness. Giant cell arteritis should be suspected in a patient over the age of 50 with the following symptoms:
- Newly onset headaches
- Abrupt onset of blurry vision
- Symptoms of polymyalgia rheumatica
- Jaw claudication
- Unexplained anemia
- Elevated erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP)
Premenopausal, Nonpregnant Women
No screening or treatment recommended for healthy, bacteriuric women.
Although asymptomatic bacteriuria increases the risk of urinary tract infection but has no effect on the long term adverse outcomes like CKD, genitourinary cancer or overall survival.[9] [10]
Studies' results indicated that the treatment wouldn't decrease the frequency for asymptomatic bacteriuria or the risk of developing symptomatic urinary tract infection.[11]
Pregnant Women
Diabetic WomenAsymptomatic bacteriuria screening or treatment is not recommended for diabetic women.
Older Persons Residing in the CommunityRoutine screening and treatment for asymptomatic bacteriuria is not indicated.
Elderly Institutionalized SubjectsNo recommendation for screening for or treatment of asymptomatic bacteriuria.
Subjects with Spinal Cord InjuriesNo benefit from screening for or treatment of asymptomatic bacteriuria Although the high prevalence of asymptomatic bacteriuria in patients with spinal cord injuries[26], but antimicrobial therapy harm outweigh benefit because of recurrent infections with more resistant strains.[27] Patients with Indwelling Urethral CathetersIt is not recommended to screen for or treat asymptomatic bacteriuria or fungiuria for short or long term catheters,[28] but antimicrobial therapy can be used for women with persistent bacteriuria 48 hours after removal of the urethral catheter.
Urologic Interventions
Immunocompromised Patients and Other PatientsPoor transplant prognosis and complications hasn't been associated with asymptomatic bacteriuria,[35][36]so there is no benefit from screening[37][38] for or treatment of asymptomatic bacteriuria in renal transplant or other solid organ[39] transplant patients.[40] There is no vaccine against the bacteria. LGV can be treated with three weeks of antibiotics. CDC STD Treatment Guidelines recommend the use of doxycyline, twice a day for 21 days. An alternative treatment is erythromycin base or azithromycin. The health care provider will determine which is best. Medical Therapy Acute Pharmacotherapy Treatment involves antibiotics and may involve drainage of the buboes or abscesses by needle aspiration or incision. Further supportive measure may need to be taken: dilatation of the rectal stricture, repair ofrectovaginal fistulae, or colostomy for rectal obstruction. Common antibiotic treatments include: tetracycline, doxycycline (all tetracyclines, including doxycycline, are contraindicated during pregnancy and in children due to effects on bone development and tooth discoloration), and erythromycin. If a patient has been treated for LGV, he/she should notify any sex partners they had sex with within 60 days of the symptom onset so they can be evaluated and treated. This will reduce the risk that their partners will develop symptoms and/or serious complications of LGV. It will reducetheir risk of becoming re-infected as well as reduce the risk of ongoing transmission in the community. The patient and all of his/her sex partners should avoid sex until the patient has completed treatment for the infection and symptoms of both the patient and their partners have disappeared. Note: Doxycycline is not recommended for use in pregnant women. Pregnant and lactating women should be treated with erythromycin. Azythromycin may prove useful for treatment of LGV in pregnancy, but no published data are available regarding its safety and efficacy. A health care provider (like a doctor or nurse) can discuss treatment options with patients. Persons with both LGV and HIV infection should receive the same LGV treatment as those who are HIV-negative. Prolonged therapy may be required, and delay in resolution of symptoms may occur among persons with HIV. As with all STD's sex partners of patients who have LGV should be examined and tested for urethral or cervical chlamydial infection. After a positive culture for chlamydia, clinical suspicion should be confirmed with testing to distinguish serotype. Antibiotic treatment should be started if they had sexual contact with the patient during the 30 days preceding onset of symptoms in the patient. Patients with a sexually transmitted disease need to be tested for other STD's. Antibiotics are not without risks and prophylaxtic broad antibiotic coverage is not recommended.[1] OverviewGenital warts may disappear without treatment, but sometimes eventually develop a fleshy, small raised growth. There is no way to predict whether they will grow or disappear. Medical TreatmentOverveiw
Some doctors inject the antiviral drug interferon-alpha directly into the warts, to treat warts that have returned after removal by traditional means. The drug is expensive, and does not reduce the rate that the warts return.
