Doxazosin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doxazosin is a alpha-adrenergic blocker that is FDA approved for the {{{indicationType}}} of benign prostatic hyperplasia (BPH) and hypertension. Common adverse reactions include edema, hypotension, nausea, dizziness, headache, somnolence, vertigo and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Benign Prostatic Hyperplasia
- Initial dose:[1]
- 1 mg once a day
- Depending on the patient's urodynamics and BPH symptomatology, dosage may be increased, with a recommended titration interval of 1-2 weeks, to:
- 2 mg once a day, followed by 4 mg once a day until the maximum dosage of 8 mg once a day.
Hypertension
- Initial dose:[1]
- 1 mg once a day
- Depending on the patient's standing blood pressure response (based on measurements taken at 2-6 hours post-dose and 24 hours post-dose), dosage may then be increased to:[1]
- 2 mg once a day, followed by 4 mg once a day, than 8 mg once a day until the maximum dosage of 16 mg once a day to achieve the desired reduction in blood pressure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxazonin in adult patients.[1]
Non–Guideline-Supported Use
Cardiac syndrome X
Disorder of the urinary system
- Dosing Information[1]
- 2 mg at bedtime, twice daily
Erectile dysfunction
- Dosing Information[1]
- 4 mg daily
Pheochromocytoma
- Dosing Information[1]
- 2—16 mg daily
Ureteric stone
- Dosing Information[1]
- 4 mg daily
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypertension
- Dosing Information
- Initial: 1 mg/day PO until the maximum dosage of 4 mg/day
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxazosin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxazosin in pediatric patients.
Contraindications
- Doxazosin tablets are contraindicated in patients with a known sensitivity to quinazolines (e.g., prazosin, terazosin), doxazosin, or any of the inert ingredients.
Warnings
Syncope and "first-dose" effect
- Doxazosin, like other alpha-adrenergic blocking agents, can cause marked hypotension, especially in the upright position, with syncope and other postural symptoms such as dizziness. Marked orthostatic effects are most common with the first dose but can also occur when there is a dosage increase, or if therapy is interrupted for more than a few days.
- To decrease the likelihood of excessive hypotension and syncope, it is essential that treatment be initiated with the 1 mg dose. The 2 mg, 4 mg, and 8 mg tablets are not for initial therapy.
- Dosage should then be adjusted slowly with evaluations and increases in dose every two weeks to the recommended dose.
- Additional antihypertensive agents should be added with caution.
- Patients being titrated with doxazosin should be cautioned to avoid situations where injury could result should syncope occur, during both the day and night.
- If syncope occurs, the patient should be placed in a recumbent position and treated supportively as necessary.
Priapism
- Rarely (probably less frequently than once in every several thousand patients), alpha-1 antagonists including doxazosin, have been associated with priapism (painful penile erection, sustained for hours and unrelieved by sexual intercourse or masturbation).
- Because this condition can lead to permanent impotence if not promptly treated, patients must be advised about the seriousness of the condition.
Adverse Reactions
Clinical Trials Experience
Benign Prostatic Hyperplasia (BPH)
- The incidence of adverse events has been ascertained from worldwide clinical trials in 965 BPH patients. The incidence rates presented below are based on combined data from seven placebo-controlled trials involving once daily administration of doxazosin in doses of 1 mg to 16 mg in hypertensives and 0.5 mg to 8 mg in normotensives. The adverse events when the incidence in the doxazosin group was at least 1% are summarized in the table below.
- No significant difference in the incidence of adverse events compared to placebo was seen except for dizziness, fatigue, hypotension, edema and dyspnea. Dizziness and dyspnea appeared to be dose-related.
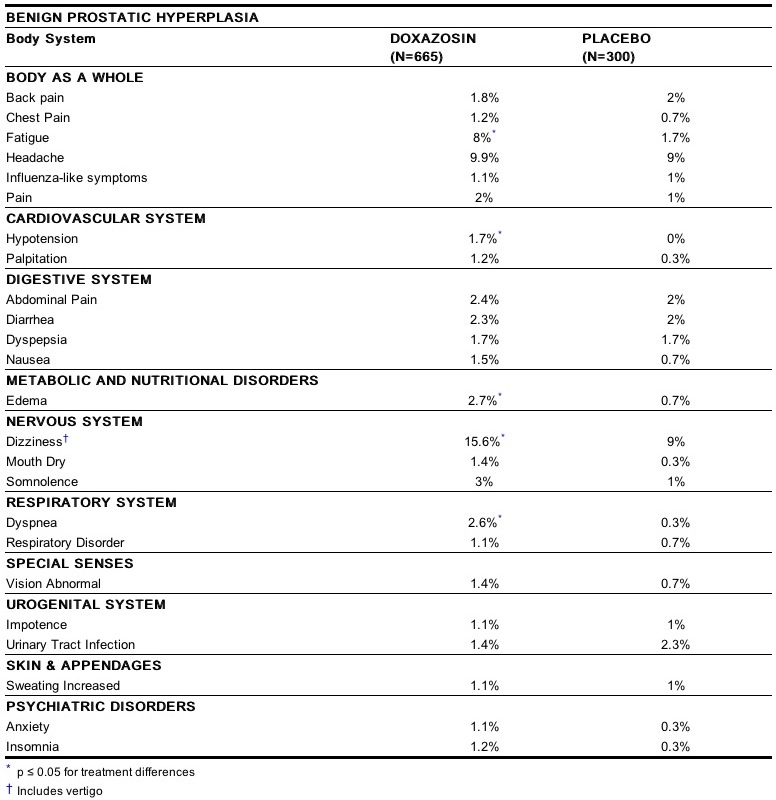
- In these placebo-controlled studies of 665 doxazosin patients, treated for a mean of 85 days, additional adverse reactions have been reported. These are less than 1% and not distinguishable from those that occurred in the placebo group.
