Ras (protein)
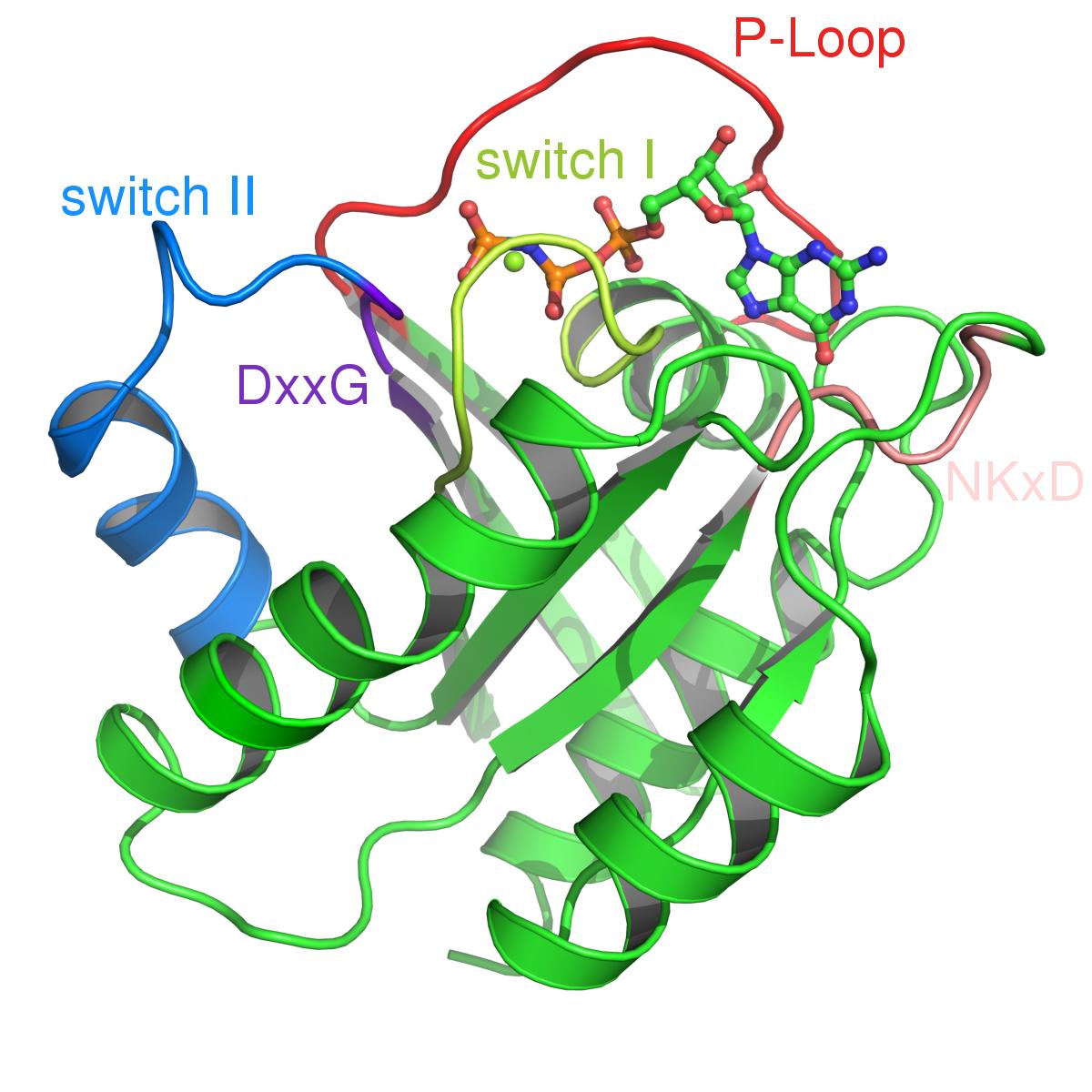 Triphosphate Structure of H-Ras p21 GNBP in complex with GppNHp and Mg2+ (PDB Code 5p21). Important sequence elements are highlighted. | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Ras | ||||||||
| Pfam | PF00071 | ||||||||
| InterPro | IPR013753 | ||||||||
| PROSITE | PDOC00859 | ||||||||
| SCOP | 5p21 | ||||||||
| SUPERFAMILY | 5p21 | ||||||||
| OPM protein | 1uad | ||||||||
| |||||||||
|
WikiDoc Resources for Ras (protein) |
|
Articles |
|---|
|
Most recent articles on Ras (protein) Most cited articles on Ras (protein) |
|
Media |
|
Powerpoint slides on Ras (protein) |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ras (protein) at Clinical Trials.gov Trial results on Ras (protein) Clinical Trials on Ras (protein) at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ras (protein) NICE Guidance on Ras (protein)
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ras (protein) Discussion groups on Ras (protein) Patient Handouts on Ras (protein) Directions to Hospitals Treating Ras (protein) Risk calculators and risk factors for Ras (protein)
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ras (protein) |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
In molecular biology, Ras is the name of a protein, the gene that encodes it, and the family and superfamily (see Ras superfamily) of proteins to which it belongs. The ras oncogene is a signal transduction protein, which means that it communicates signals to other cells. Sometimes a DNA mutation turns the signal permanently on, which leads to unlimited cell growth and cancer.[1] The Ras superfamily of small GTPases includes the Ras, Rho, Arf, Rab, and Ran families.
