Apixaban
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
DISCONTINUING Eliquis IN PATIENTS WITH NONVALVULAR ATRIAL FIBRILLATION WITHOUT ADEQUATE CONTINUOUS ANTICOAGULATION INCREASES RISK OF STROKE:
SPINAL/EPIDURAL HEMATOMA:
|
Overview
Apixaban is an anticoagulant that is FDA approved for the {{{indicationType}}} of stroke and systemic embolism in nonvalvular atrial fibrillation and prophylaxis of deep vein thrombosis following hip or knee replacement surgery. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
- Dosing Information
- The recommended dose of Eliquis is 5 mg taken orally twice daily.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Dosing Information
- The recommended dose of Eliquis is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
- In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
- In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
Dosage Adjustments
- In patients with nonvalvular atrial fibrillation: The recommended dose of Eliquis is 2.5 mg twice daily in patients with any 2 of the following characteristics:
- Age ≥80 years
- Body weight ≤60 kg
- Serum creatinine ≥1.5 mg/dL
- Coadministration with CYP3A4 and P-gp inhibitors
- For patients receiving Eliquis 5 mg twice daily when Eliquis is coadministered with drugs that are strong dual inhibitors of cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) (e.g., ketoconazole, itraconazole, ritonavir, clarithromycin), the recommended dose is 2.5 mg twice daily.
- In patients already taking 2.5 mg twice daily, coadministration of Eliquis with strong dual inhibitors of CYP3A4 and P-gp should be avoided.
Missed Dose
- If a dose of Eliquis is not taken at the scheduled time, the dose should be taken as soon as possible on the same day and twice-daily administration should be resumed. The dose should not be doubled to make up for a missed dose.
Temporary Interruption for Surgery and Other Interventions
- Eliquis should be discontinued at least 48 hours prior to elective surgery or invasive procedures with a moderate or high risk of unacceptable or clinically significant bleeding. Eliquis should be discontinued at least 24 hours prior to elective surgery or invasive procedures with a low risk of bleeding or where the bleeding would be non-critical in location and easily controlled. Bridging anticoagulation during the 24 to 48 hours after stopping Eliquis and prior to the intervention is not generally required. Eliquis should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established.
Converting from or to Eliquis
- Switching from warfarin to Eliquis
- Warfarin should be discontinued and Eliquis started when the international normalized ratio (INR) is below 2.0.
- Switching from Eliquis to warfarin: Eliquis affects INR, so that initial INR measurements during the transition to warfarin may not be useful for determining the appropriate dose of warfarin. If continuous anticoagulation is necessary, discontinue Eliquis and begin both a parenteral anticoagulant and warfarin at the time the next dose of Eliquis would have been taken, discontinuing the parenteral anticoagulant when INR reaches an acceptable range.
- Switching between Eliquis and anticoagulants other than warfarin: Discontinue one being taken and begin the other at the next scheduled dose.
Hepatic Impairment
- No dose adjustment is required in patients with mild hepatic impairment.
- Because patients with moderate hepatic impairment may have intrinsic coagulation abnormalities and there is limited clinical experience with Eliquis in these patients, dosing recommendations cannot be provided.
- Eliquis is not recommended in patients with severe hepatic impairment.
Renal Impairment
- The dosing adjustment for moderate renal impairment is described above. The recommended dose for nonvalvular atrial fibrillation patients with end-stage renal disease (ESRD) maintained on hemodialysis is 5 mg twice daily. Reduce dose to 2.5 mg twice daily if one of the following patient characteristics (age ≥80 years or body weight ≤60 kg) is present.
Administration Options
- For patients who are unable to swallow whole tablets, 5 mg and 2.5 mg Eliquis tablets may be crushed and suspended in 60 mL D5W and immediately delivered through a nasogastric tube (NGT). Information regarding the administration of crushed and suspended Eliquis tablets swallowed by mouth is not available.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Apixaban in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Venous Thromboembolism
- Dosing Information
- 2.5 mg or 5 mg PO twice daily[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Apixaban in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Apixaban in pediatric patients.