- Adverse reactions with an incidence of less than 1% but of clinical interest are (doxazosin vs. placebo):
- Cardiovascular System: angina pectoris (0.6% vs. 0.7%), postural hypotension (0.3% vs. 0.3%), syncope (0.5% vs. 0%), tachycardia (0.9% vs. 0%)
- Urogenital System: dysuria (0.5% vs. 1.3%)
- Psychiatric Disorders: libido decreased (0.8% vs. 0.3%).
- The safety profile in patients treated for up to three years was similar to that in the placebo-controlled studies.
- The majority of adverse experiences with doxazosin were mild.
Hypertension
- Doxazosin mesylate has been administered to approximately 4,000 hypertensive patients, of whom 1,679 were included in the hypertension clinical development program.
- In that program, minor adverse effects were frequent, but led to discontinuation of treatment in only 7% of patients.
- In placebo-controlled studies adverse effects occurred in 49% and 40% of patients in the doxazosin and placebo groups, respectively, and led to discontinuation in 2% of patients in each group. The major reasons for discontinuation were:
- Postural effects (2%)
- Edema, about 0.7%.
- Malaise/fatigue, about 0.7%.
- Heart rate disturbance, about 0.7%.
- In controlled hypertension clinical trials directly comparing doxazosin to placebo there was no significant difference in the incidence of side effects, except for:
- Postural effects and edema appeared to be dose related. The prevalence rates presented below are based on combined data from placebo-controlled studies involving once daily administration of doxazosin at doses ranging from 1 mg to 16 mg.
- The table below summarizes those adverse experiences (possibly/probably related) reported for patients in these hypertension studies where the prevalence rate in the doxazosin group was at least 0.5% or where the reaction is of particular interest.
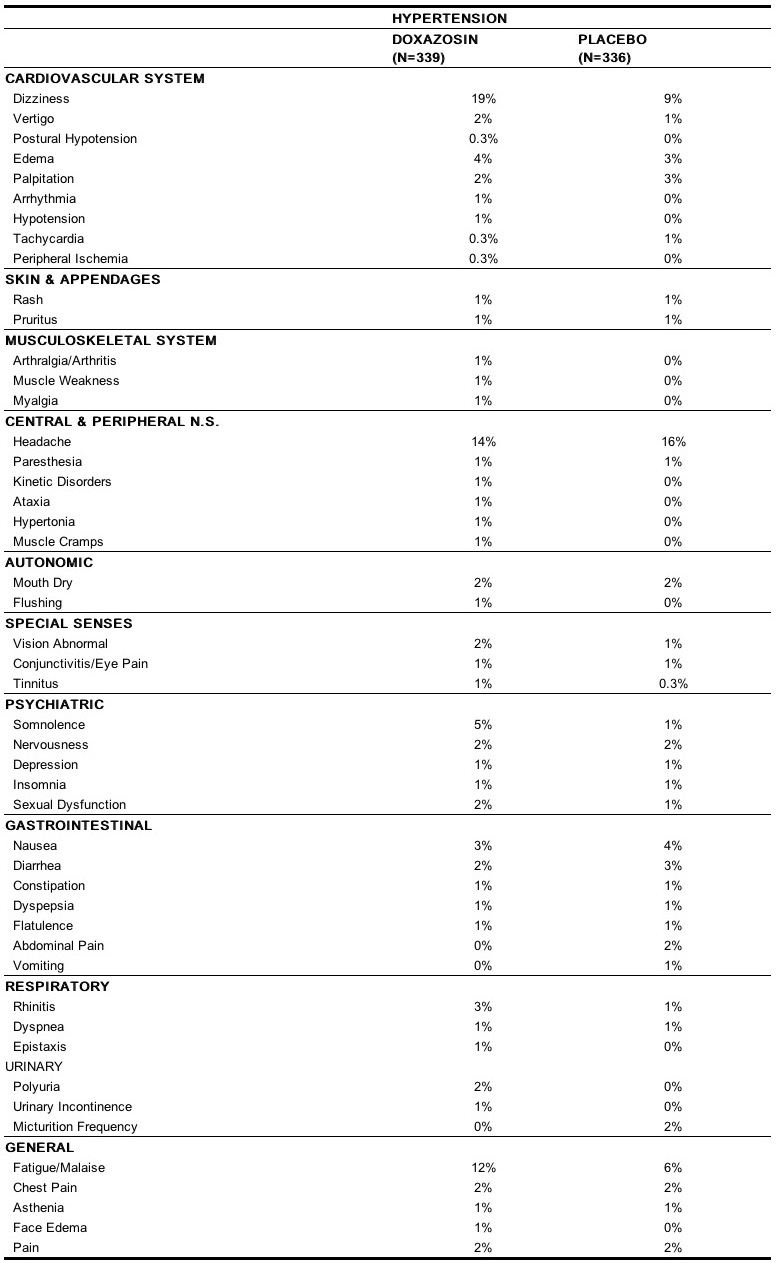
- Additional adverse reactions have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to doxazosin.
