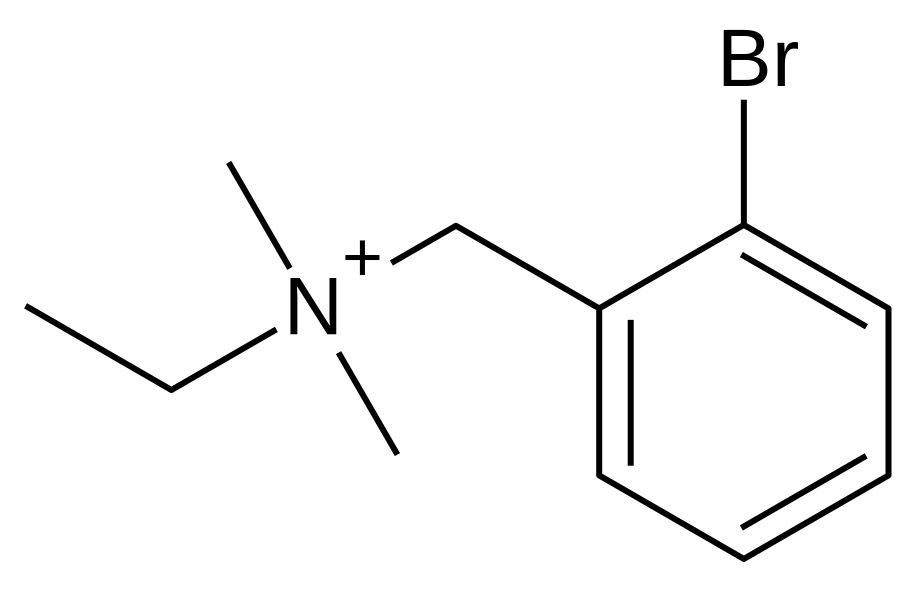
Bretylium
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a682861 |
| Pregnancy category | |
| Routes of administration | IV, IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | NA |
| Metabolism | None |
| Elimination half-life | 7-8 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C11H17BrN+ |
| Molar mass | 243.163 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Bretylium |
|
Articles |
|---|
|
Most recent articles on Bretylium |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bretylium at Clinical Trials.gov Clinical Trials on Bretylium at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bretylium
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bretylium Discussion groups on Bretylium Directions to Hospitals Treating Bretylium Risk calculators and risk factors for Bretylium
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bretylium |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bretylium (also bretylium tosylate) is an antiarrhythmic agent.[1] It blocks the release of noradrenaline from nerve terminals. In effect, it decreases output from the peripheral sympathetic nervous system. It also acts by blocking K+ channels and is considered a class III antiarrhythmic. The dose is 5–10 mg/kg and side effects are high blood pressure followed by low blood pressure and ventricular ectopy.
Originally introduced in 1959 for the treatment of hypertension.[2] Its use as an antiarrhythmic for ventricular fibrilation was discovered and patented by Marvin Bacaner in 1969 at the University of Minnesota.[3]
The American Heart Association removed Bretylium from their 2000 ECC/ACC guidelines due to its unproven efficacy and ongoing supply problems. Many have cited these supply problems as an issue of raw materials needed in the production of Bretylium. By the release of the AHA 2005 ECC/ACC guidelines there is no mention of Bretylium and it is virtually unavailable throughout most of the world.[4][5]
As of June 8, 2011 Bretylium Tosylate is permanently no longer available in the US after request of Hospira Inc. to withdraw its NDA from the market. Bretylium will remain on the FDA's discontinued drug list since its withdrawal was not the result of a safety or effectiveness concern.[6]
Uses
It was used in emergency medicine, cardiology, and other specialties throughout the 1980s-1990s for the acute management of ventricular tachycardia and ventricular fibrillation refractory to other first line treatments such as defibrillation or lidocaine.[7]
It is contraindicated in patients with AV (atrioventricular) heart block or digoxin toxicity.
Bretylium should be used only in an ICU or Emergency Department setting and should not be used elsewhere due to its dramatic actions and its predominant side effect of hypotension.
Experimental Uses
It is used in physiological and pharmacological research as an inhibitor of sympathetic transmission. Its mechanism of action is the inhibition of neurotransmitter release from sympathetic nerve terminals, both by the inhibition of action potentials in the nerve terminals and by other mechanisms.[8] Its specificity for sympathetic nerves is achieved because it is a substrate for the noradrenaline transporter;[9] hence, it accumulates inside nerve terminals which have this transporter.
Synthesis
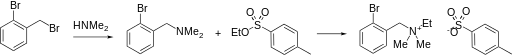
Quaternization of o-bromo-N,N-dimethylbenzylamine with ethyl-p-toluenesulfonate yields bretylium sulfonate.
References
- ↑ Tiku PE, Nowell PT (December 1991). "Selective inhibition of K(+)-stimulation of Na,K-ATPase by bretylium". Br. J. Pharmacol. 104 (4): 895–900. doi:10.1111/j.1476-5381.1991.tb12523.x. PMC 1908819. PMID 1667290.
- ↑ "the treatment of hypertension".
- ↑ "patent". Retrieved 2014-02-06.
- ↑ http://books.google.com/books?id=xco9aJ_Y9XIC&pg=PA221&lpg=PA221&dq=bretylium+raw+materials&source=bl&ots=fCgjx6lB2u&sig=UxuKAyXp6qKhKDG6riQM9gCG1hQ&hl=en&sa=X&ei=F0_lUveHAujjsASZv4DgCw&ved=0CDoQ6AEwBg#v=onepage&q=bretylium%20raw%20materials&f=false
- ↑ http://emedicine.medscape.com/article/770542-treatment
- ↑ https://www.federalregister.gov/articles/2011/12/19/2011-32367/determination-that-bretylium-tosylate-injection-50-milligramsmilliliter-was-not-withdrawn-from-sale
- ↑ "ACS". Retrieved 2008-09-23. [dead link]
- ↑ K. L. Brain & T. C. Cunnane (2008). "Bretylium abolishes neurotransmitter release without necessarily abolishing the nerve terminal action potential in sympathetic terminals". British journal of pharmacology. 153 (4): 831–839. doi:10.1038/sj.bjp.0707623. PMC 2259200. PMID 18071295.
- ↑ A. L. BOURA, F. C. COPP, W. G. DUNCOMBE, A. F. GREEN & A. McCOUBREY (June 1960). "The selective accumulation of bretylium in sympathetic ganglia and their postganglionic nerves". British journal of pharmacology and chemotherapy. 15: 265–270. doi:10.1111/j.1476-5381.1960.tb01242.x. PMC 1481934. PMID 13803289.
Template:Potassium channel blockers Template:Antiarrhythmic agents
- Pages with script errors
- All articles with dead external links
- Articles with dead external links from October 2010
- Articles with invalid date parameter in template
- CS1 maint: Multiple names: authors list
- Articles with changed KEGG identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Antiarrhythmic agents
- Potassium channel blockers
- Quaternary ammonium compounds
- Organobromides
- Drug