Langerhans cell histiocytosis differential diagnosis: Difference between revisions
Ahmed Younes (talk | contribs) No edit summary |
Ahmed Younes (talk | contribs) |
||
| Line 320: | Line 320: | ||
| style="padding: 7px 7px; background: #F5F5F5;" |The disease usually manifests in the skeleton and solitary bone lesions are encountered twice as often as multiple bone lesions.<br>The tumours can develop in any bone, but most commonly originate in the skull and jaw, followed by vertebral bodies, ribs, pelvis, and long bones.<ref name="pmid26461144">{{cite journal |vauthors=Picarsic J, Jaffe R |title=Nosology and Pathology of Langerhans Cell Histiocytosis |journal=Hematol. Oncol. Clin. North Am. |volume=29 |issue=5 |pages=799–823 |year=2015 |pmid=26461144 |doi=10.1016/j.hoc.2015.06.001 |url=}}</ref> | | style="padding: 7px 7px; background: #F5F5F5;" |The disease usually manifests in the skeleton and solitary bone lesions are encountered twice as often as multiple bone lesions.<br>The tumours can develop in any bone, but most commonly originate in the skull and jaw, followed by vertebral bodies, ribs, pelvis, and long bones.<ref name="pmid26461144">{{cite journal |vauthors=Picarsic J, Jaffe R |title=Nosology and Pathology of Langerhans Cell Histiocytosis |journal=Hematol. Oncol. Clin. North Am. |volume=29 |issue=5 |pages=799–823 |year=2015 |pmid=26461144 |doi=10.1016/j.hoc.2015.06.001 |url=}}</ref> | ||
|- | |- | ||
|} | |||
==Differential diagnosis== | |||
Langerhans cell histiocytosis must be differentiated from other cavitary lung lesions. | |||
{| class="wikitable" | |||
!Causes of | |||
lung cavities | |||
!Differentiating Features | |||
!Differentiating radiological findings | |||
!Diagnosis | |||
confirmation | |||
|- | |||
| | |||
*[[Malignancy]] ([[Lung cancer|Primary lung cance<nowiki/>r]])<ref name="pmid4353362">{{cite journal |vauthors=Chaudhuri MR |title=Primary pulmonary cavitating carcinomas |journal=Thorax |volume=28 |issue=3 |pages=354–66 |year=1973 |pmid=4353362 |pmc=470041 |doi= |url=}}</ref> | |||
| | |||
*Elderly male or female<ref name="pmid4353362">{{cite journal |vauthors=Chaudhuri MR |title=Primary pulmonary cavitating carcinomas |journal=Thorax |volume=28 |issue=3 |pages=354–66 |year=1973 |pmid=4353362 |pmc=470041 |doi= |url=}}</ref> | |||
*Chronic smokers | |||
*Presents with a [[low-grade fever]], absence of [[leukocytosis]], systemic complaints [[weight loss]], [[fatigue]] | |||
*Absence of factors that predispose to [[gastric content aspiration]], no response to [[antibiotics]] within 10 days | |||
*[[Hemoptysis]] is commonly associated with [[bronchogenic carcinoma]] | |||
| | |||