- The following adverse reactions occurred with a frequency of between 0.5% and 1%:
- Syncope
- Hypoesthesia
- Increased sweating
- Agitation
- Increased weight.
- The following additional adverse reactions were reported by < 0.5% of 3,960 patients who received doxazosin in controlled or open, short- or long-term clinical studies, including international studies.
- Cardiovascular System:
- Skin Disorders:
- Paroniria
- Amnesia
- Emotional lability
- Abnormal thinking
- Depersonalization
- Special Senses:
- Parosmia
- Earache
- Taste perversion
- Photophobia
- Abnormal lacrimation
- Increased appetite
- Fecal incontinence
- Gastroenteritis
- General Body System:
- Decreased weight
- Influenza-like symptoms.
- Doxazosin has not been associated with any clinically significant changes in routine biochemical tests. No clinically relevant adverse effects were noted on serum potassium, serum glucose, uric acid, blood urea nitrogen, creatinine or liver function tests. Doxazosin has been associated with decreases in white blood cell counts.
Postmarketing Experience
- In post-marketing experience the following additional adverse reactions have been reported:
Autonomic Nervous System
Central Nervous System
Endocrine System
Gastrointestinal System
General Body System
Heart Rate/Rhythm
Hematopoietic
Liver/Biliary System
Respiratory System
- Bronchospasm aggravated
Skin Disorders
Special Senses
Urinary System
- Hematuria
- Micturition disorder
- Nocturia
Drug Interactions
- Protein-bound drugs:
- Most (98%) of plasma doxazosin is protein bound. In vitro data in human plasma indicate that doxazosin has no effect on protein binding of digoxin, warfarin, phenytoin or indomethacin.
- There is no information on the effect of other highly plasma protein bound drugs on doxazosin binding.
- Doxazosin has been administered without any evidence of an adverse drug interaction to patients receiving thiazide diuretics, beta-blocking agents, and nonsteroidal anti-inflammatory drugs.
- In a placebo-controlled trial in normal volunteers, the administration of a single 1 mg dose of doxazosin on day 1 of a 4-day regimen of oral cimetidine (400 mg twice daily) resulted in a 10% increase in mean AUC of doxazosin (p = 0.006), and a slight but not statistically significant increase in mean Cmax and mean half-life of doxazosin. The clinical significance of this increase in doxazosin AUC is unknown.
- In clinical trials, doxazosin tablets have been administered to patients on a variety of concomitant medications; while no formal interaction studies have been conducted, no interactions were observed. Doxazosin tablets have been used with the following drugs or drug classes:
- Analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine and codeine combinations, ibuprofen, indomethacin)
- Antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole, amoxicillin)
- Antihistamines (e.g., chlorpheniramine)
- Cardiovascular agents (e.g., atenolol, hydrochlorothiazide, propranolol)
- Corticosteroids
- Gastrointestinal agents (e.g., antacids)
- Hypoglycemics and endocrine drugs
- Sedatives and tranquilizers (e.g., diazepam)
- Cold and flu remedies.
- Phosphodiesterase-5 (PDE-5):
- Concomitant administration of doxazosin tablets with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Studies in pregnant rabbits and rats at daily oral doses of up to 41 and 20 mg/kg, respectively (plasma drug concentrations 10 and 4 times human Cmax and AUC exposures with a 12 mg/day therapeutic dose), have revealed no evidence of harm to the fetus. A dosage regimen of 82 mg/kg/day in the rabbit was associated with reduced fetal survival. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, doxazosin should be used during pregnancy only if clearly needed.
Radioactivity was found to cross the placenta following oral administration of labeled doxazosin to pregnant rats.
Pregnancy Category (AUS): B3
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of doxazosin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxazosin during labor and delivery.
Nursing Mothers
Studies in lactating rats given a single oral dose of 1 mg/kg of [2-14C]-doxazosin indicate that doxazosin accumulates in rat breast milk with a maximum concentration about 20 times greater than the maternal plasma concentration. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxazosin is administered to a nursing mother.
Pediatric Use
The safety and effectiveness of doxazosin as an antihypertensive agent have not been established in children.
Geriatic Use
The safety and effectiveness profile of doxazosin in BPH was similar in the elderly (age ≥ 65 years) and younger (age < 65 years) patients.
- For hypertension:
- Clinical studies of doxazosin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Doxazosin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Doxazosin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxazosin in patients with renal impairment.
Hepatic Impairment
Doxazosin should be administered with caution to patients with evidence of impaired hepatic function or to patients receiving drugs known to influence hepatic metabolism
Females of Reproductive Potential and Males
Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of 40 mg/kg/day in rats and 120 mg/kg/day in mice revealed no evidence of carcinogenic potential. The highest doses evaluated in the rat and mouse studies are associated with AUCs (a measure of systemic exposure) that are 8 times and 4 times, respectively, the human AUC at a dose of 16 mg/day. Mutagenicity studies revealed no drug or metabolite-related effects at either chromosomal or subchromosomal levels. Studies in rats showed reduced fertility in males treated with doxazosin at oral doses of 20 (but not 5 or 10) mg/kg/day, about 4 times the AUC exposures obtained with a 12 mg/day human dose. This effect was reversible within two weeks of drug withdrawal. There have been no reports of any effects of doxazosin on male fertility in humans.