HSV=====Antiviral Drugs=Acyclovir (an antiviral drug) inhibits replication of the viral DNA, and is used both as prophylaxis (e.g., in patients with AIDS) and as therapy for herpes zoster. Other antivirals are valacyclovir and famciclovir. During the acute phase, oral acyclovir should be given. Use of acylovir is most effective in moderating the progress of the symptoms, and in preventing post-herpetic neuralgia, if started within 24 to 72 hours of the onset of symptoms, so medical care should be obtained as soon as the condition is recognized. Immunocompromised patients may respond best to intravenous acyclovir. In patients who are at high risk for recurrences, an oral dose of acyclovir, taken twice daily, is usually effective. It is also reported that the amino acid lysine inhibits the replication of herpes zoster.[42] AnalgesicsPeople with mild to moderate pain can be treated with over-the-counter analgesics. Topical lotions containing calamine can be used on the rash or blisters and may be soothing. Occasionally, severe pain may require an opioid medication, such as morphine. Once the lesions have crusted over, capsaicin cream (Zostrix) can be used. Topical lidocaine and nerve blocks may also reduce pain.[43] Administering gabapentin along with antivirals may offer relief of postherpetic neuralgia[44]. SteroidsOrally administered corticosteroids are frequently used in treatment of the infection, despite clinical trials of this treatment being unconvincing. Nevertheless, one trial studying immunocompetent patients older than 50 years of age with localized herpes zoster, suggested that administration of prednisone with aciclovir improved healing time and quality of life.[45] Upon one-month evaluation, aciclovir with prednisone increased the likelihood of crusting and healing of lesions by about twofold, when compared to placebo. This trial also evaluated the effects of this drug combination on quality of life at one month, showing that patients had less pain, and were more likely to stop the use of analgesic agents, return to usual activities and have uninterrupted sleep. However, when comparing cessation of herpes zoster-associated pain or post herpetic neuralgia, there was no difference between aciclovir plus prednisone and simply aciclovir alone. Because of the risks of corticosteroid treatment, it is recommended that this combination of drugs only be used in people more than 50 years of age, due to their greater risk of postherpetic neuralgia.[45] Other DrugsCimetidine, a common component of over-the-counter heartburn medication, has been shown to lessen the severity of herpes zoster outbreaks in several different instances.[46][47][48] This usage is considered an off-label use of the drug. In addition, cimetidine and probenecid have been shown to reduce the renal clearance of aciclovir. [49] The study showed these compounds reduce the rate, but not the extent, at which valaciclovir is converted into aciclovir. Renal clearance of aciclovir was reduced by approximately 24% and 33% respectively. In addition, respective increases in the peak plasma concentration of acyclovir of 8% and 22% were observed. The authors concluded that these effects were "not expected to have clinical consequences regarding the safety of valaciclovir". Due to the tendency of aciclovir to precipitate in renal tubules, combining these drugs should only occur under the supervision of a physician. ==VZV== 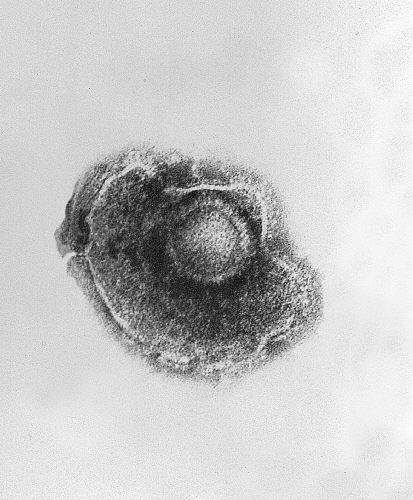
Complementary TherapiesDigestive Enzymes are available on prescription and in some over the counter preparations. Before the availability of antivirals, oral pancreatic enzyme therapy in shingles was used in some countries and later subjected to clinical and scientific research. A large scale multi-centre clinical study, using an oral preparation of such enzymes, has shown promising results.