c-Raf
| V-raf-1 murine leukemia viral oncogene homolog 1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1c1y. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | RAF1 ; CRAF; Raf-1; c-Raf | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 48145 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
c-raf is gene that codes for a protein kinase. That protein is sometimes called c-Raf and will be called "Raf-1" here. The Raf-1 protein functions in the MAPK/ERK signal transduction pathway as part of a protein kinase cascade. Raf-1 is a serine/threonine-specific kinase (EC 2.7.11.1). Raf-1 is a MAP kinase kinase kinase (MAP3K) which functions downstream of the Ras family of membrane associated GTPases to which it binds directly. Once activated Raf-1 can phosphorylate to activate the dual specificity protein kinases MEK1 and MEK2 which in turn phosphorylate to activate the serine/threonine specific protein kinases ERK1 and ERK2. Activated ERKs are pleiotropic effectors of cell physiology and play an important role in the control of gene expression involved in the cell division cycle, apoptosis, cell differentiation and cell migration. [Contributed text][1]
Discovery and role in cancer
The first raf gene that was found was the oncogene v-raf.[2] Normal (non-oncogenic) cellular homologs of v-raf were soon found to be conserved components of eukaryotic genomes and it was shown that they could mutate and become oncogenes.[3] A-Raf (Online Mendelian Inheritance in Man (OMIM) 311010) and B-Raf (Online Mendelian Inheritance in Man (OMIM) 164757) are two protein kinases with similar sequences to Raf-1. Mutations in B-Raf genes are found in several types of cancer. The Raf kinases are targets for anticancer drug development.[4]
Regulation of Raf kinase activity
Raf-1 was shown to bind efficiently to Ras only when Ras is bound to GTP, not GDP.[5] In the MAPK/ERK pathway Raf-1 becomes activated when it binds to Ras.[6] It is thought that phosphorylation of Raf-1 (at sites such as serine-338) upon binding of Raf-1 to Ras locks Raf-1 into an activated conformation that is then independent of binding to Ras for the continued activity of Raf-1.[7] Several MAPK kinase kinase kinases have been suggested to be important for phosphorylation of Raf-1 as well as positive feedback phosphorylation by MAPK (ERK).[8]
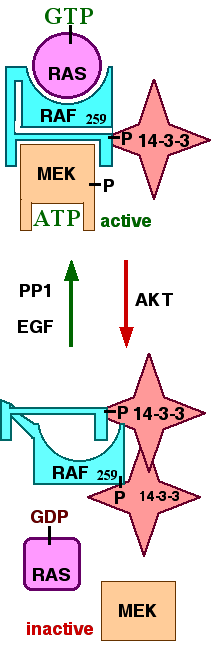
Binding of 14-3-3ζ to phosphorylated serine-259 of Raf-1 is associated with inhibition of Raf-1 kinase activity. As shown in the figure (to the right), it is thought that a 14-3-3 dimer can bind to two phosphoserines of Raf-1 when it is inactive. Dephosphorylation of serine-259 has been associated with activation of Raf-1.[9] In the model shown, the binding of GTP to Ras and the dephosphorylation of serine-259 of Raf-1 allows Raf-1 to take on a conformation that allows binding of Raf-1 to Ras-GTP. This represents a conformation in which Raf-1 can phosphorylate the downstream target MEK.
Targets of Raf-1
In the MAPK/ERK pathway Raf-1 phosphorylates and activates MEK, a MAPK kinase.[10] This allows Raf-1 to function as part of a kinase cascade: Raf-1 phosphorylates MEK which phosphorylates MAPK (see MAPK/ERK pathway).
See also
- Sorafenib - a Raf inhibitor
External links
- Domain structure diagrams for Raf-1, A-Raf and B-Raf.
- c-raf+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- ↑ "Entrez Gene: RAF1 v-raf-1 murine leukemia viral oncogene homolog 1".
- ↑ G. E. Mark and U. R. Rapp (1984) "Primary structure of v-raf: relatedness to the src family of oncogenes" in Science Volume 224, pages 285-289. Template:Entrez Pubmed
- ↑ K. Shimizu, Y. Nakatsu, S. Nomoto and M. Sekiguchi. (1986) "Structure of the activated c-raf-1 gene from human stomach cancer" in Int. Symp. Princess Takamatsu Cancer Res. Fund Volume 17, pages 85-91. Template:Entrez Pubmed
- ↑ S. S. Sridhar, D. Hedley and L. L. Siu (2005) "Raf kinase as a target for anticancer therapeutics" in Molecular cancer therapeutics Volume 4, pages 677-685. Template:Entrez Pubmed
- ↑ X. F. Zhang, J. Settleman, J. M. Kyriakis, E. Takeuchi-Suzuki, S. J. Elledge, M. S. Marshall, J. T. Bruder, U. R. Rapp and J. Avruch (1993) "Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1" in Nature Volume 364, pages 308-313.Template:Entrez Pubmed
- ↑ K. Terai and M. Matsuda (2005) "Ras binding opens c-Raf to expose the docking site for mitogen-activated protein kinase kinase" in EMBO reports Volume 6, page 251-255. Template:Entrez Pubmed
- ↑ J. Avruch, A. Khokhlatchev, J. M. Kyriakis, Z. Luo, G. Tzivion, D. Vavvas X. F. Zhang (2001) "Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade" in Recent Progress in Hormone Research Volume 56, pages 127-155.Template:Entrez Pubmed
- ↑ V. Balan, D. T. Leicht, J. Zhu, K. Balan, A. Kaplun, V. Singh-Gupta, J. Qin, H. Ruan, M. J. Comb and G. Tzivion (2006) "Identification of novel in vivo Raf-1 phosphorylation sites mediating positive feedback Raf-1 regulation by extracellular signal-regulated kinase" in Molecular biology of the cell Volume 17, pages 1141-1153. Template:Entrez Pubmed
- ↑ P. Rodriguez-Viciana, J. Oses-Prieto, A. Burlingame, M. Fried and F. McCormick (2006) "A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity" Molecular Cell Volume 22, pages 217-230. Template:Entrez Pubmed
- ↑ J. M. Kyriakis, H. App, X. F. Zhang, P. Banerjee, D. L. Brautigan, U. R. Rapp and J. Avruch (1992) "Raf-1 activates MAP kinase-kinase" in Nature Volume 358, pages 417-421.Template:Entrez Pubmed
Further reading
- Li P, Wood K, Mamon H; et al. (1991). "Raf-1: a kinase currently without a cause but not lacking in effects". Cell. 64 (3): 479–82. PMID 1846778.
- Reed JC, Zha H, Aime-Sempe C; et al. (1997). "Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death". Adv. Exp. Med. Biol. 406: 99–112. PMID 8910675.
- Geyer M, Fackler OT, Peterlin BM (2001). "Structure--function relationships in HIV-1 Nef". EMBO Rep. 2 (7): 580–5. doi:10.1093/embo-reports/kve141. PMID 11463741.
- Dhillon AS, Kolch W (2002). "Untying the regulation of the Raf-1 kinase". Arch. Biochem. Biophys. 404 (1): 3–9. PMID 12127063.
- Greenway AL, Holloway G, McPhee DA; et al. (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. PMID 12734410.
- Chen H, Kunnimalaiyaan M, Van Gompel JJ (2006). "Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1". Thyroid. 15 (6): 511–21. doi:10.1089/thy.2005.15.511. PMID 16029117.