Immunocompromised Patients
There is no FDA guidance one the use of Doxazosin in patients who are immunocompromised.
Precautions in certain conditions
- Prostate Cancer:
- Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients on or previously treated with alpha-1 blockers. This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's surgeon should be prepared for possible modifications to their surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.
- While syncope is the most severe orthostatic effect of doxazosin, other symptoms of lowered blood pressure, such as dizziness, lightheadedness, or vertigo can occur, especially at initiation of therapy or at the time of dose increases.
- These symptoms were common in clinical trials in hypertension, occurring in up to 23% of all patients treated and causing discontinuation of therapy in about 2%.
- In placebo-controlled titration trials in hypertension, orthostatic effects were minimized by beginning therapy at 1 mg per day and titrating every two weeks to 2, 4, or 8 mg per day. There was an increased frequency of orthostatic effects in patients given 8 mg or more, 10%, compared to 5% at 1 mg to 4 mg and 3% in the placebo group.
- Benign Prostatic Hyperplasia:
- In placebo-controlled trials in BPH, the incidence of orthostatic hypotension with doxazosin was 0.3% and did not increase with increasing dosage (to 8 mg/day).
- The incidence of discontinuations due to hypotensive or orthostatic symptoms was 3.3% with doxazosin and 1% with placebo. The titration interval in these studies was one to two weeks.
- Patients in occupations in which orthostatic hypotension could be dangerous should be treated with particular caution. As alpha-1 antagonists can cause orthostatic effects, it is important to evaluate standing blood pressure two minutes after standing and patients should be advised to exercise care when arising from a supine or sitting position.
- If hypotension occurs, the patient should be placed in the supine position and, if this measure is inadequate, volume expansion with intravenous fluids or vasopressor therapy may be used. A transient hypotensive response is not a contraindication to further doses of doxazosin.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Drug Monitoring of doxazosin in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doxazosin and IV administrations.
Overdosage
- Experience with doxazosin overdosage is limited.
- Two adolescents who each intentionally ingested 40 mg doxazosin with diclofenac or acetaminophen, were treated with gastric lavage with activated charcoal and made full recoveries.
- A two-year-old child who accidentally ingested 4 mg doxazosin was treated with gastric lavage and remained normotensive during the five-hour emergency room observation period.
- A six-month-old child accidentally received a crushed 1 mg tablet of doxazosin and was reported to have been drowsy.
- A 32-year-old female with chronic renal failure, epilepsy and depression intentionally ingested 60 mg doxazosin (blood level 0.9 mcg/mL; normal values in hypertensives = 0.02 mcg/mL); death was attributed to a grand mal seizure resulting from hypotension.
- A 39-year-old female who ingested 70 mg doxazosin, alcohol and flurazepam developed hypotension which responded to fluid therapy.
- The oral LD50 of doxazosin is greater than 1000 mg/kg in mice and rats. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of fluid. As doxazosin is highly protein bound, dialysis would not be indicated.
Pharmacology
| |
| |
Doxazosin
| |
| Systematic (IUPAC) name | |
| (RS)-2-{4-[(2,3-Dihydro-1,4-benzodioxin-2-yl)carbonyl]piperazin-1-yl}-6,7-dimethoxyquinazolin-4-amine | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 451.475 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 65% |
| Protein binding | 98% |
| Metabolism | Hepatic |
| Half life | 22 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | oral |
Mechanism of Action
Hypertension
- The mechanism of action of doxazosin mesylate is selective blockade of the alpha-1 (postjunctional) subtype of adrenergic receptors.
- Studies in normal human subjects have shown that doxazosin competitively antagonized the pressor effects of phenylephrine (an alpha-1 agonist) and the systolic pressor effect of norepinephrine.
- Doxazosin and prazosin have similar abilities to antagonize phenylephrine.
- The antihypertensive effect of doxazosin results from a decrease in systemic vascular resistance. The parent compound doxazosin is primarily responsible for the antihypertensive activity. The low plasma concentrations of known active and inactive metabolites of doxazosin (2-piperazinyl, 6'- and 7'-hydroxy and 6- and 7-O-desmethyl compounds) compared to parent drug indicate that the contribution of even the most potent compound (6'-hydroxy) to the antihypertensive effect of doxazosin in man is probably small. The 6'- and 7'-hydroxy metabolites have demonstrated antioxidant properties at concentrations of 5 mcM, in vitro.
- Administration of doxazosin results in a reduction in systemic vascular resistance. In patients with hypertension there is little change in cardiac output. Maximum reductions in blood pressure usually occur 2 to 6 hours after dosing and are associated with a small increase in standing heart rate. Like other alpha1-adrenergic blocking agents, doxazosin has a greater effect on blood pressure and heart rate in the standing position.
