Nabilone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 229: | Line 229: | ||
*[[Lack of effect]] | *[[Lack of effect]] | ||
*[[Face edema]] | *[[Face edema]] | ||
|drugInteractions=Potential interactions between Cesamet 2 mg, and [[diazepam]] 5 mg; [[sodium secobarbital]] 100 mg; [[alcohol]] 45 mL (absolute laboratory alcohol); or [[codeine]] 65 mg, were evaluated in 15 subjects. Only a single combination was utilized at any one time. The subjects were evaluated according to physiologic (i.e., [[heart rate]] and [[blood pressure]]), psychometric, psychomotor, and subjective parameters. In this study, as expected, the depressant effects of the combinations were additive. Psychomotor function was particularly impaired with concurrent use of [[diazepam]]. Caution must thus be used when administering nabilone in combination with any [[CNS depressant]]. | |||
Nabilone is purportedly highly bound to [[plasma proteins]], and therefore, might displace other protein-bound drugs. Therefore, practitioners should monitor patients for a change in dosage requirements when administering nabilone to patients receiving other highly protein-bound drugs. Published reports of drug-drug interactions involving cannabinoids are summarized in the following table. | |||
[[file:Nabilone Drug interactions.png|none|450px]] | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Teratology studies conducted in pregnant rats at doses up to 12 mg/kg/day (about 16 times the human dose on a body surface area basis) and in pregnant rabbits at doses up to 3.3 mg/kg/day (about 9 times the human dose on a body surface area basis) did not disclose any evidence for a teratogenic potential of nabilone. However, there was dose related developmental toxicity in both species as evidenced by increases in embryo lethality, fetal resorptions, decreased fetal weights and pregnancy disruptions. In rats, postnatal developmental toxicity was also observed. There are no adequate and well-controlled studies in pregnant women. Because animal studies cannot rule out the possibility of harm, Cesamet should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
|useInNursing=It is not known whether this drug is excreted in breast milk. Because many drugs including some cannabinoids are excreted in breast milk it is not recommended that Cesamet be given to nursing mothers. | |||
|useInPed=Safety and effectiveness have not been established in patients younger than 18 years of age. Caution is recommended in prescribing Cesamet to children because of psychoactive effects. | |||
|useInGeri=Clinical studies of Cesamet did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Cesamet should be used with caution in elderly patients aged 65 and over because they are generally more sensitive to the psychoactive effects of drugs and Cesamet can elevate supine and standing heart rates and cause postural hypotension. | |||
|overdose======Signs and Symptoms===== | |||
Signs and symptoms of overdosage are an extension of the psychotomimetic and physiologic effects of Cesamet. | |||
=====Treatment===== | |||
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient. | |||
Overdosage may be considered to have occurred, even at prescribed dosages, if disturbing psychiatric symptoms are present. In these cases, the patient should be observed in a quiet environment and supportive measures, including reassurance, should be used. Subsequent doses should be withheld until patients have returned to their baseline mental status; routine dosing may then be resumed if clinically indicated. In such instances, a lower initiating dose is suggested. In controlled clinical trials, alterations in mental status related to the use of Cesamet resolved within 72 hours without specific medical therapy. In overdose settings, attention should be paid to vital signs, since both hypertension and hypotension have been known to occur; tachycardia and orthostatic hypotension were most commonly reported. | |||
No cases of overdosage with more than 10 mg/day of nabilone were reported during clinical trials. Signs and symptoms that would be expected to occur in large overdose situations are psychotic episodes, including hallucinations, anxiety reactions, respiratory depression, and coma. If psychotic episodes occur, the patient should be managed conservatively, if possible. For moderate psychotic episodes and anxiety reactions, verbal support and comforting may be sufficient. In more severe cases, antipsychotic drugs may be useful; however, the utility of antipsychotic drugs in cannabinoid psychosis has not been systematically evaluated. Support for their use is drawn from limited experience using antipsychotic agents to manage cannabis overdoses. Because of the potential for drug-drug interactions (e.g., additive CNS depressant effects due to nabilone and chlorpromazine), such patients should be closely monitored. | |||
Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, as well as other laboratory values and physical assessments. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal. | |||
The use of forced diuresis, peritoneal dialysis, hemodialysis, charcoal hemoperfusion, or cholestyramine has not been reported. In the presence of normal renal function, most of a dose of nabilone is eliminated through the biliary system. Treatment for respiratory depression and comatose state consists in symptomatic and supportive therapy. Particular attention should be paid to the occurrence of hypothermia. If the patient becomes hypotensive, consider fluids, inotropes, and/or vasopressors. The estimated oral median lethal dose in female mice is between 1,000 and 2,000 mg/kg; in the female rat, it is greater than 2,000 mg/kg, | |||
|alcohol=Alcohol-Nabilone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Nabilone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 14:44, 19 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nabilone is a Cannabinoid and antihemetic that is FDA approved for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Common adverse reactions include hypotension, xerostomia, asthenia, ataxia, dyssomnia, headache, poor concentration, somnolence, vertigo, visual disturbance, dysphoric mood and euphoria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Nausea and Vomiting in Cancer Therapy
The usual adult dosage is 1 or 2 mg 2 times a day. On the day of chemotherapy, the initial dose should be given 1 to 3 hours before the chemotherapeutic agent is administered. To minimize side effects, it is recommended that the lower starting dose be used and that the dose be increased as necessary. A dose of 1 or 2 mg the night before may be useful. The maximum recommended daily dose is 6 mg given in divided doses 3 times a day.