History
The RAS genes were first identified as the transforming oncogenes, responsible for the cancer-causing activities of the Harvey (the HRAS oncogene) and Kirsten (KRAS) sarcoma viruses, by Edward M. Scolnick and colleagues at the National Institutes of Health (NIH). These viruses were discovered originally in rats during the 1960's by Jennifer Harvey and Werner Kirsten, respectively. In 1982, activated and transforming human RAS genes were discovered in human cancer cells by Geoffrey M. Cooper at Harvard, Mariano Barbacid and Stuart A. Aaronson at the NIH and by Robert A. Weinberg of MIT. Subsequent studies identified a third human RAS gene, designated NRAS, for its initial identification in human neuroblastoma cells.
Functions
The three human RAS genes encode highly related 188 to 189 amino acid proteins, designated H-Ras, N-Ras and K-Ras4A and K-Ras4B (the two K-Ras proteins arise from alternative gene splicing). Ras proteins function as binary molecular switches that control intracellular signaling networks. Ras-regulated signal pathways control such processes as actin cytoskeletal integrity, proliferation, differentiation, cell adhesion, apoptosis, and cell migration.
Ras and ras-related proteins are often deregulated in cancers, leading to increased invasion and metastasis, and decreased apoptosis.
Ras activates a number of pathways but an especially important one seems to be the mitogen-activated protein (MAP) kinases, which themselves transmit signals downstream to other protein kinases and gene regulatory proteins.[2]
Activated and inactivated forms
Ras is a G protein (specifically a small GTPase): a regulatory GTP hydrolase that cycles between two conformations – an activated or inactivated form, respectively RAS-GTP and RAS-GDP.
It is activated by guanine exchange factors (GEFs, eg. CDC25, SOS1 and SOS2, SDC25 in yeast), which are themselves activated by mitogenic signals and through feedback from Ras itself. A GEF usually heightens the dissociation rate of the nucleotide – while not changing the association rate (effectively lower the affinity of the nucleotide) – thereby promoting its exchange. The cellular concentration of GTP is much higher than that of GDP so the exchange is usually GDP vs. GTP.
It is inactivated by GTPase-activating proteins (GAPs, the most frequently cited one being RasGAP), which increase the rate of GTP hydrolysis, returning RAS to its GDP-bound form, simultaneously releasing an inorganic phosphate.
Attachments
Ras is attached to the cell membrane by prenylation, and in health is a key component in many pathways which couple growth factor receptors to downstream mitogenic effectors involved in cell proliferation or differentiation.[3] The C-terminal CaaX box of Ras first gets farnesylated at its Cys residue in the cytosol and then inserted into the membrane of the endoplasmatic reticulum. The Tripeptid (aaX) is then cleaved from the C-terminus by a specific prenyl-protein specific endoprotease, the new C-terminus is then methylated by a methyltransferase. The so processed Ras is now transported to the plasma membrane. Most Ras forms are now further palmityolated, while K-Ras with its long positively charged stretch interacts electrostaticly with the membrane.
Ras in cancer
Mutations in the Ras family of proto-oncogenes (comprising H-Ras, N-Ras and K-Ras) are very common, being found in 20% to 30% of all human tumours.[4]
Inappropriate activation of the gene
Inappropriate activation of the gene has been shown to play a key role in signal transduction, proliferation and malignant transformation.[2]
Mutations in a number of different genes as well as RAS itself can have this effect. Oncogenes such as p210BCR-ABL or the growth receptor erbB are upstream of Ras, so if they are constitutively activated their signals will transduce through Ras.