Contraindications
- Active pathological bleeding
- Severe hypersensitivity reaction to Eliquis (e.g., anaphylactic reactions)
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
DISCONTINUING Eliquis IN PATIENTS WITH NONVALVULAR ATRIAL FIBRILLATION WITHOUT ADEQUATE CONTINUOUS ANTICOAGULATION INCREASES RISK OF STROKE:
SPINAL/EPIDURAL HEMATOMA:
|
- Increased Risk of Stroke with Discontinuation of Eliquis in Patients with Nonvalvular Atrial Fibrillation
- Discontinuing Eliquis in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from Eliquis to warfarin in clinical trials in patients with nonvalvular atrial fibrillation. If Eliquis must be discontinued for a reason other than pathological bleeding, consider coverage with another anticoagulant.
- Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding.
- Concomitant use of drugs affecting hemostasis increases the risk of bleeding. These include aspirin and other antiplatelet agents, other anticoagulants, heparin, thrombolytic agents, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs).
- Patients should be made aware of signs and symptoms of blood loss and instructed to report them immediately or go to an emergency room. Eliquis should be discontinued in patients with active pathological hemorrhage.
- There is no established way to reverse the anticoagulant effect of apixaban, which can be expected to persist for at least 24 hours after the last dose, i.e., for about two half-lives. A specific antidote for Eliquis is not available. Hemodialysis does not appear to have a substantial impact on apixaban exposure. Protamine sulfate and vitamin K would not be expected to affect the anticoagulant activity of apixaban. There is no experience with antifibrinolytic agents (tranexamic acid, aminocaproic acid) in individuals receiving apixaban. There is neither scientific rationale for reversal nor experience with systemic hemostatics (desmopressin and aprotinin) in individuals receiving apixaban. Use of procoagulant reversal agents such as prothrombin complex concentrate, activated prothrombin complex concentrate, or recombinant factor VIIa may be considered but has not been evaluated in clinical studies. Activated oral charcoal reduces absorption of apixaban, thereby lowering apixaban plasma concentration.
- Spinal/Epidural Anesthesia or Puncture
- When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
- The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 24 hours after the last administration of Eliquis. The next dose of Eliquis should not be administered earlier than 5 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture. If traumatic puncture occurs, delay the administration of Eliquis for 48 hours.
- Patients are to be frequently monitored for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
- Patients with Prosthetic Heart Valves
- The safety and efficacy of Eliquis have not been studied in patients with prosthetic heart valves. Therefore, use of Eliquis is not recommended in these patients.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
- The safety of Eliquis was evaluated in the ARISTOTLE and AVERROES studies, including 11,284 patients exposed to Eliquis 5 mg twice daily and 602 patients exposed to Eliquis 2.5 mg twice daily. The duration of Eliquis exposure was ≥12 months for 9375 patients and ≥24 months for 3369 patients in the two studies. In ARISTOTLE, the mean duration of exposure was 89 weeks (>15,000 patient-years). In AVERROES, the mean duration of exposure was approximately 59 weeks (>3000 patients-years).
- The most common reason for treatment discontinuation in both studies was for bleeding-related adverse reactions; in ARISTOTLE this occurred in 1.7% and 2.5% of patients treated with Eliquis and warfarin, respectively, and in AVERROES, in 1.5% and 1.3% on Eliquis and aspirin, respectively.
Bleeding in Patients with Nonvalvular Atrial Fibrillation in ARISTOTLE and AVERROES
- Tables 1 and 2 show the number of patients experiencing major bleeding during the treatment period and the bleeding rate (percentage of subjects with at least one bleeding event per year) in ARISTOTLE and AVERROES.
- Major bleeding was defined as clinically overt bleeding that was accompanied by one or more of the following: a decrease in hemoglobin of 2 g/dL or more; a transfusion of 2 or more units of packed red blood cells; bleeding that occurred in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that was fatal. Intracranial hemorrhage included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
- In ARISTOTLE, the results for major bleeding were generally consistent across most major subgroups including age, weight, CHADS2 score (a scale from 0 to 6 used to estimate risk of stroke, with higher scores predicting greater risk), prior warfarin use, geographic region, Eliquis dose, type of atrial fibrillation (AF), and aspirin use at randomization (Figure 1). Subjects treated with apixaban with diabetes bled more (3.0% per year) than did subjects without diabetes (1.9% per year).