Cesamet may be administered 2 or 3 times a day during the entire course of each cycle of chemotherapy and, if needed, for 48 hours after the last dose of each cycle of chemotherapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nabilone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nabilone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nabilone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nabilone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nabilone in pediatric patients.
Contraindications
Cesamet is contraindicated in any patient who has a history of hypersensitivity to any cannabinoid.
Warnings
- The effects of Cesamet may persist for a variable and unpredictable period of time following its oral administration. *Adverse psychiatric reactions can persist for 48 to 72 hours following cessation of treatment.
- Cesamet has the potential to affect the CNS, which might manifest itself in dizziness, drowsiness, euphoria “high”, ataxia, anxiety, disorientation, depression, hallucinations and psychosis.
- Cesamet can cause tachycardia and orthostatic hypotension.
- Because of individual variation in response and tolerance to the effects of Cesamet, patients should remain under supervision of a responsible adult especially during initial use of Cesamet and during dose adjustments.
- Patients receiving treatment with Cesamet should be specifically warned not to drive, operate machinery, or engage in any hazardous activity while receiving Cesamet.
Cesamet should not be taken with alcohol, sedatives, hypnotics, or other psychoactive substances because these substances can potentiate the central nervous system effects of nabilone.
Adverse Reactions
Clinical Trials Experience
Commonly Encountered Reactions
During controlled clinical trials of Cesamet, virtually all patients experienced at least one adverse reaction. The most commonly encountered events were drowsiness, vertigo, dry mouth, euphoria (feeling “high”), ataxia, headache, and concentration difficulties.
Comparative Incidence of Reactions
Accurate estimates of the incidence of adverse events associated with the use of any drug are difficult to obtain. Estimates are influenced by factors such as drug dose, detection technique, setting, and physician judgments, among others. Consequently, the tables presented below are presented solely to indicate the relative frequency of adverse events reported in representative controlled clinical studies conducted to evaluate the safety and efficacy of Cesamet under relatively similar conditions of use. The figures cited cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice, in which patient characteristics and other factors may differ from those that prevailed in the clinical trials. These incidence figures also cannot be compared with those obtained from other clinical studies involving related drug products because each group of drug trials is conducted under a different set of conditions. Finally, it is important to emphasize that these tabulations do not reflect the relative severity and/or clinical importance of the adverse events.
The following tables list in order of decreasing frequency the adverse reactions encountered by a substantial proportion of patients treated with Cesamet participating in representative controlled clinical trials.