*A coin-shaped lesion with thick wall(>15mm) is seen on CXR with less ground glass opacities<ref name="pmid8572761">{{cite journal |vauthors=Mouroux J, Padovani B, Elkaïm D, Richelme H |title=Should cavitated bronchopulmonary cancers be considered a separate entity? |journal=Ann. Thorac. Surg. |volume=61 |issue=2 |pages=530–2 |year=1996 |pmid=8572761 |doi=10.1016/0003-4975(95)00973-6 |url=}}</ref> <ref name="pmid16183941">{{cite journal |vauthors=Onn A, Choe DH, Herbst RS, Correa AM, Munden RF, Truong MT, Vaporciyan AA, Isobe T, Gilcrease MZ, Marom EM |title=Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome |journal=Radiology |volume=237 |issue=1 |pages=342–7 |year=2005 |pmid=16183941 |doi=10.1148/radiol.2371041650 |url=}}</ref> | |||
*[[Bronchoalveolar lavage]] [[cytology]] shows malignant cells | |||
| | |||
*[[Biopsy]] of lung | |||
|- | |||
| | |||
*Pulmonary [[Tuberculosis, pulmonary|Tuberculosis]] | |||
| | |||
*Mostly in endemic areas | |||
*Symptoms include [[productive cough]], [[night sweats]], [[fever]], and [[weight loss]] | |||
| | |||
*CXR and CT demonstrates [[Internal|cavities]] in the upper lobe of the lung | |||
| | |||
*[[Sputum]] smear positive for [[acid-fast bacilli]] and nucleic acid amplification tests (NAAT) is used on sputum or any sterile fluid for rapid diagnosis and is positive for mycobacteria. | |||
|- | |||
| | |||
*[[Necrotizing Pulmonary Infections|Necrotizing]] [[Pneumonia]] | |||
| | |||
*Any age group | |||
*Acute, [[fulminant]] life threating complication of prior infection | |||
*>100.4 °F fever, with [[Hemodynamically unstable|hemodynamic]] instability | |||
*Worsening [[pneumonia]]-like symptoms | |||
| | |||
*CXR demonstrates multiple cavitary lesions | |||
*[[Pleural effusion]] and [[empyema]] are common findings | |||
| | |||
*[[Complete blood count|CBC]] is positive for causative organism | |||
|- | |||
| | |||
*Loculated [[empyema]] | |||
| | |||
* Children and elderly are at risk | |||
*Pleuritic [[chest pain]], [[dry cough]], [[fever]] with chills | |||
*Dullness to [[Percussion of the lungs|percussion]] decreased [[breath sounds]], and reduced vocal resonance on examination | |||
| | |||
*[[Empyema]] appears lenticular in shape and has a thin wall with smooth luminal margins | |||
| | |||
*[[Thoracocentesis]] | |||
|- | |||
| | |||
*[[Granulomatosis with polyangiitis]] ([[Wegener's granulomatosis|Wegener's]])<ref name="pmid10377211">{{cite journal |vauthors=Langford CA, Hoffman GS |title=Rare diseases.3: Wegener's granulomatosis |journal=Thorax |volume=54 |issue=7 |pages=629–37 |year=1999 |pmid=10377211 |pmc=1745525 |doi= |url=}}</ref> | |||
| | |||
*Women are more commonly effected than man<ref name="pmid12541109">{{cite journal |vauthors=Lee KS, Kim TS, Fujimoto K, Moriya H, Watanabe H, Tateishi U, Ashizawa K, Johkoh T, Kim EA, Kwon OJ |title=Thoracic manifestation of Wegener's granulomatosis: CT findings in 30 patients |journal=Eur Radiol |volume=13 |issue=1 |pages=43–51 |year=2003 |pmid=12541109 |doi=10.1007/s00330-002-1422-2 |url=}}</ref> | |||
*Kidneys are also involved | |||
