Restrictive lung disease
|
Restrictive Lung Disease Microchapters |
|
Differentiating Restrictive Lung Disease from other Diseases |
|---|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aditya Ganti M.B.B.S. [2], Akshun Kalia M.B.B.S.[3], Usama Talib, BSc, MD [4], Cafer Zorkun, M.D., Ph.D. [5]
Overview
Restrictive lung disease (RLD) is a group of diseases characterized by an inability to attain complete expansion of the lungs. This may result from an abnormality in the parenchyma of the lungs or an abnormality outside the lung parenchyma (such as fluid accumulation or musculoskeletal abnormalities) hindering normal lung expansion and thus ability to ventilate normally. Restrictive lung diseases presents with a restrictive pattern on pulmonary function test and includes a decrease in total lung capacity (TLC), residual volume (RV), forced vital capacity (FVC), forced expiatory volume (FEV1), and a normal to increased FEV1/FVC ratio. Unlike obstructive lung disease (characterized by air trapping within the lungs), restrictive lung diseases result in decreased lung volumes and a lower than normal amount of air within the lungs. RLDs include acute respiratory distress syndrome, hypersensitivity pneumonitis, occupational lung diseases, pleural effusion, interstitial lung disease, sarcoidosis, and neuromuscular diseases such as scoliosis, muscular dystrophy, amyotropic lateral sclerosis (ALS), and myasthenia gravis.
Classification
Various diseases that present with a restrictive pattern on pulmonary function tests include:
- Acute respiratory distress syndrome
- Hypersensitivity pneumonitis
- Occupational lung diseases
- Pleural Effusion
- Interstitial lung disease
- Sarcoidosis
- Neuromuscular diseases
Spirometry Findings in Various Lung Conditions
Spirometry can help distinguish restrictive lung disease from obstructive lung diseases. On spirometry the findings include:[1][2]
| Pulmonary Function Tests (PFT) | Obstructive Lung Disease | Restrictive Lung Disease | 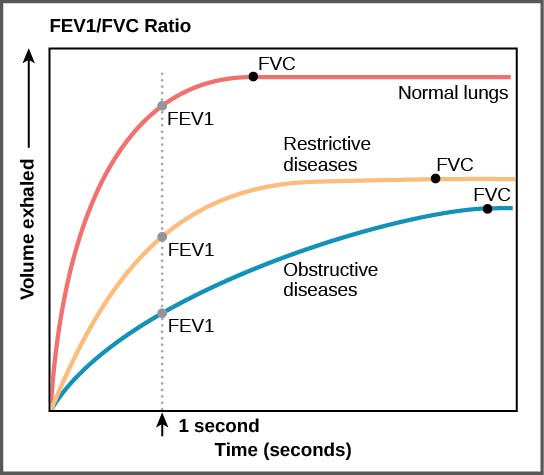 |
|---|---|---|---|
| Total lung capacity (TLC) | ↑ | ↓ | |
| Residual volume (RV) | ↑ | ↓ | |
| Forced vital capacity (FVC) | ↓ | ↓ | |
| Forced expiratory volume
in 1st second (FEV1) |
↓↓ | ↓ | |
| FEV1/FVC ratio | ↓ | N to ↑ | |
| Maximum voluntary ventilation | ↓ | ↓ |
Approach to Lung Disorders
| Spirometry | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low FEV1/FVC ratio | Normal to high FEV1/FVC ratio | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obstructive Lung Disease | Restrictive Lung Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bronchodilator therapy | DLCO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased FEV1 | No change in FEV1 | Normal DLCO | Decreased DLCO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asthma | COPD | Chest wall disorders | Interstitial Lung Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Differentiating Restrictive Lung Disease from other Diseases
Restrictive lung disease must be differentiated from other diseases that cause dyspnea, cough, hemoptysis, and fever such as ARDS, hypersensitivity pneumonitis, pneumoconiosis, sarcoidosis, pleural effusion, interstitial lung disease (ILD), lymphocytic interstitial pneumonia, obesity, pulmonary eosinophilia, and neuromuscular disorders.