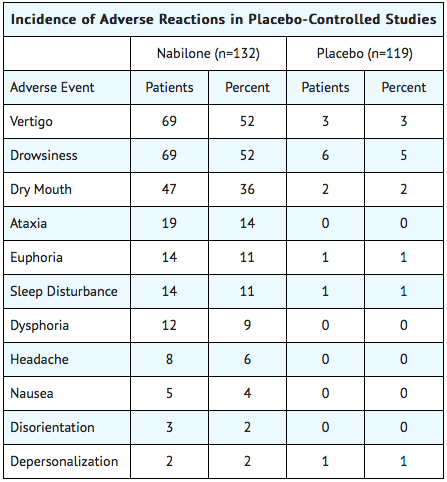
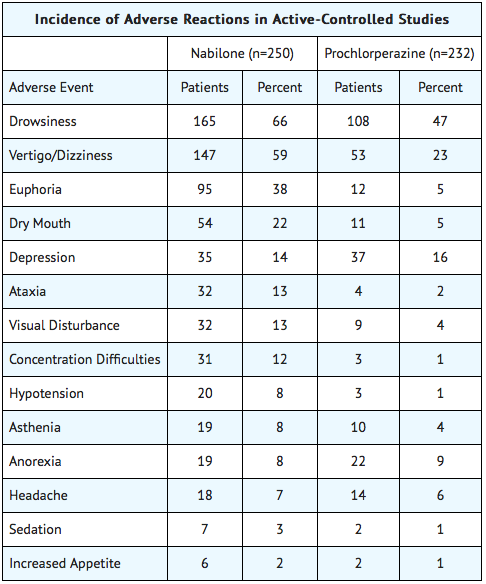
Adverse Reactions by Body System
Blood and Hematopoietic
Cardiovascular
- Orthostatic hypotension
- Hypotension
- Tachycardia
- Syncope
- Palpitation
- Flushing
- Hypertension
- Arrhythmia
- Cerebral vascular accident
Eye and Ear
- Vision disturbance
- Ear tightness
- Eye irritation
- Eye dryness
- Equilibrium dysfunction
- Tinnitus
- Eye disorder
- Amblyopia
- Eye swelling
- Eyelid diseases
- Pupil dilation
- Photophobia
- Visual field defect
Gastrointestinal
- Dry mouth
- Nausea
- Anorexia
- Vomiting
- Diarrhea
- Abdominal pain
- Constipation
- Aphthous ulcer
- Mouth irritation
- Gastritis
- Dyspepsia
Genitourinary
Infection
Metabolic and Endocrine
Musculoskeletal
Nervous System
- Drowsiness
- Vertigo
- Ataxia
- Decreased concentration
- Sedation
- Hallucinations
- Paresthesia
- Tremor
- Memory disturbance
- Perception disturbance
- Convulsions
- Dystonia
- Numbness
- Akathisia
Psychiatric
- Euphoria (feeling “high”)
- Sleep disturbance
- Depression
- Confusion
- Disorientation
- Anxiety
- Depersonalization syndrome
- Speech disorder
- Abnormal dreams
- Insomnia
- Mood swings
- Inebriated feeling
- Toxic psychosis
- Paranoia
- Apathy
- Thought disorder
- Withdrawal
- Panic disorder
- Phobic neurosis
- Emotional disorder
- Hyperactivity
Respiratory
- Dyspnea
- Pharyngitis
- Nasal congestion
- Sinus headache
- Thick tongue
- Dry throat
- Dry nose
- Wheezing
- Nosebleed
- Cough
- Voice change
- Chest pain
Skin and Appendages
Miscellaneous and Ill-Defined Conditions
- Headache
- Fatigue
- Lightheadedness
- Coordination disturbance
- Asthenia
- Dysphoria
- Dizziness
- Taste change
- Excessive appetite
- Chills
- Excessive sweating
- Nervousness
- Malaise
- Postural dizziness
- Twitch
- Irritability
- Fever
- Inhibited walking
- Unconsciousness
- Hypotonia
- Impaired urination
Postmarketing Experience
Cesamet has been marketed internationally since 1982. The following adverse reactions listed in order of decreasing frequency by body system have been reported since Cesamet has been marketed. All events are listed regardless of causality assessment.
Blood and Hematopoietic
Cardiovascular
Eye and Ear
Gastrointestinal
Nervous System
- Hallucinations
- CNS depression
- CNS stimulation
- Ataxia
- Stupor
- Vertigo
- Convulsion
- Circumoral paresthesia
Psychiatric
- Somnolence
- Confusion
- Euphoria
- Depression
- Dysphoria
- Depersonalization
- Anxiety
- Psychosis
- Emotional lability
Miscellaneous and Ill-Defined Conditions
Drug Interactions
Potential interactions between Cesamet 2 mg, and diazepam 5 mg; sodium secobarbital 100 mg; alcohol 45 mL (absolute laboratory alcohol); or codeine 65 mg, were evaluated in 15 subjects. Only a single combination was utilized at any one time. The subjects were evaluated according to physiologic (i.e., heart rate and blood pressure), psychometric, psychomotor, and subjective parameters. In this study, as expected, the depressant effects of the combinations were additive. Psychomotor function was particularly impaired with concurrent use of diazepam. Caution must thus be used when administering nabilone in combination with any CNS depressant.
Nabilone is purportedly highly bound to plasma proteins, and therefore, might displace other protein-bound drugs. Therefore, practitioners should monitor patients for a change in dosage requirements when administering nabilone to patients receiving other highly protein-bound drugs. Published reports of drug-drug interactions involving cannabinoids are summarized in the following table.