*Upper respiratory tract symptoms, perforation of [[nasal septum]], [[chronic sinusitis]], [[otitis media]], [[mastoiditis]]. | |||
*Lower respiratory tract symptoms (e.g. [[hemoptysis]], [[cough]], [[dyspnea]]) | |||
*Renal symptoms, [[hematuria]], red cell [[casts]] | |||
| | |||
*Pulmonary nodules with cavities and infiltrates are a frequent manifestation on CXR | |||
| | |||
*Positive for [[P-ANCA]] | |||
*Biopsy of the tissue involved shows necrotizing [[granulomas]]<ref name="pmid10377211">{{cite journal |vauthors=Langford CA, Hoffman GS |title=Rare diseases.3: Wegener's granulomatosis |journal=Thorax |volume=54 |issue=7 |pages=629–37 |year=1999 |pmid=10377211 |pmc=1745525 |doi= |url=}}</ref> | |||
|- | |||
| | |||
*[[Rheumatoid nodule]] | |||
| | |||
*Elderly females of 40-50 age group | |||
*Manifestation of [[rheumatoid arthritis]] | |||
*Presents with other systemic symptoms including symmetric [[arthritis]] of the small joints of the hands and feet with morning stiffness are common manifestations | |||
| | |||
*Pulmonary nodules with cavitation are located in the upper lobe ([[Caplan syndrome]]) on X-ray | |||
| | |||
*Positive for both [[rheumatoid factor]] and anticyclic citrullinated peptide [[Antibody|antibody.]] | |||
|- | |||
| | |||
*[[Sarcoidosis]] | |||
| | |||
*More common in African-American females | |||
*Often [[asymptomatic]] except for [[Lymphadenopathy|enlarged lymph nodes]]<ref name="pmid11734441">{{cite journal |vauthors=Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R |title=Clinical characteristics of patients in a case control study of sarcoidosis |journal=Am. J. Respir. Crit. Care Med. |volume=164 |issue=10 Pt 1 |pages=1885–9 |year=2001 |pmid=11734441 |doi=10.1164/ajrccm.164.10.2104046 |url=}}</ref> | |||
*Associated with [[restrictive lung disease]] | |||
*[[Erythema nodosum]] | |||
*[[Lupus pernio]] (skin lesions on face resembling lupus) | |||
*[[Bell's palsy|Bell palsy]] | |||
*[[Epithelioid]] [[granuloma]]<nowiki/>s containing microscopic [[Schaumann bodies|Schaumann]] and asteroid bodies | |||
| | |||
*On CXR bilateral [[Lymphadenopathy|adenopathy]] and coarse reticular opacities are seen | |||
*CT of the chest demonstrates extensive [[Hilar lymphadenopathy|hilar]] and mediastinal adenopathy | |||
*Additional findings on CT include [[fibrosis]] (honeycomb, linear, or associated with bronchial distortion), pleural thickening, and ground-glass opacities.<ref name="pmid2748828">{{cite journal |vauthors=Brauner MW, Grenier P, Mompoint D, Lenoir S, de Crémoux H |title=Pulmonary sarcoidosis: evaluation with high-resolution CT |journal=Radiology |volume=172 |issue=2 |pages=467–71 |year=1989 |pmid=2748828 |doi=10.1148/radiology.172.2.2748828 |url=}}</ref> | |||
| | |||
*Biopsy of lung shows non-[[caseating]] [[granuloma]] | |||
|- | |||
| | |||
