Epoprostenol detailed information
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not applicable (IV only) |
| Metabolism | To 6-keto-PGF1α and 6,15-diketo-13,14-dihydro-PGF1α |
| Elimination half-life | 6 minutes (in vitro) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
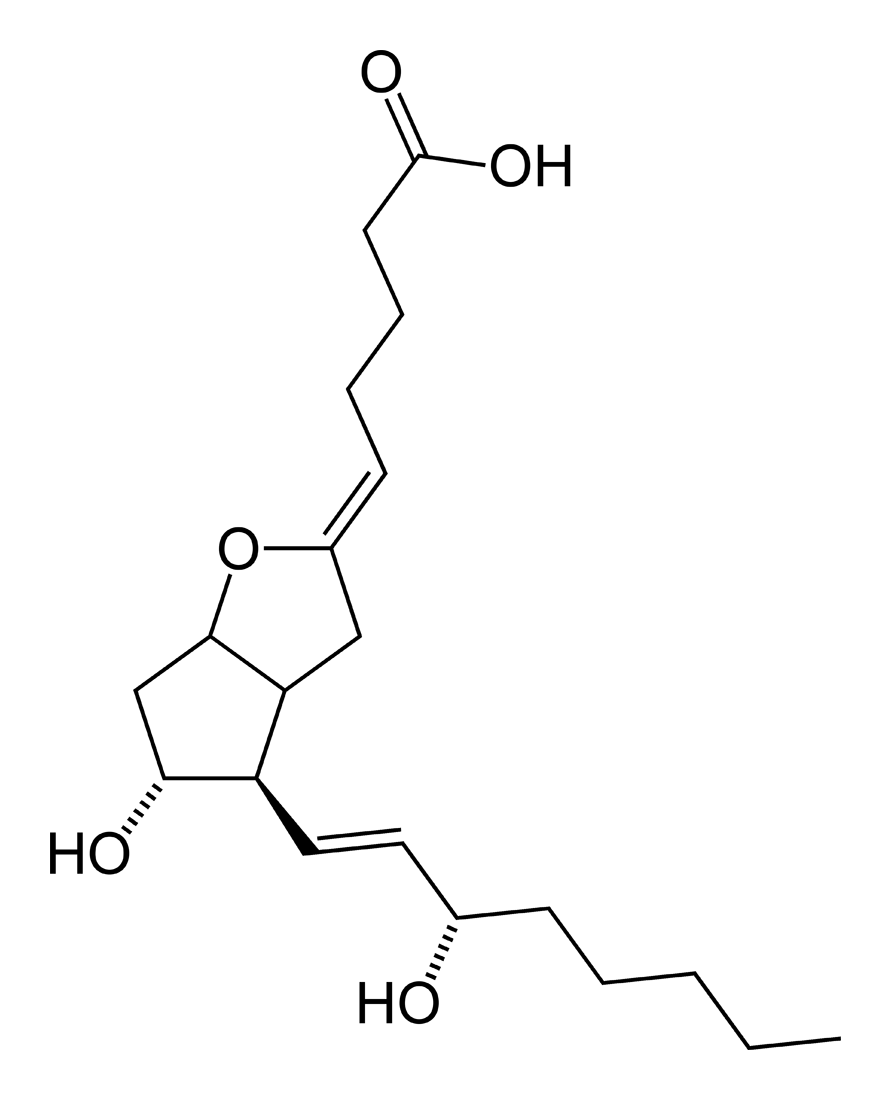
| Formula | C20H32O5 |
| Molar mass | 352.465 g/mol |
| 3D model (JSmol) | |
| |
Epoprostenol is a synthetic form of prostacyclin, and is used to treat pulmonary hypertension. It is sold under the trade name Flolan.
Clinical pharmacology
As an analogue of prostacyclin PGI2, epoprostenol effects vasodilation, which in turn lowers the blood pressure. Epoprotstenol also inhibits platelet aggregation, though the role this phenomenon may play in relation to pulmonary hypertension has yet to be determined.
Administration
Epoprostenol is given via continuous infusion that requires a semi-permanent central venous catheter. This means that the patient must be attached to an infusion pump at all times. This delivery system can cause sepsis and thrombosis. Because epoprostenol is unstable, it must be kept cold, even during administration. Since it has a half-life of 3 to 5 minutes, the infusion has to be continuous (24/7), and an interruption can lead to a potentially fatal rebound of symptoms.
History
Epoprostenol was developed by GlaxoSmithKline and approved in the USA as a medicine in 1995.
Marketing
It was licensed to Myogen, which was subsequently acquired by Gilead Sciences. Flolan is marketed in the United States by Gilead Sciences and elsewhere by GlaxoSmithKline.
Template:PAH rx Template:Prostaglandins
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Orphan drugs
- Prostaglandins