[50] [51] The results of another clinical study support the concept that oral enzyme therapy is beneficial in diseases characterized in part by TGF-beta overproduction that included shingles patients. [52] TGF-β has also been found to be elevated in instances of VZV infection. [53] [54] Anti-viral medicationNucleoside analogsTreatment is available in the form of antiviral medications such as nucleoside analogs, which reduce the duration of symptoms of a herpex simplex virus outbreak and accelerate healing. Nucleoside analogs are molecules which possess a similarity to natural nucleotides - the building-blocks of DNA and RNA. Active herpes simplex virus will replicate; a virus replicating in the presence of these analogs will incorporate them into its DNA, so that its genetic material will contain defects and mutations. As a result, the next generation of virus will be damaged and reduced in number. Nucleoside analogs are typically used at the first symptoms of an viral outbreak to reduce the duration of the outbreak and improve healing of the lesion. Treatment taken prior to the appearance of lesions may avert or reduce the symptoms of the outbreak. Occasionally nucleoside analogs are used as a daily suppressive therapy, and taken daily for several years. Suppressive therapy reduces frequency of symptoms and recurrence of outbreaks. In addition, suppressive therapy reduces subclinical viral shedding, lowering the risk of transmission through sexual contact or kissing. Common nucleoside analogs are listed in the table above. Of these, Ganciclovir is known to have cytotoxic effects on infected cells but Acyclovir is not known to have this effect.[55]
Fusion inhibitorsFusion inhibitors prevent "fusion" of the viral envelope with the cell membrane. This prevents viral entry to the cell. Helicase-primase inhibitorsOne of three key protein structures involved in HSV DNA replication is the Helicase-Primase structure. New research compounds which bind to this megamolecule show remarkable effectiveness against HSV. In particular, BAY 57-1293 has shown positive results in animal models of HSV infection.[58] Dietary supplementsThe amino acid lysine has demonstrated the ability to reduce the duration of infection through inhibiting the replication of the HSV. When foods high in lysine (such as lentils) are consumed in preference to foods high in arginine, HSV replication may be inhibited; conversely, consuming foods high in arginine (such as nuts or peanuts) may interfere with the therapeutic use of lysine.[59] However, according to the American Social Health Association: "While some studies have suggested that lysine supplements can reduce the frequency of recurrences or healing time, other trials have been unable to replicate those results. Therefore, there is not sufficient information to discern how effective it may be, in addition to what the effective dosages or frequency of L-lysine may be."[56] Other anti-viral medicationUndecylenic acid (Castor oil derivative) is proven to have anti-bacterial and anti-viral properties that are effective on viral skin infections such as the herpes simplex virus (HSV). Butylated hydroxytoluene (BHT), commonly available as a food preservative, has been shown in vitro to inactivate enveloped viruses including herpes.[60][61] In-vivo studies of topical application to animals confirmed the anti-viral activity of BHT during outbreaks.[62] BHT has not been clinically tested and approved to treat herpes in humans. Drug ResistanceResistance of HSVes in cell culture has been reported for nucleosides in the range of 10-2 to 10-4 and for Helicase-Primase inhibitors in the range of 10-4 to 10-6. However, in the clinic roughly 1-2% of the patients are infected by nucleoside-resistant HSVes. In the immunocompromised patient population such as transplant, AIDS or cancer patients the resistance rate can reach up to 10%.
ChlamydiaChlamydia is a genus of obligate intracellular bacteria in the family Chlamydiaceae, order Chlamydiales, class and phylum Chlamydiae. Several species of Chlamydia are pathogenic to humans causing pneumonia, eye infections, and sexually transmitted disease, which is called Chlamydia 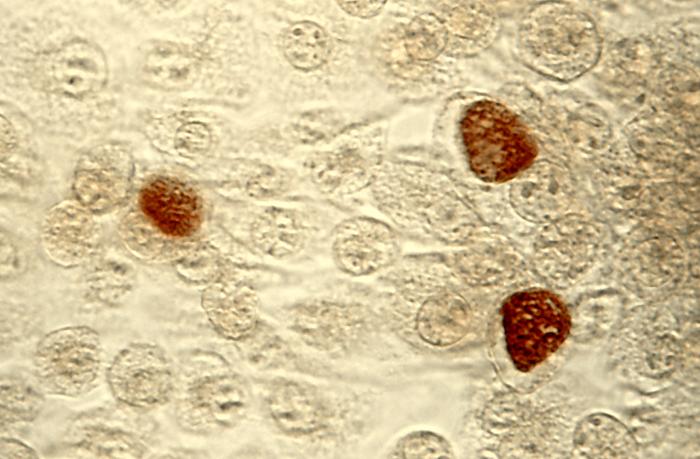 C. trachomatis infection can be effectively cured with antibiotics once it is detected. Current Centers for Disease Control guidelines provide for the following treatments:
Untested Treatments
β-lactams are not suitable drugs for the treatment of chlamydia. While they have the ability to halt growth of the organism (i.e. are microbistatic), these antibiotics do not eliminate the bacteria. Once treatment is stopped, the bacteria will begin to grow once more. (See below for Persistence.)