*[[Bronchiolitis obliterans]] ([[Cryptogenic organizing pneumonia]])<ref name="pmid9724431">{{cite journal |vauthors=Murphy J, Schnyder P, Herold C, Flower C |title=Bronchiolitis obliterans organising pneumonia simulating bronchial carcinoma |journal=Eur Radiol |volume=8 |issue=7 |pages=1165–9 |year=1998 |pmid=9724431 |doi=10.1007/s003300050527 |url=}}</ref><ref name="pmid19561910">{{cite journal |vauthors=Al-Ghanem S, Al-Jahdali H, Bamefleh H, Khan AN |title=Bronchiolitis obliterans organizing pneumonia: pathogenesis, clinical features, imaging and therapy review |journal=Ann Thorac Med |volume=3 |issue=2 |pages=67–75 |year=2008 |pmid=19561910 |pmc=2700454 |doi=10.4103/1817-1737.39641 |url=}}</ref> | |||
| | |||
*Rare condition and mimics [[asthma]], [[pneumonia]] and [[emphysema]] | |||
*It is caused by [[drug]] or [[toxin]] exposure, [[autoimmune diseases]], [[viral infections]], or [[radiation injury]] | |||
*People working in industries are at high risk | |||
*Presents with [[Fever|feve]]<nowiki/>r, [[cough]], [[wheezing]], and [[shortness of breath]] over weeks to months<ref name="pmid2805873">{{cite journal |vauthors=Cordier JF, Loire R, Brune J |title=Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients |journal=Chest |volume=96 |issue=5 |pages=999–1004 |year=1989 |pmid=2805873 |doi= |url=}}</ref> | |||
| | |||
*Common appearance on CT is patchy [[Consolidation (medicine)|consolidation,]]<nowiki/>often accompanied by ground-glass opacities and nodules.<ref name="pmid8109493">{{cite journal |vauthors=Lee KS, Kullnig P, Hartman TE, Müller NL |title=Cryptogenic organizing pneumonia: CT findings in 43 patients |journal=AJR Am J Roentgenol |volume=162 |issue=3 |pages=543–6 |year=1994 |pmid=8109493 |doi=10.2214/ajr.162.3.8109493 |url=}}</ref> | |||
| | |||
*Biopsy of the lung<ref name="pmid19561910">{{cite journal |vauthors=Al-Ghanem S, Al-Jahdali H, Bamefleh H, Khan AN |title=Bronchiolitis obliterans organizing pneumonia: pathogenesis, clinical features, imaging and therapy review |journal=Ann Thorac Med |volume=3 |issue=2 |pages=67–75 |year=2008 |pmid=19561910 |pmc=2700454 |doi=10.4103/1817-1737.39641 |url=}}</ref> | |||
*[[Pulmonary function tests]] demonstrate low fev1/fvc | |||
|- | |||
| | |||
*[[Langerhans cell histiocytosis|Langerhans]] cell [[Langerhans cell histiocytosis|Histiocytosis]]<ref name="pmid22429393">{{cite journal |vauthors=Suri HS, Yi ES, Nowakowski GS, Vassallo R |title=Pulmonary langerhans cell histiocytosis |journal=Orphanet J Rare Dis |volume=7 |issue= |pages=16 |year=2012 |pmid=22429393 |pmc=3342091 |doi=10.1186/1750-1172-7-16 |url=}}</ref> | |||
| | |||
*Exclusively afflicts smokers, with a peak age of onset of between 20 and 40 years | |||
*Clinical presentation varies, but symptoms generally include months of dry [[cough]], [[fever]], [[night sweats]], and [[weight loss]] | |||
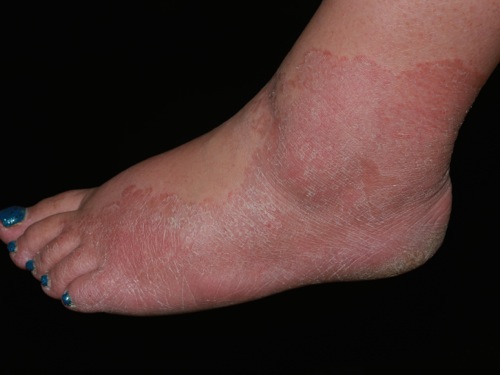