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Other Adverse Reactions
- Hypersensitivity reactions (including drug hypersensitivity, such as skin rash, and anaphylactic reactions, such as allergic edema) and syncope were reported in <1% of patients receiving Eliquis.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- The safety of Eliquis has been evaluated in 1 Phase II and 3 Phase III studies including 5924 patients exposed to Eliquis 2.5 mg twice daily undergoing major orthopedic surgery of the lower limbs (elective hip replacement or elective knee replacement) treated for up to 38 days.
- In total, 11% of the patients treated with Eliquis 2.5 mg twice daily experienced adverse reactions.
- bleeding results during the treatment period in the Phase III studies are shown in Table 3. bleeding was assessed in each study beginning with the first dose of double-blind study drug.
- Adverse reactions occurring in ≥1% of patients undergoing hip or knee replacement surgery in the 1 Phase II study and the 3 Phase III studies are listed in Table 4.
- Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of ≥0.1% to <1%:
- Blood and lymphatic system disorders: thrombocytopenia (including platelet count decreases)
- Vascular disorders: hypotension (including procedural hypotension)
- Respiratory, thoracic, and mediastinal disorders: epistaxis
- Gastrointestinal disorders: gastrointestinal hemorrhage (including hematemesis and melena), hematochezia
- Hepatobiliary disorders: liver function test abnormal, blood alkaline phosphatase increased, blood bilirubin increased
- Renal and urinary disorders: hematuria (including respective laboratory parameters)
- Injury, poisoning, and procedural complications: wound secretion, incision-site hemorrhage (including incision-site hematoma), operative hemorrhage
- Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of <0.1%:
- Gingival bleeding, hemoptysis, hypersensitivity, muscle hemorrhage, ocular hemorrhage (including conjunctival hemorrhage), rectal hemorrhage
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Apixaban in the drug label.
Drug Interactions
- Apixaban is a substrate of both CYP3A4 and P-gp. Inhibitors of CYP3A4 and P-gp increase exposure to apixaban and increase the risk of bleeding. Inducers of CYP3A4 and P-gp decrease exposure to apixaban and increase the risk of stroke.
Strong Dual Inhibitors of CYP3A4 and P-gp
- For patients receiving 5 mg twice daily, the dose of Eliquis should be decreased to 2.5 mg twice daily when it is coadministered with drugs that are strong dual inhibitors of CYP3A4 and P-gp (e.g., ketoconazole, itraconazole, ritonavir, or clarithromycin).
- In patients already taking Eliquis at a dose of 2.5 mg twice daily, avoid coadministration with strong dual inhibitors of CYP3A4 and P-gp.
Strong Dual Inducers of CYP3A4 and P-gp
- Avoid concomitant use of Eliquis with strong dual inducers of CYP3A4 and P-gp (e.g., rifampin, carbamazepine, phenytoin, St. John's wort) because such drugs will decrease exposure to apixaban.
Anticoagulants and Antiplatelet Agents
- Coadministration of antiplatelet agents, fibrinolytics, heparin, aspirin, and chronic NSAID use increases the risk of bleeding.
- APPRAISE-2, a placebo-controlled clinical trial of apixaban in high-risk, post-acute coronary syndrome patients treated with aspirin or the combination of aspirin and clopidogrel, was terminated early due to a higher rate of bleeding with apixaban compared to placebo. The rate of ISTH major bleeding was 2.77% per year with apixaban versus 0.62% per year with placebo in patients receiving single antiplatelet therapy and was 5.91% per year with apixaban versus 2.50% per year with placebo in those receiving dual antiplatelet therapy.
- In ARISTOTLE, concomitant use of aspirin increased the bleeding risk on Eliquis from 1.8% per year to 3.4% per year and the bleeding risk on warfarin from 2.7% per year to 4.6% per year. In this clinical trial, there was limited (2.3%) use of dual antiplatelet therapy with Eliquis.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- There are no adequate and well-controlled studies of Eliquis in pregnant women. Treatment is likely to increase the risk of hemorrhage during pregnancy and delivery. Eliquis should be used during pregnancy only if the potential benefit outweighs the potential risk to the mother and fetus.
- Treatment of pregnant rats, rabbits, and mice after implantation until the end of gestation resulted in fetal exposure to apixaban, but was not associated with increased risk for fetal malformations or toxicity. No maternal or fetal deaths were attributed to bleeding. Increased incidence of maternal bleeding was observed in mice, rats, and rabbits at maternal exposures that were 19, 4, and 1 times, respectively, the human exposure of unbound drug, based on area under plasma-concentration time curve (AUC) comparisons at the maximum recommended human dose (MRHD) of 10 mg (5 mg twice daily).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Apixaban in women who are pregnant.