Besides supportive care, infant botulism can be treated with human botulism immune globulin (BabyBIG), when available. Supply is extremely limited, but is available through the California Department of Health Services. This dramatically decreases the length of illness for most infants. Paradoxically, antibiotics (especially aminoglycosides or clindamycin) may cause dramatic acceleration of paralysis as the affected bacteria release toxin. Visual stimulation should be performed during the time the infant is paralyzed as well, in order to promote the normal development of visual pathways in the brain during this critical developmental period. Furthermore each case of food-borne botulism is a potential public health emergency in that it is necessary to identify the source of the outbreak and ensure that all persons who have been exposed to the toxin have been identified, and that no contaminated food remains. There are two primary Botulinum Antitoxins available for treatment of wound and foodborne botulism. Trivalent (A,B,E) Botulinum Antitoxin is derived from equine sources utilizing whole antibodies (Fab & Fc portions). This antitoxin is available from the local health department via the CDC. The second antitoxin is heptavalent (A,B,C,D,E,F,G) Botulinum Antitoxin which is derived from "despeciated" equine IgG antibodies which have had the Fc portion cleaved off leaving the F(ab')2 portions. This is a less immunogenic antitoxin that is effective against all known strains of botulism where not contraindicated. This is available from the US Army. On June 1, 2006 the US Department of Health and Human Services awarded a $363 million contract with Cangene Corporation for 200,000 doses of Heptavalent Botulinum Antitoxin over five years for delivery into the Strategic National Stockpile beginning in 2007.[64] Antimicrobial regimen
Pharmacotherapy brucellaAcute PharmacotherapiesThe gold standard treatment for adults is daily intramuscular injections of streptomycin 1 g for 14 days and oral doxycycline 100 mg twice daily for 45 days (concurrently). Gentamicin 5 mg/kg by intramuscular injection once daily for 7 days is an acceptable substitute when streptomycin is not available or difficult to obtain.[67] Another widely used regimen is doxycycline plus rifampin twice daily for at least 6 weeks. This regimen has the advantage of oral administration. A triple therapy of doxycycline, together with rifampin and cotrimoxazole has been used succefully to treat neurobrucellosis. [68] Doxycycline is able to cross the blood-brain barrier, but requires the addition of two other drugs to prevent relapse. Ciprofloxacin and co-trimoxazole therapy is associated with an unacceptably high rate of relapse. In brucellic endocarditis surgery is required for an optimal outcome. Even with optimal antibrucellic therapy relapses still occur in 5-10 percent of patients with Malta fever. Experiments have shown that cotrimoxyzol and rifampin are both safe drugs to use in treatment of pregnant women who have Brucellosis.
Acute Pharmacotherapies gonorrhea
Several antibiotics can successfully cure gonorrhea in adolescents and adults. However, drug-resistant strains of gonorrhea are increasing in many areas of the world, including the United States, and successful treatment of gonorrhea is becoming more difficult. Because many people with gonorrhea also have chlamydia, another sexually transmitted disease, antibiotics for both infections are usually given together. Persons with gonorrhea should be tested for other STDs. It is important to take all of the medication prescribed to cure gonorrhea. Although medication will stop the infection, it will not repair any permanent damage done by the disease. People who have had gonorrhea and have been treated can get the disease again if they have sexual contact with persons infected with gonorrhea. If a person's symptoms continue even after receiving treatment, he or she should return to a doctor to be reevaluated. The mainstay of treatment is the appropriate use of antibiotics. While penicillin was the most common antibiotic used to treat gonorrhea up until the 1970s, an increase in antibiotic resistance has led to a decline in its use. Recommendations for first choice treatment of gonorrhea must depend on local information on resistance patterns and it is not possible to make treatment recommendations that are applicable to all parts of the world. The Centers for Disease Control and Prevention (CDC) released a report on Thursday, April 12, 2007 officially adding gonorrhea to a list of super bugs that are now resistant to common antibiotics according to CDC. Antibiotics that may be used to treat gonorrhea include:
These drugs are all given as a single dose. The level of tetracycline resistance in Neisseria gonorrheae is now so high as to make it completely ineffective in most parts of the world. The fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin) cannot be used in pregnancy. It is important to refer all sexual partners to be checked for gonorrhea to prevent spread of the disease and to prevent the patient from becoming re-infected with gonorrhea. Patients should also be offered screening for other sexually transmitted infections. In areas where co-infection with chlamydia is common, doctors may prescribe a combination of antibiotics, such as ceftriaxone with doxycycline or azithromycin, to treat both diseases. Penicillin is ineffective at treating rectal gonorrhea: this is because other bacteria within the rectum produce β-lactamases that destroy penicillin. All current treatments are less effective at treating gonorrhea of the throat, so the patient must be rechecked by throat swab 72 hours or more after being given treatment, and then retreated if the throat swab is still positive. Although gonorrhea usually does not require follow-up (with the exception of rectal or pharyngeal disease), patients are usually advised to phone for results five to seven days after diagnosis to confirm that the antibiotic they received was likely to be effective. Patients are advised to abstain from sex during this time. Drug resistant strains are known to exist. United States recommendationsThe United States does not have a federal system of sexual health clinics, and the majority of infections are treated in family practices. A third-generation cephalosporin antibiotic such as ceftriaxone is recommended for use in most areas. Since some areas such as Hawaii and California have very high levels of resistance to fluoroquinolone antibiotics (ciprofloxacin, ofloxacin, levofloxacin) they are no longer used empirically to treat infections originating in these areas. United Kingdom recommendationsIn the United Kingdom, the majority of patients with gonorrhea are treated in dedicated sexual health clinics. The current recommendation is for ceftriaxone or cefixime as first line therapy; no resistance to either drug has yet been reported in the UK. Levels of spectinomycin resistance in the UK are less than 1%, which would make it a good choice in theory, but intramuscular spectinomycin injection is very painful. Azithromycin (given as a single dose of 2 g) is recommended if there is concurrent infection with chlamydia. A single dose of oral ciprofloxacin 500 mg is effective if the organism is known to be sensitive, but fluoroquinolones were removed from the UK recommendations for empirical therapy in 2003 because of increasing resistance rates. In 2005, resistance rates for ciprofloxacin were 22% for the whole of the UK (42% for London, 10% for the rest of the UK).[69]
antrax
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [13]; Associate Editor(s)-in-Chief: Jesus Rosario Hernandez, M.D. [14] OverviewBacillus anthracis is a Gram-positive, facultatively anaerobic, rod-shaped bacterium of the genus Bacillus. An endospore forming bacterium, B. anthracis is a natural soil-dwelling organism, as well as the causative agent of anthrax.[70] Each cell is about 1 by 6 μm in size. Historical backgroundB. anthracis was the first bacterium conclusively demonstrated to cause disease, by Robert Koch in 1877.