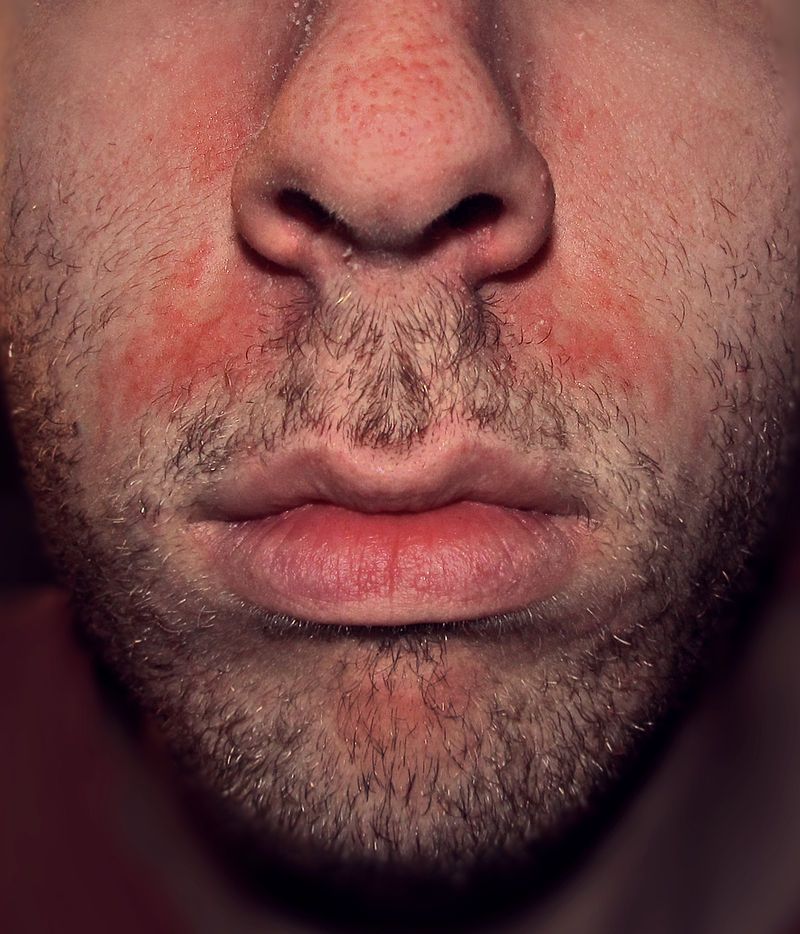
*Skin is involved in 80% of the cases, scaly [[erythematous rash]] is typical | |||
| | |||
*Thin-walled cystic cavities are the usual radiographic manifestation, observed in over 50% of patients by either CXR or CT scans.<ref name="pmid2787035">{{cite journal |vauthors=Moore AD, Godwin JD, Müller NL, Naidich DP, Hammar SP, Buschman DL, Takasugi JE, de Carvalho CR |title=Pulmonary histiocytosis X: comparison of radiographic and CT findings |journal=Radiology |volume=172 |issue=1 |pages=249–54 |year=1989 |pmid=2787035 |doi=10.1148/radiology.172.1.2787035 |url=}}</ref> | |||
| | |||
*Biopsy of the lung | |||
|} | |} | ||
Revision as of 00:02, 22 September 2017
|
Langerhans cell histiocytosis Microchapters |
|
Differentiating Langerhans cell histiocytosis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Langerhans cell histiocytosis differential diagnosis On the Web |
|
American Roentgen Ray Society Images of Langerhans cell histiocytosis differential diagnosis |
|
Langerhans cell histiocytosis differential diagnosis in the news |
|
Blogs on Langerhans cell histiocytosis differential diagnosis |
|
Directions to Hospitals Treating Langerhans cell histiocytosis |
|
Risk calculators and risk factors for Langerhans cell histiocytosis differential diagnosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Haytham Allaham, M.D. [2]
Overview
Langerhans cell histiocytosis must be differentiated from other diseases that cause bone pain, cutaneous lesions, hepatosplenomegaly, and palpable lymph nodes, such as cat-scratch disease, mastocytosis, sinus histiocytosis with massive lymphadenopathy.[1][2]
Differentiating Langerhans cell histiocytosis from other Diseases
- Langerhans cell histiocytosis must be differentiated from other diseases that cause bone pain, cutaneous lesions, hepatosplenomegaly, and palpable lymph nodes, such as:[1][2]
- Lymphoma
- Mastocytosis
- Cat-scratch disease
- Hypersensitivity reaction
- Acrodermatitis enteropathica
- Erdheim-Chester disease
- Wiskott-Aldrich syndrome
- Seborrheic dermatitis
- Sinus histiocytosis with massive lymphadenopathy
- Kimura disease
- Eosinophilic pustular folliculitis
- Erythema toxicum neonatorum
- Acropustulosis of infancy
| Disease | Rash Characteristics | Signs and Symptoms | Associated Conditions | Images |
|---|---|---|---|---|
| Cutaneous T cell lymphoma/Mycosis fungoides[3] |
|
|
||
| Pityriasis rosea[4] |
|
|
||
| Pityriasis lichenoides chronica |
|
|
||
| Nummular dermatitis[7] |
|
|
|
|
| Secondary syphilis[8] |
|
|||
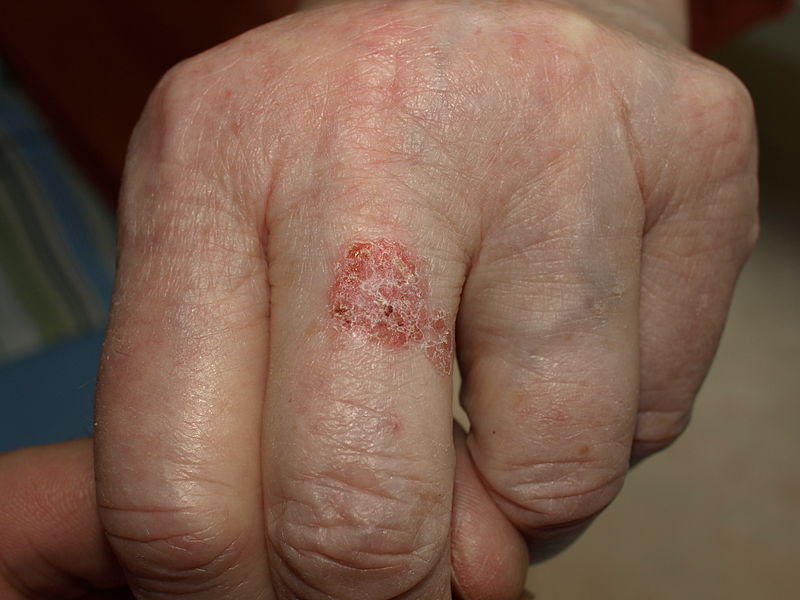
| Bowen’s disease[9] |
|
|
||
| Exanthematous pustulosis[11] |
|
|
||
| Hypertrophic lichen planus[13] |
|
|
|
|
| Sneddon–Wilkinson disease[15] |
|
|
||
| Small plaque parapsoriasis[19] |
|
|
|
|
| Intertrigo[21] |
|
|
||
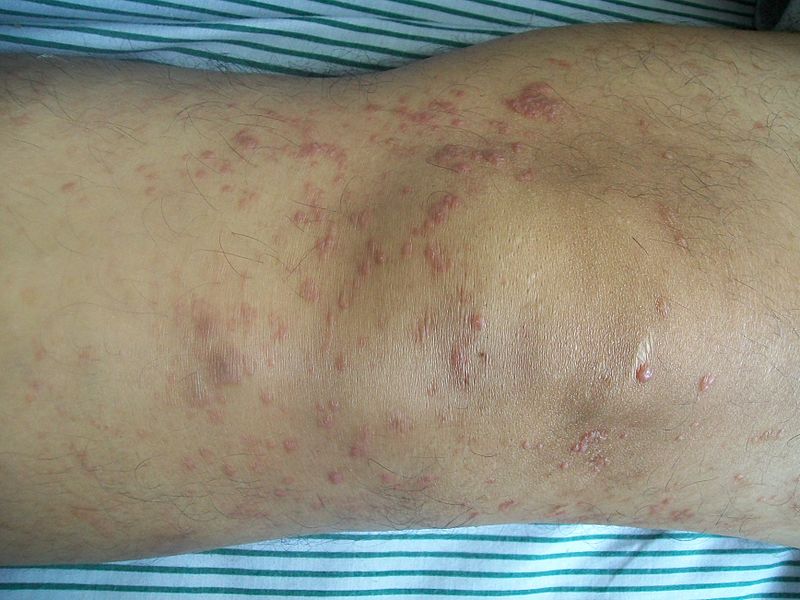