- In a pooled analysis of placebo-controlled hypertension studies with about 300 hypertensive patients per treatment group, doxazosin, at doses of 1 mg to 16 mg given once daily, lowered blood pressure at 24 hours by about 10/8 mmHg compared to placebo in the standing position and about 9/5 mmHg in the supine position. Peak blood pressure effects (1 to 6 hours) were larger by about 50% to 75% (i.e., trough values were about 55% to 70% of peak effect), with the larger peak-trough differences seen in systolic pressures. There was no apparent difference in the blood pressure response of caucasians and blacks or of patients above and below age 65. In these predominantly normocholesterolemic patients doxazosin produced small reductions in total serum cholesterol (2% to 3%), LDL cholesterol (4%), and a similarly small increase in HDL/total cholesterol ratio (4%). The clinical significance of these findings is uncertain. In the same patient population, patients receiving doxazosin gained a mean of 0.6 kg compared to a mean loss of 0.1 kg for placebo patients.
Structure
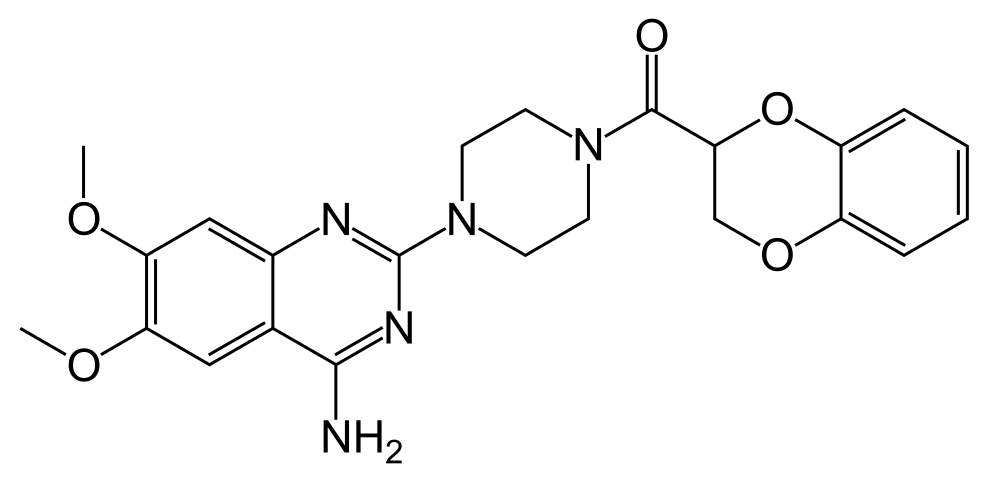
Doxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha-1 subtype of alpha adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. The molecular formula for doxazosin mesylate is C23H25N5O5 • CH4O3S and the molecular weight is 547.6. It has the following structure:
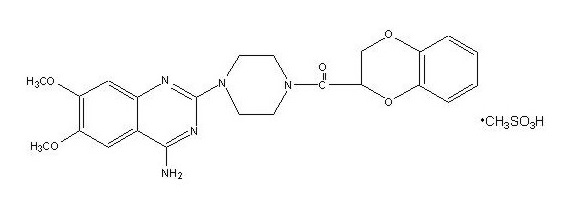
Pharmacodynamics
Benign Prostatic Hyperplasia
- Benign prostatic hyperplasia (BPH) is a common cause of urinary outflow obstruction in aging males. Severe BPH may lead to urinary retention and renal damage. A static and a dynamic component contribute to the symptoms and reduced urinary flow rate associated with BPH.
- The static component is related to an increase in prostate size caused, in part, by a proliferation of smooth muscle cells in the prostatic stroma. However, the severity of BPH symptoms and the degree of urethral obstruction do not correlate well with the size of the prostate.
- The dynamic component of BPH is associated with an increase in smooth muscle tone in the prostate and bladder neck. The degree of tone in this area is mediated by the alpha-1 adrenoceptor, which is present in high density in the prostatic stroma, prostatic capsule and bladder neck.
- Blockade of the alpha-1 receptor decreases urethral resistance and may relieve the obstruction and BPH symptoms.
- In the human prostate, doxazosin antagonizes phenylephrine (alpha1 agonist)-induced contractions, in vitro, and binds with high affinity to the alpha1c adrenoceptor. The receptor subtype is thought to be the predominant functional type in the prostate. Doxazosin acts within 1 to 2 weeks to decrease the severity of BPH symptoms and improve urinary flow rate. Since alpha1 adrenoceptors are of low density in the urinary bladder (apart from the bladder neck), doxazosin should maintain bladder contractility.
- The efficacy of doxazosin was evaluated extensively in over 900 patients with BPH in double-blind, placebo-controlled trials. Doxazosin treatment was superior to placebo in improving patient symptoms and urinary flow rate.
- Significant relief with doxazosin was seen as early as one week into the treatment regimen, with doxazosin treated patients (N = 173) showing a significant (p < 0.01) increase in maximum flow rate of 0.8 mL/sec compared to a decrease of 0.5 mL/sec in the placebo group (N = 41). In long-term studies improvement was maintained for up to 2 years of treatment. In 66% to 71% of patients, improvements above baseline were seen in both symptoms and maximum urinary flow rate.
- In three placebo-controlled studies of 14 to 16 weeks duration obstructive symptoms (hesitation, intermittency, dribbling, weak urinary stream, incomplete emptying of the bladder) and irritative symptoms (nocturia, daytime frequency, urgency, burning) of BPH were evaluated at each visit by patient-assessed symptom questionnaires. The bothersomeness of symptoms was measured with a modified Boyarsky questionnaire. Symptom severity/frequency was assessed using a modified Boyarsky questionnaire or an AUA-based questionnaire. Uroflowmetric evaluations were performed at times of peak (2 to 6 hours post-dose) and/or trough (24 hours post-dose) plasma concentrations of doxazosin.
