Mitogen-activated protein kinase
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
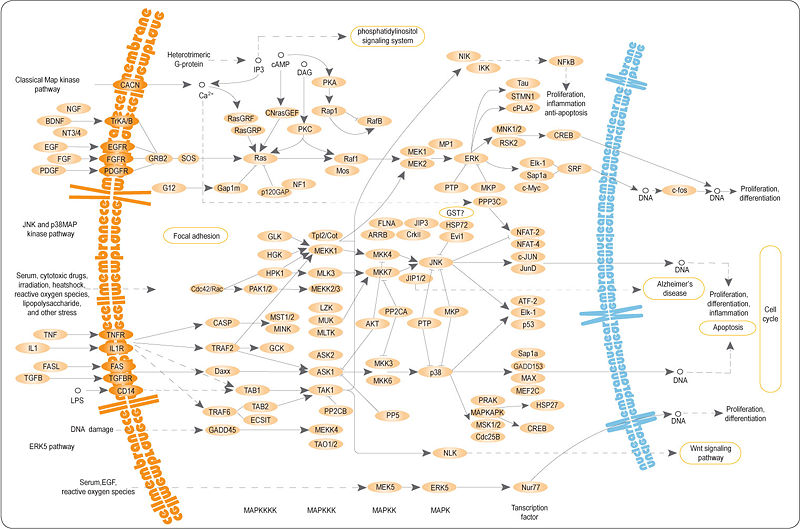
Overview
Mitogen-activated protein (MAP) kinases (EC 2.7.11.24) are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, cell survival, and apoptosis.[10]
Function
MAPK is involved in the action of most nonnuclear oncogenes. It is responsible for cell response to growth factors such as BDNF or nerve growth factor. Extracellular stimuli lead to activation of a MAP kinase via a signaling cascade ("MAPK cascade") composed of MAP kinase, MAP kinase kinase (MKK or MAP2K), and MAP kinase kinase kinase (MKKK or MAP3K, EC 2.7.11.25).
A MAP3K that is activated by extracellular stimuli phosphorylates a MAP2K on its serine and threonine residues, and then this MAP2K activates a MAP kinase through phosphorylation on its serine and tyrosine residues. This MAP kinase signaling cascade has been evolutionarily well-conserved from yeast to mammals.
Groups
To date, six distinct groups of MAPKs have been characterized in mammals:
- extracellular signal-regulated kinases (ERK1, ERK2). The ERKs (also known as classical MAP kinases) signaling pathway is preferentially activated in response to growth factors and phorbol ester (a tumor promoter), and regulates cell proliferation and cell differentiation.
- c-Jun N-terminal kinases (JNKs), (MAPK8,MAPK9,MAPK10) also known as stress-activated protein kinases (SAPKs).
- p38 isoforms. (MAPK11, MAPK12(= ERK6), MAPK13, MAPK14) Both JNK and p38 signaling pathways are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation and apoptosis.
- ERK5. ERK5 (MAPK7), which has been found recently, is activated both by growth factors and by stress stimuli, and it participates in cell proliferation.
- ERK3/4. ERK3 (MAPK6) and ERK4 (MAPK4) are structurally related atypical MAPKs posses SEG motif in activation loop and displaying major differences only in the C-terminal extension. ERK3 and ERK4 mostly cytoplasmic protein which binds, translocates and activates the MK5 (PRAK, MAP2K5). ERK3 is known as an unstable unlike ERK4 which is relativily stable.[11]
- ERK7/8. (MAPK15) This is newest member of MAPKs and behaves like atypical MAPKs. It possesses a long C terminus similar to ERK3/4.
See also
- MAPK/ERK pathway
- Anthra(1,9-cd)pyrazol-6(2H)-one - inhibitor
- MAPK1
- MAPK3
- MAPK14
References
- ↑ Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999). "Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms". Science. 286 (5443): 1358–62. doi:10.1126/science.286.5443.1358. PMID 10558990.
- ↑ Chadee DN, Yuasa T, Kyriakis JM (2002). "Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2". Mol. Cell. Biol. 22 (3): 737–49. doi:10.1128/MCB.22.3.737-749.2002. PMID 11784851.
- ↑ Chang L, Karin M (2001). "Mammalian MAP kinase signalling cascades". Nature. 410 (6824): 37–40. doi:10.1038/35065000. PMID 11242034.
- ↑ Chen YR, Tan TH (2000). "The c-Jun N-terminal kinase pathway and apoptotic signaling (review)". Int. J. Oncol. 16 (4): 651–62. PMID 10717232.
- ↑ Hazzalin CA, Mahadevan LC (2002). "MAPK-regulated transcription: a continuously variable gene switch?". Nat. Rev. Mol. Cell Biol. 3 (1): 30–40. doi:10.1038/nrm715. PMID 11823796.
- ↑ Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD (1997). "BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C". EMBO J. 16 (23): 7054–66. doi:10.1093/emboj/16.23.7054. PMID 9384584.
- ↑ Kiefer F, Tibbles LA, Anafi M, Janssen A, Zanke BW, Lassam N, Pawson T, Woodgett JR, Iscove NN (1996). "HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway". EMBO J. 15 (24): 7013–25. PMID 9003777.
- ↑ Pearson G, English JM, White MA, Cobb MH (2001). "ERK5 and ERK2 cooperate to regulate NF-kappaB and cell transformation". J. Biol. Chem. 276 (11): 7927–31. doi:10.1074/jbc.M009764200. PMID 11118448.
- ↑ Weston CR, Lambright DG, Davis RJ (2002). "Signal transduction. MAP kinase signaling specificity". Science. 296 (5577): 2345–7. doi:10.1126/science.1073344. PMID 12089430.
- ↑ Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001). "Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions". Endocr. Rev. 22 (2): 153–83. doi:10.1210/er.22.2.153. PMID 11294822.
- ↑ Kant S, Schumacher S, Singh MK, Kispert A, Kotlyarov A, Gaestel M. (2006). "Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5". J Biol Chem. 281 (46): 35511–9. doi:10.1074/jbc.M606693200. PMID 16973613.
External links
- Table of names for mitogen-activated kinases.
- Knock-in/out mouse models for MAPKs
- MAPK cascade picture: http://wwwmgs.bionet.nsc.ru/mgs/gnw/genenet/viewer/MAPK%20cascade.html
- Seger R, Krebs E (1995). "The MAPK signaling cascade". FASEB J. 9 (9): 726–35. PMID 7601337.
- Mitogen-Activated+Protein+Kinases at the US National Library of Medicine Medical Subject Headings (MeSH)
de:MAP-Kinase ur:انقسامیہ مفاعل لحمیاتی حرمرہ Template:WikiDoc Sources