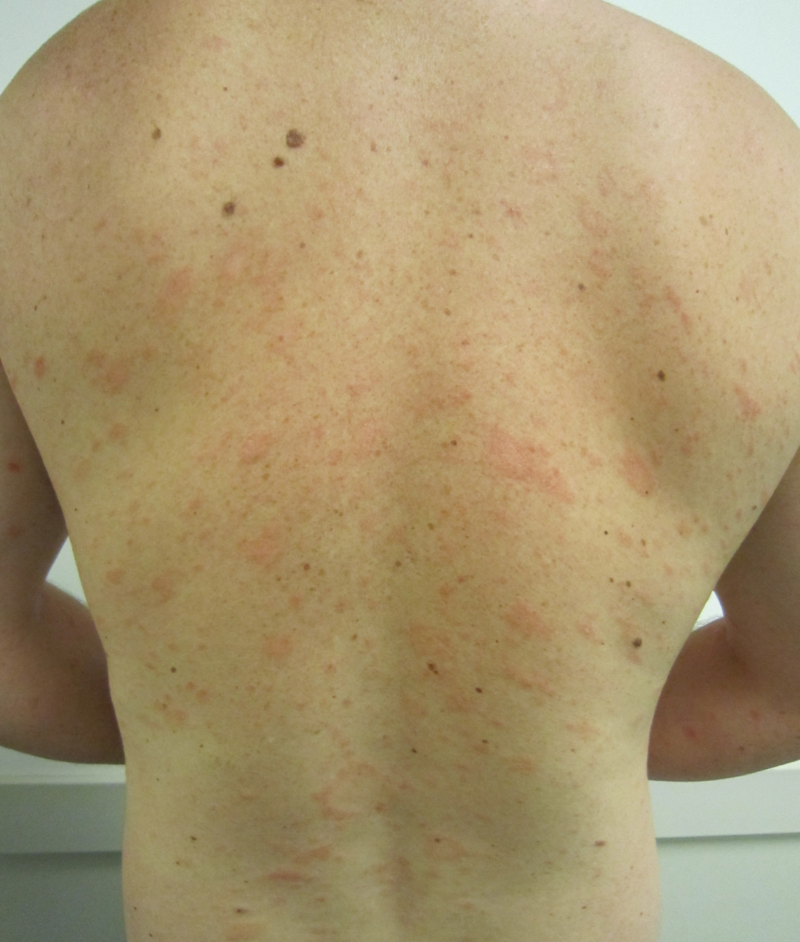
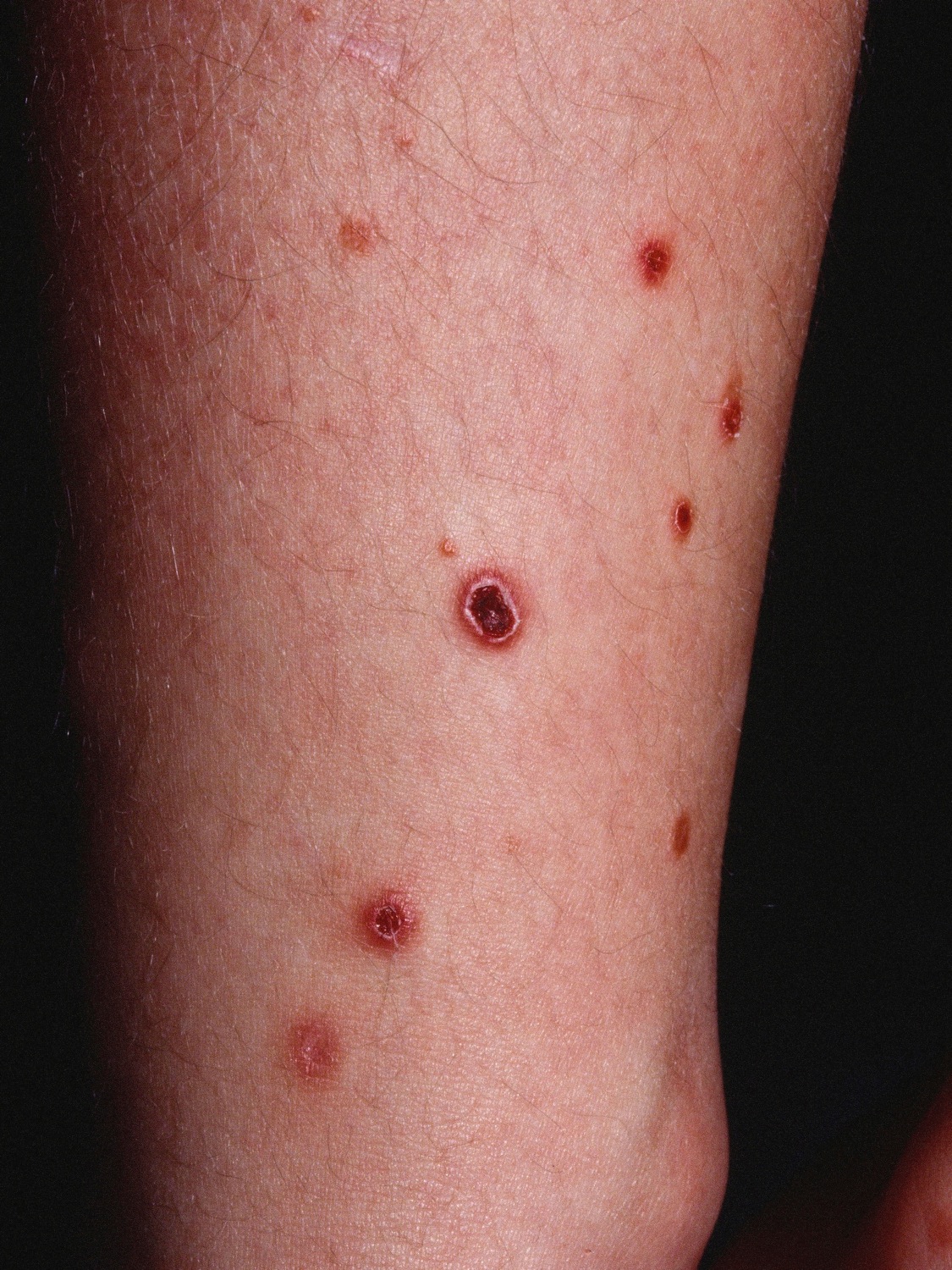
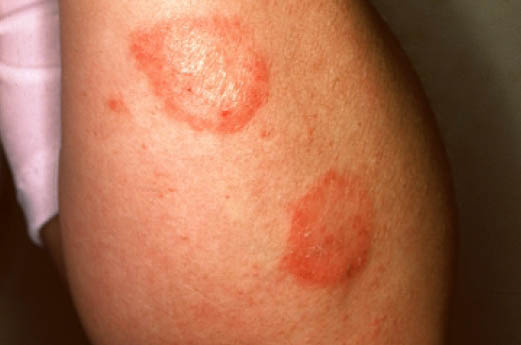
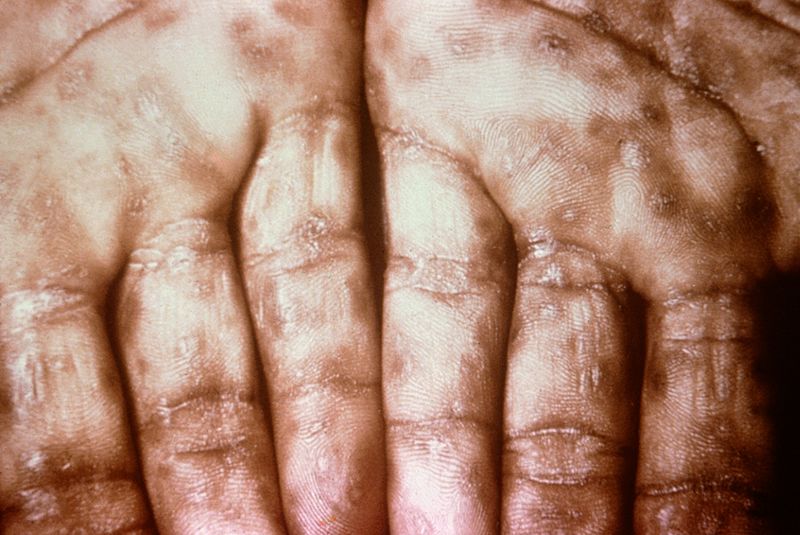
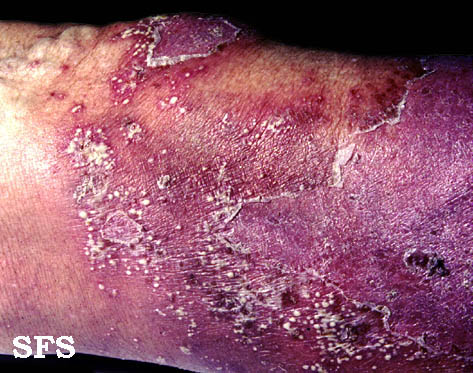
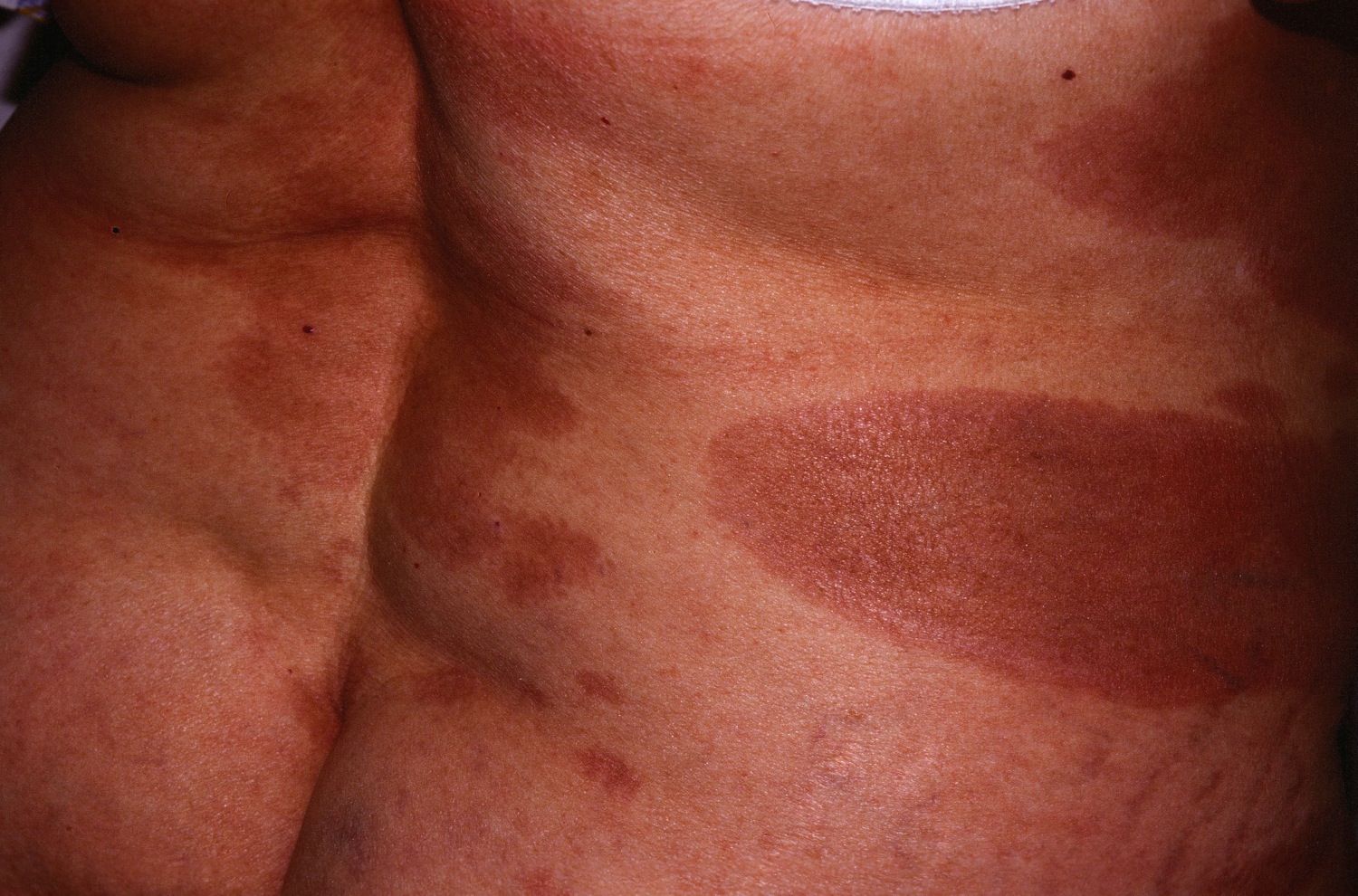
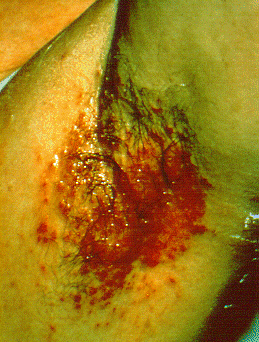
| Langerhans cell histiocytosis[22] |
|
|
|
|
| Tinea manuum/pedum/capitis[26] |
|
|
|
|
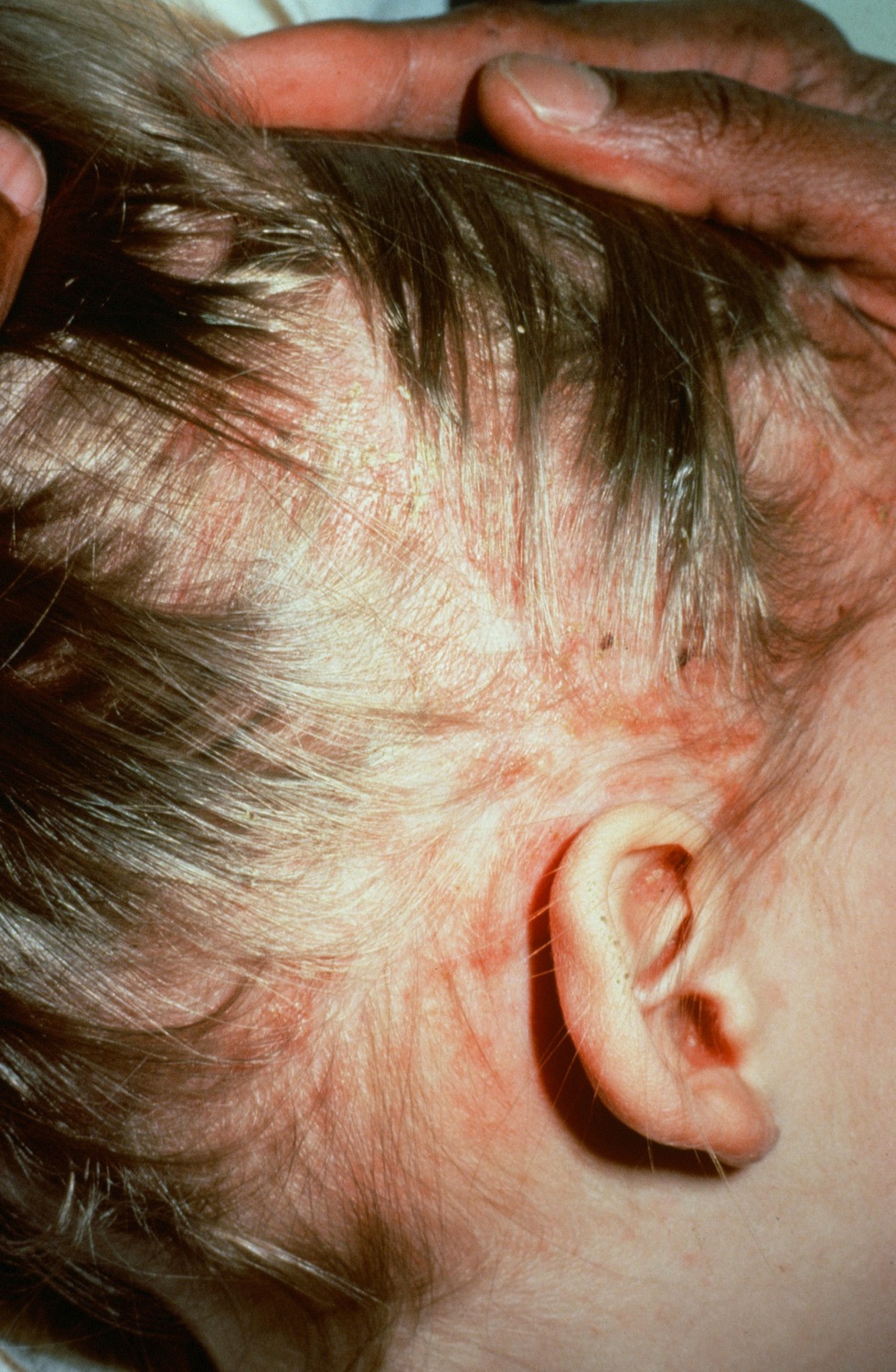
| Seborrheic dermatitis |
|
|
Langerhans cell histiocytosis must be differentiated from other diseases that cause bone pain, edema, and erythema.
| Disease | Findings |
|---|---|
| Soft tissue infection (Commonly cellulitis) |
History of skin warmness, swelling and erythema. Bone probing is the definite way to differentiate them.[29][30] |
| Osteonecrosis (Avascular necrosis of bone) |
Previous history of trauma, radiation, use of steroids or biphosphonates are suggestive to differentiate osteonecrosis from ostemyelitis.[31][32] MRI is diagnostic.[33][34] |
| Charcot joint | Patients with Charcot joint commonly develop skin ulcerations that can in turn lead to secondary osteomyelitis. Contrast-enhanced MRI may be diagnostically useful if it shows a sinus tract, replacement of soft tissue fat, a fluid collection, or extensive marrow abnormalities. Bone biopsy is the definitive diagnostic modality.[35] |
| Bone tumors | May present with local pain and radiographic changes consistent with osteomyelitis. Tumors most likely to mimic osteomyelitis are osteoid osteomas and chondroblastomas that produce small, round, radiolucent lesions on radiographs.[36] |
| Gout | Gout presents with joint pain and swelling. Joint aspiration and crystals in synovial fluid is diagnostic for gout.[37] |
| SAPHO syndrome (Synovitis, acne, pustulosis, hyperostosis, and osteitis) |
SAPHO syndrome consists of a wide spectrum of neutrophilic dermatosis associated with aseptic osteoarticular lesions. It can mimic osteomyelitis in patients who lack the characteristic findings of pustulosis and synovitis. The diagnosis is established via clinical manifestations; bone culture is sterile in the setting of osteitis. |
| Sarcoidosis | It involves most frequently the pulmonary parenchyma and mediastinal lymph nodes, but any organ system can be affected. Bone involvement is often bilateral and bones commonly affected include the middle and distal phalanges (producing “sausage finger”), wrist, skull, vertebral column, and long bones. |
| Langerhans' cell histiocytosis | The disease usually manifests in the skeleton and solitary bone lesions are encountered twice as often as multiple bone lesions. The tumours can develop in any bone, but most commonly originate in the skull and jaw, followed by vertebral bodies, ribs, pelvis, and long bones.[38] |
Differential diagnosis
Langerhans cell histiocytosis must be differentiated from other cavitary lung lesions.