[71] The species name anthracis is from the Greek anthrakis (ἄνθραξ), meaning coal and referring to the most common form of the disease, cutaneous anthrax, in which large black skin lesions are formed. PathogenicityUnder conditions of environmental stress, B. anthracis bacteria naturally produce endospores which rest in the soil and can survive for decades in this state. When ingested by a cattle, sheep, or other herbivores, the bacteria begin to reproduce inside the animal and eventually kill it, then continue to reproduce in its carcass. Once the nutrients are exhausted, new endospores are produced and the cycle repeats.[72] B. anthracis has at least 89 known strains, ranging from highly virulent strains with biological warfare and bioterrorism applications (Ames and Vollum) to benign strains used for inoculations (Sterne). The strains differ in presence and activity of various genes, determining their virulence and production of antigens and toxins. The form associated with the 2001 anthrax attacks produced both toxin (consisting of three proteins: the protective antigen, the edema factor and the lethal factor) and a capsule (consisting of a polymer of glutamic acid). Infection with anthrax requires the presence of all three of these exotoxins.[73] The bacterium can be cultivated in ordinary nutrient medium under aerobic or anaerobic conditions. TreatmentInfections with B. anthracis can be treated with β-lactam antibiotics such as penicillin, and others which are active against Gram-positive bacteria.[74] References
Gallery
ReferencesAntibiotic TreatmentCutaneous Anthrax without Systemic InvolvementChoice of Antibiotics
Duration of Treatment
Systemic Anthrax When Meningitis Has Been ExcludedChoice of Antibiotics
Duration of Treatment
Follow–up Oral Treatment for Systemic DiseaseOnce patients with systemic illness who were exposed to aerosolized spores have completed initial combination treatment, they should be transitioned to single-agent oral treatment to prevent relapse from surviving B. anthracis spores.[1] Systemic Anthrax with Possible/Confirmed MeningitisChoice of Antibiotics
Duration of Treatment
Follow–up Oral Treatment for Systemic DiseaseOnce patients with systemic illness who were exposed to aerosolized spores have completed initial combination treatment, they should be transitioned to single-agent oral treatment to prevent relapse from surviving B. anthracis spores. Dosage of Antibiotics▸ Click on the following categories to expand treatment regimens.[2][3][4]
piss
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [15]; Associate Editor(s)-in-Chief: Aditya Govindavarjhulla, M.B.B.S. [16] OverviewPsittacosis is an infection caused by the obligatory intracellular bacterium Chlamydia psittaci. It is apparently acquired from the birds (parrots). CausesPsittacosis is an infection caused by the obligatory intracellular bacterium Chlamydia psittaci. It is apparently acquired from the birds (parrots). Ornithosis is infection from any kind bird. References
plam
This page is about clinical aspects of the disease. For microbiologic aspects of the causative organism(s), see Plasmodium.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [17]; Associate Editor(s)-In-Chief: Yazan Daaboul, Serge Korjian, Alison Leibowitz [18] OverviewMalaria is a vector-borne infectious disease caused by protozoan parasites. P. vivax is the most common cause of infection, responsible for about 80% of all malaria cases. P. falciparum, the most significant cause of disease, is responsible for about 15% of infections and 90% of deaths.[1][2] CausesP. vivax is the most common cause of infection, responsible for about 80% of all malaria cases. P. falciparum, the most significant cause of disease, is responsible for about 15% of infections and 90% of deaths.[3] The remainder of human malaria infections are caused by P. ovale, P. malariae, and P. knowlesi.
References
q fever microb