| Disease | Clinical manifestations | Diagnosis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| History | Symptoms | Physical exam | Lab findings | PFT | Imaging | Gold standard | |||||||||||
| History/Exposure | Dyspnea | Cough | Hemoptysis | Fever | Cyanosis | Clubbing | JVD | Peripheral edema | Auscultation | Other prominent findings | DLCO | CXR | CT | ||||
| Acute Respiratory Distress Syndrome (ARDS)[3] | + | +/– | – | – | +/– | – | +/– | – |
|
|
|
↓ |
|
|
| ||
| Hypersensitivity Pneumonitis[4] |
|
+ | + | – | + | – | + | – | – |
|
|
|
↓ |
|
|
| |
| Pneumoconiosis[6] | Silicosis[7][8] | Occupational history
|
+ | + | +/– | – | + | + | + | – |
|
|
|
↓ |
|
|
|
| Asbestosis[9] |
|
+ | + | +/– | – | + | + | + | – |
|
|
↓ |
|
| |||
| Berylliosis[10] |
|
+ | + | +/– | – | + | + | + | – |
|
– |
|
↓ |
|
| ||
| Byssinosis [11] |
|
+ | + | +/– | – | + | + | + | – |
|
|
|
↓ |
|
| ||
| Disease | Clinical manifestations | Diagnosis | |||||||||||||||
| History | Symptoms | Physical exam | Lab findings | PFT | Imaging | Gold standard | |||||||||||
| History/Exposure | Dyspnea | Cough | Hemoptysis | Fever | Cyanosis | Clubbing | JVD | Peripheral edema | Auscultation | Other prominent findings | DLCO | CXR | CT | ||||
| Sarcoidosis
(stage 2–5)[12] |
|
+ | + | + | + | – | – | – | – |
|
|
|
↓ |
|
|
||
| Pleural Effusion | Transudate
Exudate |
+ | + | +/– | +/– | +/– | +/– | +/– | +/– |
|
|
|
NL | Supine:
Lateral decubitus:
|
|
||
| Interstitial lung disease[14] | ++ | + | + | – | +/– | +/– | +/– | +/– |
|
Depending on the underlying cause: | ↓ |
|
|
| |||
| Lymphocytic Interstitial Pneumonia[15] | + | + | + | + | – | + | – | – |
|
|
NL |
|
|
| |||
| Obesity[16][17] | + | + | – | – | – | – | – | + | – | NL |
|
|
| ||||
| Pulmonary Eosinophilia[18] | Infections | + | + | + | + | + | – | + | + |
|
|
↓ |
|
|
| ||
| Disease | Clinical manifestations | Diagnosis | |||||||||||||||
| History | Symptoms | Physical exam | Lab findings | PFT | Imaging | Gold standard | |||||||||||
| History/Exposure | Dyspnea | Cough | Hemoptysis | Fever | Cyanosis | Clubbing | JVD | Peripheral edema | Auscultation | Other prominent findings | DLCO | CXR | CT | ||||
| Neuromuscular diseases[19] | Scoliosis[20] |
|
+ | – | – | – | – | – | – | – |
|
|
|
NL |
|
|
|
| Muscular dystrophy |
|
+ | – | – | – | – | – | – | – |
|
NL |
|
|
||||
| ALS[21] |
|
+ | – | – | – | – | – | – | – |
|
|
|
NL |
|
|
| |
| Myasthenia gravis[22] |
|
+ | – | – | + | – | – | – | – |
|
|
|
NL |
|
|
||
References
- ↑ Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (November 2005). "Interpretative strategies for lung function tests". Eur. Respir. J. 26 (5): 948–68. doi:10.1183/09031936.05.00035205. PMID 16264058.
- ↑ Mehrparvar AH, Sakhvidi MJ, Mostaghaci M, Davari MH, Hashemi SH, Zare Z (2014). "Spirometry values for detecting a restrictive pattern in occupational health settings". Tanaffos. 13 (2): 27–34. PMC 4260070. PMID 25506373.
- ↑ Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H (June 2013). "Acute respiratory distress syndrome: new definition, current and future therapeutic options". J Thorac Dis. 5 (3): 326–34. doi:10.3978/j.issn.2072-1439.2013.04.05. PMC 3698298. PMID 23825769.
- ↑ Spagnolo P, Rossi G, Cavazza A, Bonifazi M, Paladini I, Bonella F, Sverzellati N, Costabel U (2015). "Hypersensitivity Pneumonitis: A Comprehensive Review". J Investig Allergol Clin Immunol. 25 (4): 237–50, quiz follow 250. PMID 26310038.
- ↑ Yi ES (November 2002). "Hypersensitivity pneumonitis". Crit Rev Clin Lab Sci. 39 (6): 581–629. doi:10.1080/10408360290795583. PMID 12484500.