| Causes of
lung cavities |
Differentiating Features | Differentiating radiological findings | Diagnosis
confirmation |
|---|---|---|---|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
| |
|
|
||
|
|
| |
|
|
|
References
- ↑ 1.0 1.1 Langerhans cell histiocytosis. PathologyOutlines (2015) http://www.pathologyoutlines.com/topic/lymphnodesLCH.html Accessed on February, 4 2016
- ↑ 2.0 2.1 Langerhans Cell Histiocytosis. National Organization for Rare Disoders (2015) http://rarediseases.org/rare-diseases/langerhans-cell-histiocytosis/ Accessed on February, 4 2016
- ↑ "Mycosis Fungoides and the Sézary Syndrome Treatment (PDQ®)—Patient Version - National Cancer Institute".
- ↑ Mahajan K, Relhan V, Relhan AK, Garg VK (2016). "Pityriasis Rosea: An Update on Etiopathogenesis and Management of Difficult Aspects". Indian J Dermatol. 61 (4): 375–84. doi:10.4103/0019-5154.185699. PMC 4966395. PMID 27512182.
- ↑ Prantsidis A, Rigopoulos D, Papatheodorou G, Menounos P, Gregoriou S, Alexiou-Mousatou I, Katsambas A (2009). "Detection of human herpesvirus 8 in the skin of patients with pityriasis rosea". Acta Derm. Venereol. 89 (6): 604–6. doi:10.2340/00015555-0703. PMID 19997691.
- ↑ Smith KJ, Nelson A, Skelton H, Yeager J, Wagner KF (1997). "Pityriasis lichenoides et varioliformis acuta in HIV-1+ patients: a marker of early stage disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR)". Int. J. Dermatol. 36 (2): 104–9. PMID 9109005.
- ↑ Jiamton S, Tangjaturonrusamee C, Kulthanan K (2013). "Clinical features and aggravating factors in nummular eczema in Thais". Asian Pac. J. Allergy Immunol. 31 (1): 36–42. PMID 23517392.
- ↑ "STD Facts - Syphilis".
- ↑ Neagu TP, Ţigliş M, Botezatu D, Enache V, Cobilinschi CO, Vâlcea-Precup MS, GrinŢescu IM (2017). "Clinical, histological and therapeutic features of Bowen's disease". Rom J Morphol Embryol. 58 (1): 33–40. PMID 28523295.
- ↑ Murao K, Yoshioka R, Kubo Y (2014). "Human papillomavirus infection in Bowen disease: negative p53 expression, not p16(INK4a) overexpression, is correlated with human papillomavirus-associated Bowen disease". J. Dermatol. 41 (10): 878–84. doi:10.1111/1346-8138.12613. PMID 25201325.
- ↑ Szatkowski J, Schwartz RA (2015). "Acute generalized exanthematous pustulosis (AGEP): A review and update". J. Am. Acad. Dermatol. 73 (5): 843–8. doi:10.1016/j.jaad.2015.07.017. PMID 26354880.
- ↑ Schmid S, Kuechler PC, Britschgi M, Steiner UC, Yawalkar N, Limat A, Baltensperger K, Braathen L, Pichler WJ (2002). "Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation". Am. J. Pathol. 161 (6): 2079–86. doi:10.1016/S0002-9440(10)64486-0. PMC 1850901. PMID 12466124.
- ↑ Ankad BS, Beergouder SL (2016). "Hypertrophic lichen planus versus prurigo nodularis: a dermoscopic perspective". Dermatol Pract Concept. 6 (2): 9–15. doi:10.5826/dpc.0602a03. PMC 4866621. PMID 27222766.
- ↑ Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W (2009). "Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis". Arch Dermatol. 145 (9): 1040–7. doi:10.1001/archdermatol.2009.200. PMID 19770446.
- ↑ Lutz ME, Daoud MS, McEvoy MT, Gibson LE (1998). "Subcorneal pustular dermatosis: a clinical study of ten patients". Cutis. 61 (4): 203–8. PMID 9564592.
- ↑ Kasha EE, Epinette WW (1988). "Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) in association with a monoclonal IgA gammopathy: a report and review of the literature". J. Am. Acad. Dermatol. 19 (5 Pt 1): 854–8. PMID 3056995.
- ↑ Delaporte E, Colombel JF, Nguyen-Mailfer C, Piette F, Cortot A, Bergoend H (1992). "Subcorneal pustular dermatosis in a patient with Crohn's disease". Acta Derm. Venereol. 72 (4): 301–2. PMID 1357895.
- ↑ Sauder MB, Glassman SJ (2013). "Palmoplantar subcorneal pustular dermatosis following adalimumab therapy for rheumatoid arthritis". Int. J. Dermatol. 52 (5): 624–8. doi:10.1111/j.1365-4632.2012.05707.x. PMID 23489057.
- ↑ Lambert WC, Everett MA (1981). "The nosology of parapsoriasis". J. Am. Acad. Dermatol. 5 (4): 373–95. PMID 7026622.
- ↑ Väkevä L, Sarna S, Vaalasti A, Pukkala E, Kariniemi AL, Ranki A (2005). "A retrospective study of the probability of the evolution of parapsoriasis en plaques into mycosis fungoides". Acta Derm. Venereol. 85 (4): 318–23. doi:10.1080/00015550510030087. PMID 16191852.
- ↑ Janniger CK, Schwartz RA, Szepietowski JC, Reich A (2005). "Intertrigo and common secondary skin infections". Am Fam Physician. 72 (5): 833–8. PMID 16156342.
- ↑ Satter EK, High WA (2008). "Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society". Pediatr Dermatol. 25 (3): 291–5. doi:10.1111/j.1525-1470.2008.00669.x. PMID 18577030.
- ↑ Stull MA, Kransdorf MJ, Devaney KO (1992). "Langerhans cell histiocytosis of bone". Radiographics. 12 (4): 801–23. doi:10.1148/radiographics.12.4.1636041. PMID 1636041.
- ↑ Sholl LM, Hornick JL, Pinkus JL, Pinkus GS, Padera RF (2007). "Immunohistochemical analysis of langerin in langerhans cell histiocytosis and pulmonary inflammatory and infectious diseases". Am. J. Surg. Pathol. 31 (6): 947–52. doi:10.1097/01.pas.0000249443.82971.bb. PMID 17527085.