Coxiella burnetii is a species of intracellular, pathogenic bacteria, and is the causative agent of Q fever. The genus Coxiella is morphologically similar to the rickettsia, but with a variety of genetic and physiological differences. C. burnetii are small Gram negative bacteria with two growth phases, as well as a spore form which lies idle in soil.[1] It can survive standard disinfectants, and is resistant to many other environmental changes.[2] PathogenesisThe ID50 (the dose needed to infect 50% of experimental subjects) is one via inhalation— i.e. inhalation of one organism will yield disease in 50% of the population. Disease occurs in two states: An acute state presents with headaches, chills, and respiratory symptoms, and an insidious chronic stage. While most infections clear up spontaneously, treatment with tetracycline or doxycycline appears to reduce the symptomatic duration and reduce the likelihood of chronic infection. A combination of erythromycin and rifampin is highly effective in curing and prevention of disease and so is vaccination with Q-vax vaccine (CSL).
References
Anti-malarial Agents
*Used in combination with quinine or quinidine
Initial Assessment & Severe MalariaThe first step in the management of patients with malaria is to conduct a clinical assessment of status and disease severity, as well as determination of the degree of parasitemia. Signs of severe malarial disease include any of the following: Prostration, impaired consciousness/coma, respiratory distress, convulsions, shock, pulmonary edema, acute respiratory distress syndrome (ARDS), jaundice, abnormal bleeding, severe anemia, hemolysis, hemoglobinuria, acute kidney injury, metabolic acidosis, disseminated intravascular coagulopathy, parasitemia >5%. Patients with severe disease require rapid resuscitation and medical therapy. The most vital step in the management is immediate initiation of appropriate parenteral treatment. Unlike patients who appear stable clinically, patients with severe malaria do not require speciation prior to initiation of medical therapy. The therapeutic regimen in patients with severe malaria consists of intravenous quinidine gluconate plus either tetracycline, doxycycline, or clindamycin.[1] Other supportive measures include admission to the intensive care unit, continuous monitoring of cardiac function, glycemia, parasitemia, hemoglobin and electrolytes. Exchange transfusions may also be considered in patients with a degree of parasitemia >10%. Uncomplicated MalariaIn patients with clinically and bacteriologically uncomplicated malaria, speciation is required to tailor medical therapy. For most non-falciparum species, chloroquine remains the first line therapeutic agent. It is important to add primaquine to the treatment regimen in patients with documented P. vivax and P. ovale infections to eradicate liver hypnozoites (dormant liver spores that are responsible for recurrence). Care should be taken in patients with G6PD deficiency as large doses of primaquine can cause significant hemolysis. Patients infected with P. malaria do not require primaquine as the species is not capable of forming hypnozoites.[2] Patients diagnosed with P. falciparum malaria require hospitalization given the risk of progression to severe malaria. These patients have to be monitored on daily basis with a blood film and a full physical exam. The choice of drug in these patients depends on two main factors: the area of acquisition of the parasite, and the center at which the patient is being treated.[1] Despite being the mainstay of therapy since its introduction, empiric treatment with chloroquine in patients with P. falciparum is no longer recommended due to a sharp increase in resistance. A detailed travel history is important to determine where the infection was acquired. Most malaria endemic countries have reported chloroquine resistant strains, with the exception of Central America west of Panama Canal, Mexico, Hispaniola, certain parts of China, and the Middle East (see figure below). If acquired in any of the latter sites then treatment with chloroquine is adequate. Acquisition from all other endemic countries requires other therapeutic regimens such as oral quinine with either tetracycline, doxycycline, or clindamycin as a first line therapy in the United States, otherwise atovaquone-proguanil or mefloquine if the primary regimen is unavailable. Worldwide, the