- ↑ Gay SE, Kazerooni EA, Toews GB, Lynch JP, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, Popovich J, Hyzy R, Martinez FJ (1998). "Idiopathic pulmonary fibrosis: predicting response to therapy and survival". Am. J. Respir. Crit. Care Med. 157 (4 Pt 1): 1063–72. doi:10.1164/ajrccm.157.4.9703022. PMID 9563720.
- ↑ du Bois RM (2006). "Evolving concepts in the early and accurate diagnosis of idiopathic pulmonary fibrosis". Clin. Chest Med. 27 (1 Suppl 1): S17–25, v–vi. doi:10.1016/j.ccm.2005.08.001. PMID 16545629.
- ↑ Neghab M, Mohraz MH, Hassanzadeh J (2011). "Symptoms of respiratory disease and lung functional impairment associated with occupational inhalation exposure to carbon black dust". J Occup Health. 53 (6): 432–8. PMID 21996929.
- ↑ Billings CG, Howard P (April 2000). "Asbestos exposure, lung cancer and asbestosis". Monaldi Arch Chest Dis. 55 (2): 151–6. PMID 10949878.
- ↑ Sood A (December 2009). "Current treatment of chronic beryllium disease". J Occup Environ Hyg. 6 (12): 762–5. doi:10.1080/15459620903158698. PMC 2774897. PMID 19894178.
- ↑ McL Niven R, Pickering CA (June 1996). "Byssinosis: a review". Thorax. 51 (6): 632–7. PMC 1090498. PMID 8693449.
- ↑ Carmona EM, Kalra S, Ryu JH (July 2016). "Pulmonary Sarcoidosis: Diagnosis and Treatment". Mayo Clin. Proc. 91 (7): 946–54. doi:10.1016/j.mayocp.2016.03.004. PMID 27378039.
- ↑ Iannuzzi MC, Rybicki BA, Teirstein AS (November 2007). "Sarcoidosis". N. Engl. J. Med. 357 (21): 2153–65. doi:10.1056/NEJMra071714. PMID 18032765.
- ↑ Boros PW, Franczuk M, Wesolowski S (2004). "Value of spirometry in detecting volume restriction in interstitial lung disease patients. Spirometry in interstitial lung diseases". Respiration. 71 (4): 374–9. doi:10.1159/000079642. PMID 15316211.
- ↑ Honda O, Johkoh T, Ichikado K, Tomiyama N, Maeda M, Mihara N, Higashi M, Hamada S, Naito H, Yamamoto S, Nakamura H (1999). "Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT". AJR Am J Roentgenol. 173 (1): 71–4. doi:10.2214/ajr.173.1.10397102. PMID 10397102.
- ↑ Zammit C, Liddicoat H, Moonsie I, Makker H (2010). "Obesity and respiratory diseases". Int J Gen Med. 3: 335–43. doi:10.2147/IJGM.S11926. PMC 2990395. PMID 21116339.
- ↑ O’Neill, Donal (2015). "Measuring obesity in the absence of a gold standard". Economics & Human Biology. 17: 116–128. doi:10.1016/j.ehb.2015.02.002. ISSN 1570-677X.
- ↑ de Górgolas M, Casado V, Renedo G, Alen JF, Fernández Guerrero ML (2009). "Nodular lung schistosomiais lesions after chemotherapy for dysgerminoma". Am. J. Trop. Med. Hyg. 81 (3): 424–7. PMID 19706907.
- ↑ Polkey MI, Lyall RA, Moxham J, Leigh PN (January 1999). "Respiratory aspects of neurological disease". J. Neurol. Neurosurg. Psychiatry. 66 (1): 5–15. PMC 1736177. PMID 9886443.
- ↑ Bowen RE, Scaduto AA, Banuelos S (September 2008). "Decreased body mass index and restrictive lung disease in congenital thoracic scoliosis". J Pediatr Orthop. 28 (6): 665–8. doi:10.1097/BPO.0b013e3181841ffd. PMID 18724205.
- ↑ Vitacca M, Clini E, Facchetti D, Pagani M, Poloni M, Porta R, Ambrosino N (July 1997). "Breathing pattern and respiratory mechanics in patients with amyotrophic lateral sclerosis". Eur. Respir. J. 10 (7): 1614–21. PMID 9230256.
- ↑ Roy TM, Walker JF, Farrow JR (April 1991). "Respiratory failure associated with myasthenia gravis". J Ky Med Assoc. 89 (4): 169–73. PMID 2040830.