- The results from the three placebo-controlled studies (N = 609) showing significant efficacy with 4 mg and 8 mg doxazosin are summarized in Table 1. In all three studies, doxazosin resulted in statistically significant relief of obstructive and irritative symptoms compared to placebo. Statistically significant improvements of 2.3 to 3.3 mL/sec in maximum flow rate were seen with doxazosin in Studies 1 and 2, compared to 0.1 to 0.7 mL/sec with placebo.
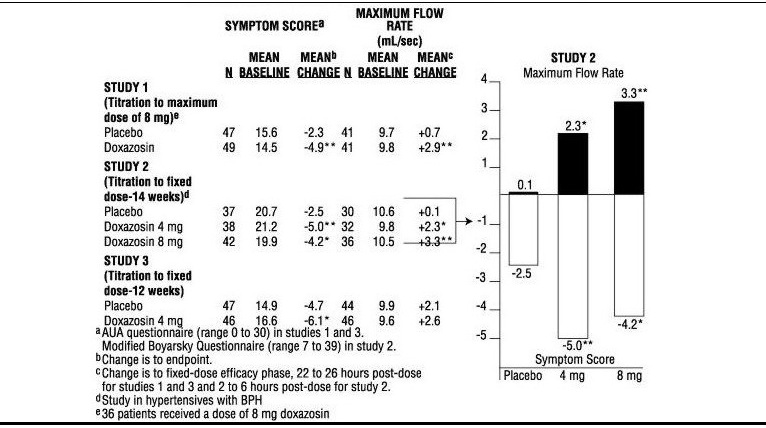
- In one fixed dose study, doxazosin therapy (4 mg to 8 mg, once daily) resulted in a significant and sustained improvement in maximum urinary flow rate of 2.3 to 3.3 mL/sec compared to placebo (0.1 mL/sec). In this study, the only study in which weekly evaluations were made, significant improvement with doxazosin vs. placebo was seen after one week.
- The proportion of patients who responded with a maximum flow rate improvement of ≥ 3 mL/sec was significantly larger with doxazosin (34% to 42%) than placebo (13% to 17%). A significantly greater improvement was also seen in average flow rate with doxazosin (1.6 mL/sec) than with placebo (0.2 mL/sec). The onset and time course of symptom relief and increased urinary flow from Study 1 are illustrated in figure below.
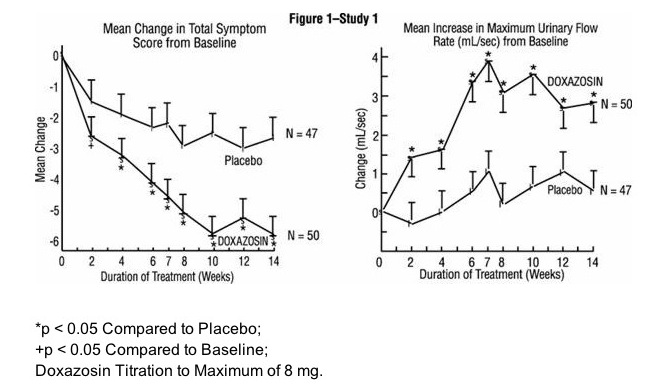
- In BPH patients (N = 450) treated for up to 2 years in open-label studies, doxazosin therapy resulted in significant improvement above baseline in urinary flow rates and BPH symptoms. The significant effects of doxazosin were maintained over the entire treatment period.
- Although blockade of alpha-1 adrenoceptors also lowers blood pressure in hypertensive patients with increased peripheral vascular resistance, doxazosin treatment of normotensive men with BPH did not result in a clinically significant blood pressure lowering effect. The proportion of normotensive patients with a sitting systolic blood pressure less than 90 mmHg and/or diastolic blood pressure less than 60 mmHg at any time during treatment with doxazosin 1 mg to 8 mg once daily was 6.7% with doxazosin and not significantly different (statistically) from that with placebo (5%).
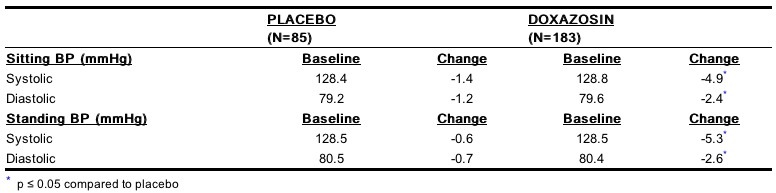
Pharmacokinetics
- After oral administration of therapeutic doses, peak plasma levels of doxazosin mesylate occur at about 2 to 3 hours.
- Bioavailability is approximately 65%, reflecting first pass metabolism of doxazosin by the liver. The effect of food on the pharmacokinetics of doxazosin was examined in a crossover study with 12 hypertensive subjects. Reductions of 18% in mean maximum plasma concentration and 12% in the area under the concentration-time curve occurred when doxazosin was administered with food. Neither of these differences was statistically or clinically significant.