The tumour suppressor gene NF1 encodes a Ras-GAP – its mutation in neurofibromatosis will mean that Ras is less likely to be inactivated. Ras can also be amplified, although this only occurs occasionally in tumours.
Finally, Ras oncogenes can be activated by point mutations so that its GTPase reaction can no longer be stimulated by GAP – this increases the half life of active Ras-GTP mutants.[3]
Constitutively active Ras
Constitutively active Ras (RasD) is one which contains mutations that prevent GTP hydrolysis, thus locking Ras in a permanently 'On' state.
The most common mutations are found at residue G12 in the P-loop and the catalytic residue Q61.
- The glycine to valine mutation at residue 12 renders the GTPase domain of Ras insensitive to inactivation by GAP and thus stuck in the "on state". Ras requires a GAP for inactivation as it is a relatively poor catalyst on its own, as opposed to other G-domain-containing proteins such as the alpha subunit of heterotrimeric G proteins.
- Residue 61[5] is responsible for stabilizing the transition state for GTP hydrolysis. Because enzyme catalysis in general is achieved by lowering the energy barrier between substrate and product, mutation of Q61 necessarily reduces the rate of intrinsic Ras GTP hydrolysis to physiologically meaningless levels.
See also "dominant negative" mutants such as S17N and D119N.
Human proteins containing Ras domain
ARHE; ARHGAP5; CDC42; DIRAS1; DIRAS2; DIRAS3; ERAS; GEM; GRLF1; HRAS; KRAS; LOC393004; MRAS; NKIRAS1; NRAS; RAB10; RAB11A; RAB11B; RAB12; RAB13; RAB14; RAB15; RAB17; RAB18; RAB19; RAB1A; RAB1B; RAB2; RAB20; RAB21; RAB22A; RAB23; RAB24; RAB25; RAB26; RAB27A; RAB27B; RAB28; RAB2B; RAB30; RAB31; RAB32; RAB33A; RAB33B; RAB34; RAB35; RAB36; RAB37; RAB38; RAB39; RAB39B; RAB3A; RAB3B; RAB3C; RAB3D; RAB40A; RAB40AL; RAB40B; RAB40C; RAB41; RAB42; RAB43; RAB4A; RAB4B; RAB5A; RAB5B; RAB5C; RAB6A; RAB6B; RAB6C; RAB7A; RAB7B; RAB7L1; RAB8A; RAB8B; RAB9; RAB9B; RABL2A; RABL2B; RABL4; RAC1; RAC2; RAC3; RALA; RALB; RAN; RANP1; RAP1A; RAP1B; RAP2A; RAP2B; RAP2C; RASD1; RASD2; RASEF; RASL11A; RASL12; RBJ; REM1; REM2; RERG; RHEB; RHEBL1; RHOA; RHOB; RHOBTB1; RHOBTB2; RHOC; RHOD; RHOF; RHOG; RHOH; RHOJ; RHOQ; RHOU; RHOV; RIT1; RIT2; RND1; RND2; RND3; RRAD; RRAS; RRAS2; TC4;
References
- ↑ David S. Goodsell, Physician Education: The Molecular Perspective: The ras Oncogene, The Oncologist, Vol. 4, No. 3, 263-264, June 1999 Introductory article on molecular biology of ras oncogene for physicians. Illustrated. Full text free.
- ↑ 2.0 2.1 Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000). "Chapter 25, Cancer". Molecular cell biology (4th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-3706-X.
- ↑ 3.0 3.1 Reuter C, Morgan M, Bergmann L (2000). "Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies?". Blood. 96 (5): 1655–69. PMID 10961860.
- ↑ Bos J (1989). "ras oncogenes in human cancer: a review". Cancer Res. 49 (17): 4682–9. PMID 2547513.
- ↑ Omim - Neuroblastoma Ras Viral Oncogene Homolog; Nras
External links
- "Brain tumour findings offer hope of new strategy Canadian Cancer Society says" at ncic.cancer.ca
- "Novel cancer treatment gets NCI support" at arstechnica.com
- ras+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- ras+Genes at the US National Library of Medicine Medical Subject Headings (MeSH)