MAPK/ERK pathway
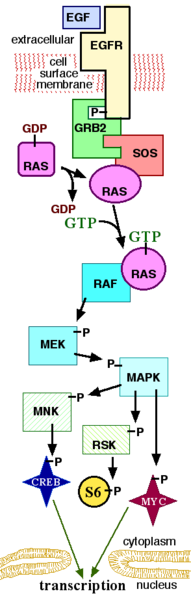
The MAPK/ERK pathway is a signal transduction pathway that couples intracellular responses to the binding of growth factors to cell surface receptors. This pathway is very complex and includes many protein components [1]. The basic pathway shown in the figure (to the right) and described below includes the major components of the pathway. In many cell types, activation of this pathway promotes cell division.
Coupling cell surface receptors to G proteins
Receptor-linked tyrosine kinases such as the epidermal growth factor receptor (EGFR) are activated by extracellular ligands. Binding of epidermal growth factor (EGF) to the EGFR activates the tyrosine kinase activity of the cytoplasmic domain of the receptor. The EGFR becomes phosphorylated on tyrosines. Docking proteins such as GRB2 contain SH2 domains that bind to the phosphotyrosines of the activated receptor [2]. GRB2 binds to the guanine nucleotide exchange factor SOS by way of an SH3 domain of GRB2. When the GRB2-SOS complex docks to phosphorylated EGFR, SOS becomes activated [3]. Activated SOS promotes the removal of GDP from Ras. Ras can then bind GTP and become active. Other small G proteins can be activated in a similar way, but are not discussed further here.
Kinase cascade
Activated Ras activates the protein kinase activity of RAF kinase [4], a serine/threonine-selective protein kinase. RAF kinase phosphorylates and activates MEK, another serine/threonine kinase. MEK phosphorylates and activates mitogen-activated protein kinase (MAPK).
Technically, RAF, MEK and MAPK are all mitogen-activated kinases, as is MNK (see below). MAPK was originally called "extracellular signal-regulated kinase" (ERK) and microtubule-associated protein kinase (MAPK). One of the first proteins known to be phosphorylated by ERK was a microtubule-associated protein. As discussed below, many additional targets for phosphorylation by MAPK were later found and the protein was re-named "mitogen-activated protein kinase" (MAPK). The series of kinases from RAF to MEK to MAPK is an example of a protein kinase cascade. Such series of kinases provide opportunities for feedback regulation and signal amplification.
Regulation of translation and transcription
Three of the many proteins that are phosphorylated by MAPK are shown in the Figure. One effect of MAPK activation is to alter the translation of mRNA to proteins. MAPK phosphorylates 40S ribosomal protein S6 kinase (RSK). This activates RSK which in turn phosphorylates ribosomal protein S6 [5]. Mitogen-activated protein kinases that phosphorylate ribosomal protein S6 were the first to be isolated [4].
MAPK regulates the activities of several transcription factors. MAPK can phosphorylate C-myc. MAPK phosphorylates and activates MNK which in turn phosphorylates CREB. MAPK also regulates the transcription of the Fos gene. By altering the levels and activities of transcription factors, MAPK leads to altered transcription of genes that are important for the cell cycle.
See also
External links
- Kyoto Encyclopedia of Genes and Genomes - MAPK pathway
- MAP+Kinase+Signaling+System at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- ↑ R. J. Orton, O. E. Sturm, V. Vyshemirsky, M. Calder, D. R. Gilbert and W. Kolch (2005) "Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway" in The Biochemical journal Volume 392, pages 249-261. Review.Template:Entrez Pubmed.
- ↑ W. X. Schulze, L. Deng and M. Mann (2005) "Phosphotyrosine interactome of the ErbB-receptor kinase family" in Molecular systems biology Volume 1, May 25.Template:Entrez Pubmed.
- ↑ N. Zarich, J. L. Oliva, N. Martinez, R. Jorge, A. Ballester, S. Gutierrez-Eisman, S. Garcia-Vargas and J. N. Rojas (2006) "Grb2 Is a Negative Modulator of the Intrinsic Ras-GEF Activity of hSos1" in Molecular Biology of the Cell Epub ahead of print.Template:Entrez Pubmed.
- ↑ 4.0 4.1 J. Avruch, A. Khokhlatchev, J. M. Kyriakis, Z. Luo, G. Tzivion, D. Vavvas and X. F. Zhang (2001) "Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade" in Recent Progress in Hormone Research Volume 56, pages 127-155. Template:Entrez Pubmed.
- ↑ M. Pende, S. H. Um, V. Mieulet, M. Sticker, V. L. Goss, J. Mestan, M. Mueller, S. Fumagalli, S. C. Kozma and G. Thomas.(2004) "S6K1,(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway" in Molecular and Cellular Biology Volume 24, pages 3112-3124. Template:Entrez Pubmed.