- ↑ Grois N, Pötschger U, Prosch H, Minkov M, Arico M, Braier J, Henter JI, Janka-Schaub G, Ladisch S, Ritter J, Steiner M, Unger E, Gadner H (2006). "Risk factors for diabetes insipidus in langerhans cell histiocytosis". Pediatr Blood Cancer. 46 (2): 228–33. doi:10.1002/pbc.20425. PMID 16047354.
- ↑ Al Hasan M, Fitzgerald SM, Saoudian M, Krishnaswamy G (2004). "Dermatology for the practicing allergist: Tinea pedis and its complications". Clin Mol Allergy. 2 (1): 5. doi:10.1186/1476-7961-2-5. PMC 419368. PMID 15050029.
- ↑ Schwartz RA, Janusz CA, Janniger CK (2006). "Seborrheic dermatitis: an overview". Am Fam Physician. 74 (1): 125–30. PMID 16848386.
- ↑ Misery L, Touboul S, Vinçot C, Dutray S, Rolland-Jacob G, Consoli SG, Farcet Y, Feton-Danou N, Cardinaud F, Callot V, De La Chapelle C, Pomey-Rey D, Consoli SM (2007). "[Stress and seborrheic dermatitis]". Ann Dermatol Venereol (in French). 134 (11): 833–7. PMID 18033062.
- ↑ Bisno AL, Stevens DL (1996). "Streptococcal infections of skin and soft tissues". N. Engl. J. Med. 334 (4): 240–5. doi:10.1056/NEJM199601253340407. PMID 8532002.
- ↑ Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC (2014). "Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America". Clin. Infect. Dis. 59 (2): 147–59. doi:10.1093/cid/ciu296. PMID 24947530.
- ↑ Shigemura T, Nakamura J, Kishida S, Harada Y, Ohtori S, Kamikawa K, Ochiai N, Takahashi K (2011). "Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study". Rheumatology (Oxford). 50 (11): 2023–8. doi:10.1093/rheumatology/ker277. PMID 21865285.
- ↑ Slobogean GP, Sprague SA, Scott T, Bhandari M (2015). "Complications following young femoral neck fractures". Injury. 46 (3): 484–91. doi:10.1016/j.injury.2014.10.010. PMID 25480307.
- ↑ Amanatullah DF, Strauss EJ, Di Cesare PE (2011). "Current management options for osteonecrosis of the femoral head: part 1, diagnosis and nonoperative management". Am J. Orthop. 40 (9): E186–92. PMID 22022684.
- ↑ Etienne G, Mont MA, Ragland PS (2004). "The diagnosis and treatment of nontraumatic osteonecrosis of the femoral head". Instr Course Lect. 53: 67–85. PMID 15116601.
- ↑ Ahmadi ME, Morrison WB, Carrino JA, Schweitzer ME, Raikin SM, Ledermann HP (2006). "Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR imaging characteristics". Radiology. 238 (2): 622–31. doi:10.1148/radiol.2382041393. PMID 16436821.
- ↑ Lovell, Wood (2014). Lovell and Winter's pediatric orthopaedics. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-1605478142.
- ↑ Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ, Kanneganti TD, van der Meer JW (2010). "Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis". Arthritis Rheum. 62 (11): 3237–48. doi:10.1002/art.27667. PMC 2970687. PMID 20662061.
- ↑ Picarsic J, Jaffe R (2015). "Nosology and Pathology of Langerhans Cell Histiocytosis". Hematol. Oncol. Clin. North Am. 29 (5): 799–823. doi:10.1016/j.hoc.2015.06.001. PMID 26461144.
- ↑ 39.0 39.1 Chaudhuri MR (1973). "Primary pulmonary cavitating carcinomas". Thorax. 28 (3): 354–66. PMC 470041. PMID 4353362.
- ↑ Mouroux J, Padovani B, Elkaïm D, Richelme H (1996). "Should cavitated bronchopulmonary cancers be considered a separate entity?". Ann. Thorac. Surg. 61 (2): 530–2. doi:10.1016/0003-4975(95)00973-6. PMID 8572761.
- ↑ Onn A, Choe DH, Herbst RS, Correa AM, Munden RF, Truong MT, Vaporciyan AA, Isobe T, Gilcrease MZ, Marom EM (2005). "Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome". Radiology. 237 (1): 342–7. doi:10.1148/radiol.2371041650. PMID 16183941.
- ↑ 42.0 42.1 Langford CA, Hoffman GS (1999). "Rare diseases.3: Wegener's granulomatosis". Thorax. 54 (7): 629–37. PMC 1745525. PMID 10377211.
- ↑ Lee KS, Kim TS, Fujimoto K, Moriya H, Watanabe H, Tateishi U, Ashizawa K, Johkoh T, Kim EA, Kwon OJ (2003). "Thoracic manifestation of Wegener's granulomatosis: CT findings in 30 patients". Eur Radiol. 13 (1): 43–51. doi:10.1007/s00330-002-1422-2. PMID 12541109.
- ↑ Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R (2001). "Clinical characteristics of patients in a case control study of sarcoidosis". Am. J. Respir. Crit. Care Med. 164 (10 Pt 1): 1885–9. doi:10.1164/ajrccm.164.10.2104046. PMID 11734441.
- ↑ Brauner MW, Grenier P, Mompoint D, Lenoir S, de Crémoux H (1989). "Pulmonary sarcoidosis: evaluation with high-resolution CT". Radiology. 172 (2): 467–71. doi:10.1148/radiology.172.2.2748828. PMID 2748828.
- ↑ Murphy J, Schnyder P, Herold C, Flower C (1998). "Bronchiolitis obliterans organising pneumonia simulating bronchial carcinoma". Eur Radiol. 8 (7): 1165–9. doi:10.1007/s003300050527. PMID 9724431.
- ↑ 47.0 47.1 Al-Ghanem S, Al-Jahdali H, Bamefleh H, Khan AN (2008). "Bronchiolitis obliterans organizing pneumonia: pathogenesis, clinical features, imaging and therapy review". Ann Thorac Med. 3 (2): 67–75. doi:10.4103/1817-1737.39641. PMC 2700454. PMID 19561910.
- ↑ Cordier JF, Loire R, Brune J (1989). "Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients". Chest. 96 (5): 999–1004. PMID 2805873.
- ↑ Lee KS, Kullnig P, Hartman TE, Müller NL (1994). "Cryptogenic organizing pneumonia: CT findings in 43 patients". AJR Am J Roentgenol. 162 (3): 543–6. doi:10.2214/ajr.162.3.8109493. PMID 8109493.
- ↑ Suri HS, Yi ES, Nowakowski GS, Vassallo R (2012). "Pulmonary langerhans cell histiocytosis". Orphanet J Rare Dis. 7: 16. doi:10.1186/1750-1172-7-16. PMC 3342091. PMID 22429393.
- ↑ Moore AD, Godwin JD, Müller NL, Naidich DP, Hammar SP, Buschman DL, Takasugi JE, de Carvalho CR (1989). "Pulmonary histiocytosis X: comparison of radiographic and CT findings". Radiology. 172 (1): 249–54. doi:10.1148/radiology.172.1.2787035. PMID 2787035.