- Doxazosin is extensively metabolized in the liver, mainly by O-demethylation of the quinazoline nucleus or hydroxylation of the benzodioxan moiety. Although several active metabolites of doxazosin have been identified, the pharmacokinetics of these metabolites have not been characterized. In a study of two subjects administered radiolabelled doxazosin 2 mg orally and 1 mg intravenously on two separate occasions, approximately 63% of the dose was eliminated in the feces and 9% of the dose was found in the urine. On average only 4.8% of the dose was excreted as unchanged drug in the feces and only a trace of the total radioactivity in the urine was attributed to unchanged drug. At the plasma concentrations achieved by therapeutic doses approximately 98% of the circulating drug is bound to plasma proteins.
- Plasma elimination of doxazosin is biphasic, with a terminal elimination half-life of about 22 hours. Steady-state studies in hypertensive patients given doxazosin doses of 2 mg to 16 mg once daily showed linear kinetics and dose proportionality. In two studies, following the administration of 2 mg orally once daily, the mean accumulation ratios (steady-state AUC vs. first dose AUC) were 1.2 and 1.7. Enterohepatic recycling is suggested by secondary peaking of plasma doxazosin concentrations.
- In a crossover study in 24 normotensive subjects, the pharmacokinetics and safety of doxazosin were shown to be similar with morning and evening dosing regimens. The area under the curve after morning dosing was, however, 11% less than that after evening dosing and the time to peak concentration after evening dosing occurred significantly later than that after morning dosing (5.6 hr vs. 3.5 hr).
- The pharmacokinetics of doxazosin in young (< 65 years) and elderly (≥ 65 years) subjects were similar for plasma half-life values and oral clearance. Pharmacokinetic studies in elderly patients and patients with renal impairment have shown no significant alterations compared to younger patients with normal renal function. Administration of a single 2 mg dose to patients with cirrhosis (Child-Pugh Class A) showed a 40% increase in exposure to doxazosin. There are only limited data on the effects of drugs known to influence the hepatic metabolism of doxazosin. As with any drug wholly metabolized by the liver, use of doxazosin in patients with altered liver function should be undertaken with caution.
- In two placebo-controlled studies, of normotensive and hypertensive BPH patients, in which doxazosin was administered in the morning and the titration interval was two weeks and one week, respectively, trough plasma concentrations of doxazosin were similar in the two populations. Linear kinetics and dose proportionality were observed.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of doxazosin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of doxazosin in the drug label.
How Supplied
- Doxazosin Tablets, USP are available as tablets for oral administration. Each tablet contains doxazosin mesylate, USP equivalent to 1 mg, 2 mg or 4 mg of doxazosin.
- The 1 mg are available as white to off-white, round tablets debossed with M over D9 on one side of the tablet and scored on the other side. They are available as follows:
- NDC 51079-957-20 – Unit dose blister packages of 100 (10 cards of 10 tablets each).
- The 2 mg are available as pink, round tablets debossed with M over D10 on one side of the tablet and scored on the other side. They are available as follows:
- NDC 51079-958-20 – Unit dose blister packages of 100 (10 cards of 10 tablets each).
The 4 mg are available as blue, round tablets debossed with M over D11 on one side of the tablet and scored on the other side. They are available as follows:
- NDC 51079-959-20 – Unit dose blister packages of 100 (10 cards of 10 tablets each).
- PHARMACIST: Dispense a Patient Information Leaflet with each prescription.
Storage
- Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Doxazosin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Doxazosin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
For Benign Prostatic Hyperplasia
- Read this leaflet:
- Before you start taking doxazosin tablets.
- Each time you get a new prescription.
- You and your doctor should discuss this treatment and your BPH symptoms before you start taking doxazosin tablets and at your regular checkups. This leaflet does NOT take the place of discussions with your doctor.
- Doxazosin tablets are used to treat both benign prostatic hyperplasia (BPH) and high blood pressure (hypertension). This leaflet describes doxazosin tablets as treatment for BPH (although you may be taking doxazosin tablets for both your BPH and high blood pressure).
- What is BHP:
- BPH is an enlargement of the prostate gland. This gland surrounds the tube that drains the urine from the bladder. The symptoms of BPH can be caused by a tensing of the enlarged muscle in the prostate gland which blocks the passage of urine. This can lead to such symptoms as:
- Weak or start-and-stop stream when urinating.
- Delay or difficulty in the beginning of urination.
- Need to urinate often during the day and especially at night.
- Feeling that you must urinate immediately.
- Treatment options for:
- The four main treatment options for BPH are:
- If you are not bothered by your symptoms, you and your doctor may decide on a program of "watchful waiting". It is not an active treatment like taking medication or surgery but involves having regular checkups to see if your condition is getting worse or causing problems.
- Treatment with doxazosin tablets or other similar drugs. Doxazosin tablets is the medication your doctor has prescribed for you. See "What Doxazosin Tablets Do", below.
- Treatment with the medication class of 5-alpha reductase inhibitors (e.g., finasteride). It can cause the prostate to shrink. It may take 6 months or more for the full benefit of finasteride to be seen.
- What Doxazosin tablets do:
- Doxazosin tablets help relieve the symptoms of BPH (weak stream, start-and-stop stream, a feeling that your bladder is not completely empty, delay in beginning of urination, need to urinate often during the day and especially at night, and feeling that you must urinate immediately). It does not change the size of the prostate. The prostate may continue to grow; however a larger prostate is not necessarily related to more symptoms or to worse symptoms. Doxazosin tablets can decrease your symptoms and improve urinary flow, without decreasing the size of the prostate.