Labor and Delivery
- Safety and effectiveness of Eliquis during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using Eliquis in this setting.
- Treatment of pregnant rats from implantation (gestation Day 7) to weaning (lactation Day 21) with apixaban at a dose of 1000 mg/kg (about 5 times the human exposure based on unbound apixaban) did not result in death of offspring or death of mother rats during labor in association with uterine bleeding. However, increased incidence of maternal bleeding, primarily during gestation, occurred at apixaban doses of ≥25 mg/kg, a dose corresponding to ≥1.3 times the human exposure.
Nursing Mothers
- It is unknown whether apixaban or its metabolites are excreted in human milk. Rats excrete apixaban in milk (12% of the maternal dose).
- Women should be instructed either to discontinue breastfeeding or to discontinue Eliquis therapy, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Of the total subjects in the ARISTOTLE and AVERROES clinical studies, >69% were 65 and older, and >31% were 75 and older. In the ADVANCE-1, ADVANCE-2, and ADVANCE-3 clinical studies, 50% of subjects were 65 and older, while 16% were 75 and older. No clinically significant differences in safety or effectiveness were observed when comparing subjects in different age groups.
Gender
There is no FDA guidance on the use of Apixaban with respect to specific gender populations.
Race
There is no FDA guidance on the use of Apixaban with respect to specific racial populations.
Renal Impairment
- Patients with ESRD with or without hemodialysis were not studied in clinical efficacy and safety studies with Eliquis; therefore, the dosing recommendation for patients with nonvalvular atrial fibrillation is based on pharmacokinetic and pharmacodynamic (anti-Factor Xa activity) data in subjects with ESRD maintained on dialysis. The recommended dose for ESRD patients maintained with hemodialysis is 5 mg orally twice daily. For ESRD patients maintained with hemodialysis with one of the following patient characteristics, age ≥80 years or body weight ≤60 kg, reduce dose to 2.5 mg twice daily.
Hepatic Impairment
There is no FDA guidance on the use of Apixaban in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Apixaban in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Apixaban in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
- Patients are to be frequently monitored for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
IV Compatibility
There is limited information regarding IV Compatibility of Apixaban in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- There is no antidote to Eliquis. Overdose of Eliquis increases the risk of bleeding.
- In controlled clinical trials, orally administered apixaban in healthy subjects at doses up to 50 mg daily for 3 to 7 days (25 mg twice daily for 7 days or 50 mg once daily for 3 days) had no clinically relevant adverse effects.
Management
- In healthy subjects, administration of activated charcoal 2 and 6 hours after ingestion of a 20-mg dose of apixaban reduced mean apixaban AUC by 50% and 27%, respectively. Thus, administration of activated charcoal may be useful in the management of apixaban overdose or accidental ingestion.
Chronic Overdose
There is limited information regarding Chronic Overdose of Apixaban in the drug label.
Pharmacology
| |
Apixaban
| |
| Systematic (IUPAC) name | |
| 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5-dihydropyrazolo[5,4-c]pyridine-3-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 459.497 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ca. 50% |
| Metabolism | ? |
| Half life | 9–14 h |
| Excretion | 75% biliary, 25% renally |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Apixaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Apixaban inhibits free and clot-bound FXa, and prothrombinase activity. Apixaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, apixaban decreases thrombin generation and thrombus development.
Structure
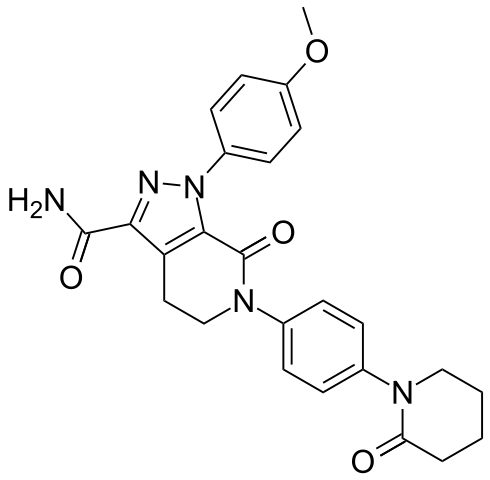
- Eliquis (apixaban), a factor Xa (FXa) inhibitor, is chemically described as 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide. Its molecular formula is C25H25N5O4, which corresponds to a molecular weight of 459.5. Apixaban has the following structural formula:
- Apixaban is a white to pale-yellow powder. At physiological pH (1.2–6.8), apixaban does not ionize; its aqueous solubility across the physiological pH range is ~0.04 mg/mL.