treatment of both complicated and uncomplicated P. falciparum malaria requires a combination therapy that includes artemisinin derivatives. According to the 2010 WHO guidelines on the treatment of malaria, the following regimens are first line for the treatment of uncomplicated P. falciparum: artemether plus lumefantrine, artesunate plus amodiaquine, artesunate plus mefloquine, and artesunate plus sulfadoxine-pyrimethamin. It is important to note that artemisin monotherapy in not recommended due to increasing resistance. For patients with severe P. falciparum malaria, artesunate IV or IM is first line followed by IV quinidine. The artemisinin derivatives clear parasites very rapidly have been shown to reduce mortality in severe malaria compared with parenteral quinine. Artemisins are not widely available in the United States and their use is not common practice. Only oral artemether plus lumefantrine is available, while IV atresunate can be obtained through the CDC part of an investigational drug protocol. [3] 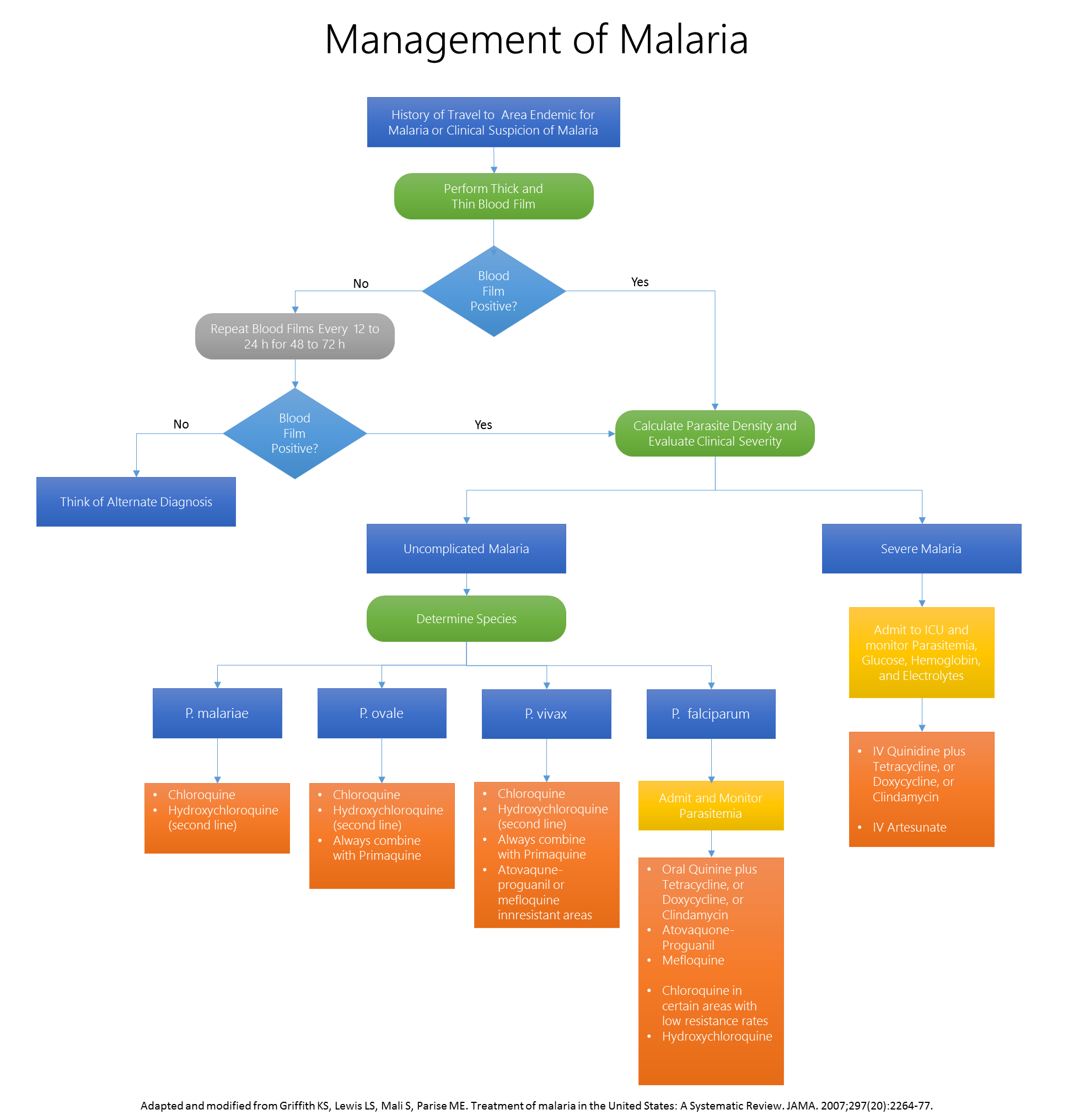 |
- ↑ 1.0 1.1 Griffith KS, Lewis LS, Mali S, Parise ME (2007). "Treatment of malaria in the United States: a systematic review". JAMA. 297 (20): 2264–77. doi:10.1001/jama.297.20.2264. PMID 17519416.
- ↑ White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014). "Malaria". Lancet. 383 (9918): 723–35. doi:10.1016/S0140-6736(13)60024-0. PMID 23953767.
- ↑ Template:Cite website
- CS1 maint: Multiple names: authors list
- Pages with reference errors
- CS1 errors: PMID
- CS1 maint: Explicit use of et al.
- CS1 maint: PMC format
- Pages with citations using unsupported parameters
- CS1 errors: dates
- CS1 maint: Unrecognized language
- CS1 maint: Extra text: authors list
- CS1 maint: Extra text
- All articles with unsourced statements
- Articles with unsourced statements from January 2009
- Articles with invalid date parameter in template
- Needs content
- Disease
- Congenital disorders
- Obstetrics
- Pediatrics
- Gynecology
- Proctology
- Pathology
- Surgery
- Types of cancer
- Rare diseases
- Pediatric cancers
- Endocrinology
- Pages with broken file links
- Bacillaceae
- Pulmonology
- Zoonoses
- Bacterial diseases
- Infectious diseases
- Apicomplexa
- Insect-borne diseases
- Malaria
- Emergency medicine
- Parasitic diseases
- Tropical disease
- Deaths from malaria
- Proteobacteria
- Infectious Disease Project

![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/e/e4/Bacillus_anthracis01.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/1/17/Bacillus_anthracis02.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/6/6d/Bacillus_anthracis03.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/0/0c/Bacillus_anthracis05.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/2/25/Bacillus_anthracis06.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/e/e4/Bacillus_anthracis07.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/8/86/Bacillus_anthracis08.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/d/d2/Bacillus_anthracis09.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/3/34/Bacillus_anthracis10.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/8/8f/Bacillus_anthracis11.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/e/ef/Bacillus_anthracis12.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/3/38/Bacillus_anthracis13.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/9/9f/Bacillus_anthracis14.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/9/9e/Bacillus_anthracis15.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/7/70/Bacillus_anthracis16.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/8/82/Bacillus_anthracis17.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/a/a2/Bacillus_anthracis18.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/b/b3/Bacillus_anthracis19.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/2/2c/Bacillus_anthracis20.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/f/f0/Bacillus_anthracis21.jpeg)
![Bacillus anthracis. From Public Health Image Library (PHIL). [1]](/images/e/e5/Bacillus_anthracis22.jpeg)