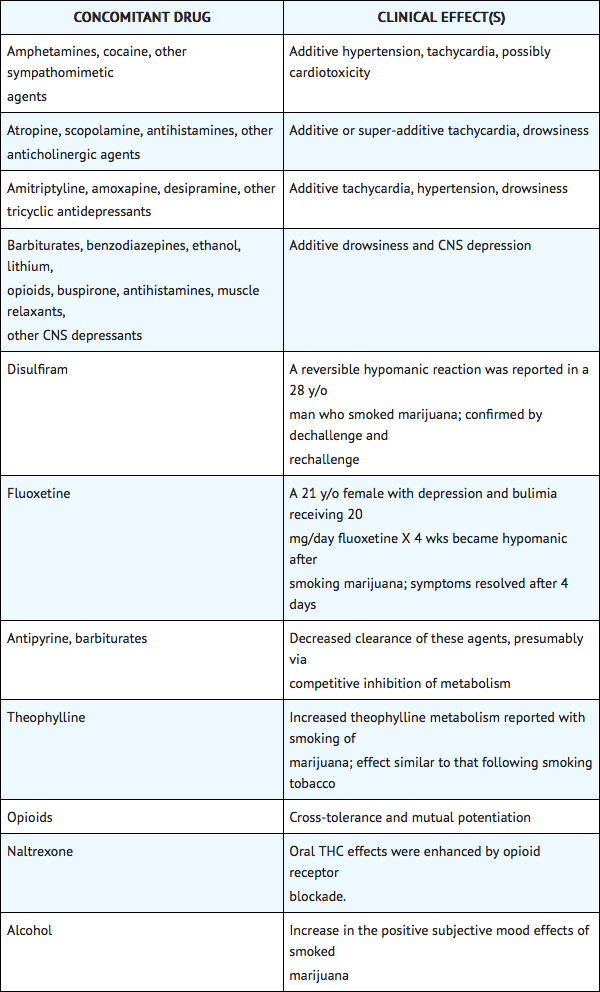
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Teratology studies conducted in pregnant rats at doses up to 12 mg/kg/day (about 16 times the human dose on a body surface area basis) and in pregnant rabbits at doses up to 3.3 mg/kg/day (about 9 times the human dose on a body surface area basis) did not disclose any evidence for a teratogenic potential of nabilone. However, there was dose related developmental toxicity in both species as evidenced by increases in embryo lethality, fetal resorptions, decreased fetal weights and pregnancy disruptions. In rats, postnatal developmental toxicity was also observed. There are no adequate and well-controlled studies in pregnant women. Because animal studies cannot rule out the possibility of harm, Cesamet should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nabilone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nabilone during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in breast milk. Because many drugs including some cannabinoids are excreted in breast milk it is not recommended that Cesamet be given to nursing mothers.
Pediatric Use
Safety and effectiveness have not been established in patients younger than 18 years of age. Caution is recommended in prescribing Cesamet to children because of psychoactive effects.
Geriatic Use
Clinical studies of Cesamet did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Cesamet should be used with caution in elderly patients aged 65 and over because they are generally more sensitive to the psychoactive effects of drugs and Cesamet can elevate supine and standing heart rates and cause postural hypotension.
Gender
There is no FDA guidance on the use of Nabilone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nabilone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nabilone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nabilone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nabilone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nabilone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Nabilone Administration in the drug label.
Monitoring
There is limited information regarding Nabilone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Nabilone and IV administrations.
Overdosage
Signs and Symptoms
Signs and symptoms of overdosage are an extension of the psychotomimetic and physiologic effects of Cesamet.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Overdosage may be considered to have occurred, even at prescribed dosages, if disturbing psychiatric symptoms are present. In these cases, the patient should be observed in a quiet environment and supportive measures, including reassurance, should be used. Subsequent doses should be withheld until patients have returned to their baseline mental status; routine dosing may then be resumed if clinically indicated. In such instances, a lower initiating dose is suggested. In controlled clinical trials, alterations in mental status related to the use of Cesamet resolved within 72 hours without specific medical therapy. In overdose settings, attention should be paid to vital signs, since both hypertension and hypotension have been known to occur; tachycardia and orthostatic hypotension were most commonly reported.
No cases of overdosage with more than 10 mg/day of nabilone were reported during clinical trials. Signs and symptoms that would be expected to occur in large overdose situations are psychotic episodes, including hallucinations, anxiety reactions, respiratory depression, and coma. If psychotic episodes occur, the patient should be managed conservatively, if possible. For moderate psychotic episodes and anxiety reactions, verbal support and comforting may be sufficient. In more severe cases, antipsychotic drugs may be useful; however, the utility of antipsychotic drugs in cannabinoid psychosis has not been systematically evaluated. Support for their use is drawn from limited experience using antipsychotic agents to manage cannabis overdoses. Because of the potential for drug-drug interactions (e.g., additive CNS depressant effects due to nabilone and chlorpromazine), such patients should be closely monitored.
Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, as well as other laboratory values and physical assessments. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
The use of forced diuresis, peritoneal dialysis, hemodialysis, charcoal hemoperfusion, or cholestyramine has not been reported. In the presence of normal renal function, most of a dose of nabilone is eliminated through the biliary system. Treatment for respiratory depression and comatose state consists in symptomatic and supportive therapy. Particular attention should be paid to the occurrence of hypothermia. If the patient becomes hypotensive, consider fluids, inotropes, and/or vasopressors. The estimated oral median lethal dose in female mice is between 1,000 and 2,000 mg/kg; in the female rat, it is greater than 2,000 mg/kg,
Pharmacology
There is limited information regarding Nabilone Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Nabilone Mechanism of Action in the drug label.
Structure
There is limited information regarding Nabilone Structure in the drug label.
Pharmacodynamics
There is limited information regarding Nabilone Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Nabilone Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Nabilone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Nabilone Clinical Studies in the drug label.
How Supplied
There is limited information regarding Nabilone How Supplied in the drug label.
Storage
There is limited information regarding Nabilone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nabilone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nabilone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Nabilone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Nabilone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nabilone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nabilone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Nabilone.png | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral form (PO)- capsule |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20% after first-pass by the liver |
| Protein binding | similar to THC (+/-97%) |
| Elimination half-life | 2 hours, with metabolites around 35 hours. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H36O3 |
| Molar mass | 372.541 g/mol |
Nabilone is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It is a synthetic cannabinoid, which mimics the main ingredient of marijuana (THC) but it has more predictable side effects and causes no or minimal euphoria. Nabilone is not derived from the cannabis plant as is dronabinol.
In Canada, the United States, the United Kingdom and Mexico, nabilone is marketed as Cesamet. It was approved in 1985 by the United States FDA for treatment of chemotherapy-induced nausea and vomiting that has not responded to conventional antiemetics. Though it was approved by the FDA in 1985, the drug only began marketing in the United States in 2006. It is also approved for use in treatment of anorexia and weight loss in patients with AIDS.
Although it doesn't have the official indication (except in Mexico), nabilone is widely used as an adjunct therapy for chronic pain management. Numerous trials and case studies have demonstrated various benefits for condition such as fibromyalgia and multiple sclerosis[citation needed].
Nabilone is a racemic mixture consisting of the (S,S) and the (R,R) isomers ("trans").
Clinical trials
The main settings that have seen published clinical trials of nabilone include movement disorders such as Parkinson's syndrome, chronic pain, dystonia and spasticity neurological disorders, fibromyalgia, multiple sclerosis, and the nausea of cancer chemotherapy.
A study comparing nabilone with metoclopramide, conducted before the development of modern 5-HT3 inhibitor anti-emetics such as ondansetron, revealed that patients taking cisplatin chemotherapy preferred metoclopramide, while patients taking carboplatin chemotherapy preferred nabilone to control nausea and vomiting. [1] Another study compared nabilone alone to nabilone with dexamethasone. The study found that the combination worked better than the single medication. [2] An older study revealed that nabilone was more effective than prochlorperazine in controlling nausea, though in this study, only 9% of nabilone patients had complete resolution of symptoms. [3] A follow-up to this study revealed similar findings. [4]
References
- ↑ Cunningham D, Bradley C, Forrest G, Hutcheon A, Adams L, Sneddon M, Harding M, Kerr D, Soukop M, Kaye S (1988). "A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues". Eur J Cancer Clin Oncol. 24 (4): 685–9. PMID 2838294.
- ↑ Niiranen A, Mattson K (1987). "Antiemetic efficacy of nabilone and dexamethasone: a randomized study of patients with lung cancer receiving chemotherapy". Am J Clin Oncol. 10 (4): 325–9. PMID 3039831.
- ↑ Herman T, Einhorn L, Jones S, Nagy C, Chester A, Dean J, Furnas B, Williams S, Leigh S, Dorr R, Moon T (1979). "Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy". N Engl J Med. 300 (23): 1295–7. PMID 375088.
- ↑ Einhorn L, Nagy C, Furnas B, Williams S. "Nabilone: an effective antiemetic in patients receiving cancer chemotherapy". J Clin Pharmacol. 21 (8-9 Suppl): 64S–69S. PMID 6271844.
Template:Antiemetics and antinauseants
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from December 2007
- Articles with invalid date parameter in template
- Cannabinoids