- If doxazosin tablets are helping you, you should notice an affect within 1 to 2 weeks after you start your medication. Doxazosin tablets have been studied in over 900 patients for up to 2 years and the drug has been shown to continue to work during long-term treatment. Even though you take doxazosin tablets and it may help you, doxazosin tablets may not prevent the need for surgery in the future.
- Other important facts:
- You should see an improvement of your symptoms within 1 to 2 weeks. In addition to your other regular checkups you will need to continue seeing your doctor regularly to check your progress regarding your BPH and to monitor your blood pressure.
- Doxazosin Mesylate is not a treatment for prostate cancer. Your doctor has prescribed doxazosin tablets for your BPH and not for prostate cancer; however, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). A higher incidence of prostate cancer has been noted in men of African-American descent. These checks should continue even if you are taking doxazosin tablets.
- How to take Doxazosin tablets and what you should know while taking Doxazosin tablets for BPH:
- Doxazosin Tablets Can Cause a Sudden Drop in Blood Pressure After the very first dose. You may feel dizzy, faint or "light-headed", especially after you stand up from a lying or sitting position. This is more likely to occur after you've taken the first few doses or if you increase your dose, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then restart treatment. If you feel very dizzy, faint or "light-headed" you should contact your doctor. Your doctor will discuss with you how often you need to visit and how often your blood pressure should be checked.
- Your blood pressure should be checked when you start taking doxazosin tablets even if you do not have high blood pressure (hypertension). Your doctor will discuss with you the details of how blood pressure is measured.
- Blood Pressure Measurement: Whatever equipment is used, it is usual for your blood pressure to be measured in the following way: measure your blood pressure after lying quietly on your back for five minutes. Then, after standing for two minutes measure your blood pressure again. Your doctor will discuss with you what other times during the day your blood pressure should be taken, such as two to six hours after a dose, before bedtime or after waking up in the morning. Note that moderate to high-intensity exercise can, over a period of time, lower your average blood pressure.
- You can take doxazosin tablets either in the morning or at bedtime and it will be equally effective. If you take doxazosin tablets at bedtime but need to get up from bed to go to the bathroom, get up slowly and cautiously until you are sure how the medication affects you. It is important to get up slowly from a chair or bed at any time until you learn how you react to doxazosin tablets.
- You should not drive or do any hazardous tasks until you are used to the effects of the medication. If you begin to feel dizzy, sit or lie down until you feel better.
- You will start with a 1 mg dose of doxazosin tablets once daily. Then the once daily dose will be increased as your body gets used to the effects of the medication. Follow your doctor's instructions about how to take doxazosin tablets. You must take it every day at the dose prescribed.
- Talk with your doctor if you don't take it for a few days for some reason; you may then need to restart the medication at a 1 mg dose. Increase your dose gradually and again be cautious about possible dizziness. Do not share doxazosin tablets with anyone else; it was prescribed only for you.
- Other side effects you could have while taking doxazosin tablets, in addition to lowering of the blood pressure, include dizziness, fatigue (tiredness), swelling of the feet and shortness of breath. Most side effects are mild. However, you should discuss any unexpected effects you notice with your doctor.
- Warning: Extremely rarely, doxazosin tablets and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
- Tell your surgeon if you take or have taken doxazosin tablets if you plan to have surgery for cataracts (clouding of the eye). During cataract surgery, a condition called Intraoperative Floppy Iris Syndrome (IFIS) can happen if you take or have taken doxazosin tablets.
- If you use doxazosin tablets with an oral erectile dysfunction medicine (phosphodiesterase-5 (PDE-5) inhibitor), it can cause a sudden drop in your blood pressure and you can become dizzy or faint. Talk with your healthcare provider before using PDE-5 inhibitors.
- Keep doxazosin tablets and all medicines out of the reach of children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. FOR MORE INFORMATION ABOUT DOXAZOSIN TABLETS AND BPH TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
Precautions with Alcohol
Alcohol-Doxazosin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cardura
- Cardura XL
Look-Alike Drug Names
- Cardura - Coumadin
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Doxazosin |Pill Name=51079-0957-20 RXNAVIMAGE10 15340AA0.jpg |Drug Name=Doxazosin |Pill Ingred=* Anhydrous lactose
- Silicone dioxide
- Magnesium stearate
- Cellulose, Microcrystalline
- Sodium lauryl sulfate
- Sodium starch glycolate type A potato|+sep=;
|Pill Imprint=M; D9 |Pill Dosage=1 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=51079-957
}}
{{#subobject:
|Page Name=Doxazosin |Pill Name=Doxazosin pill 2.jpg |Drug Name=Doxazosin |Pill Ingred=* Anhydrous lactose
- Silicone dioxide
- Magnesium stearate
- Cellulose, Microcrystalline
- Sodium lauryl sulfate
- Sodium starch glycolate type A potato
- D&C red no. 30|+sep=;
|Pill Imprint=M; D9 |Pill Dosage=1 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=51079-958
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET - 1.jpg
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-5.jpg
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-2.jpg
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-3.jpg
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-6.jpg
}}
{{#subobject:
|Label Page=Doxazosin |Label Name=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-4.jpg
}}
{{LabelImage |fileName=DOXAZOSIN (DOXAZOSIN MESYLATE) TABLET-7.jpg