- Eliquis tablets are available for oral administration in strengths of 2.5 mg and 5 mg of apixaban with the following inactive ingredients: anhydrous lactose, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. The film coating contains lactose monohydrate, hypromellose, titanium dioxide, triacetin, and yellow iron oxide (2.5 mg tablets) or red iron oxide (5 mg tablets).
Pharmacodynamics
- As a result of FXa inhibition, apixaban prolongs clotting tests such as prothrombin time (PT), INR, and activated partial thromboplastin time (aPTT). Changes observed in these clotting tests at the expected therapeutic dose, however, are small, subject to a high degree of variability, and not useful in monitoring the anticoagulation effect of apixaban.
- The Rotachrom® Heparin chromogenic assay was used to measure the effect of apixaban on FXa activity in humans during the apixaban development program. A concentration-dependent increase in anti-FXa activity was observed in the dose range tested and was similar in healthy subjects and patients with AF.
- This test is not recommended for assessing the anticoagulant effect of apixaban.
Pharmacodynamic Drug Interaction Studies
- Pharmacodynamic drug interaction studies with aspirin, clopidogrel, aspirin and clopidogrel, prasugrel, enoxaparin, and naproxen were conducted. No pharmacodynamic interactions were observed with aspirin, clopidogrel, or prasugrel. A 50% to 60% increase in anti-FXa activity was observed when apixaban was coadministered with enoxaparin or naproxen.
Specific Populations
- Renal impairment: Anti-FXa activity adjusted for exposure to apixaban was similar across renal function categories.
- Hepatic impairment: Changes in anti-FXa activity were similar in patients with mild-to-moderate hepatic impairment and healthy subjects. However, in patients with moderate hepatic impairment, there is no clear understanding of the impact of this degree of hepatic function impairment on the coagulation cascade and its relationship to efficacy and bleeding. Patients with severe hepatic impairment were not studied.
Cardiac Electrophysiology
- Apixaban has no effect on the QTc interval in humans at doses up to 50 mg.
Pharmacokinetics
- Apixaban demonstrates linear pharmacokinetics with dose-proportional increases in exposure for oral doses up to 10 mg.
Absorption
- The absolute bioavailability of apixaban is approximately 50% for doses up to 10 mg of Eliquis. Food does not affect the bioavailability of apixaban. Maximum concentrations (Cmax) of apixaban appear 3 to 4 hours after oral administration of Eliquis. At doses ≥25 mg, apixaban displays dissolution-limited absorption with decreased bioavailability. Following administration of a crushed 5 mg Eliquis tablet that was suspended in 60 mL D5W and delivered through a nasogastric tube (NGT), exposure was similar to that seen in other clinical trials involving healthy volunteers receiving a single oral 5 mg tablet dose.
Distribution
- Plasma protein binding in humans is approximately 87%. The volume of distribution (Vss) is approximately 21 liters.
Metabolism
- Approximately 25% of an orally administered apixaban dose is recovered in urine and feces as metabolites. Apixaban is metabolized mainly via CYP3A4 with minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2. O-demethylation and hydroxylation at the 3-oxopiperidinyl moiety are the major sites of biotransformation.
- Unchanged apixaban is the major drug-related component in human plasma; there are no active circulating metabolites.
Elimination
- Apixaban is eliminated in both urine and feces. Renal excretion accounts for about 27% of total clearance. Biliary and direct intestinal excretion contributes to elimination of apixaban in the feces.
- Apixaban has a total clearance of approximately 3.3 L/hour and an apparent half-life of approximately 12 hours following oral administration.
- Apixaban is a substrate of transport proteins: P-gp and breast cancer resistance protein.
Drug Interaction Studies
- In vitro apixaban studies at concentrations significantly greater than therapeutic exposures, no inhibitory effect on the activity of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2D6, CYP3A4/5, or CYP2C19, nor induction effect on the activity of CYP1A2, CYP2B6, or CYP3A4/5 were observed. Therefore, apixaban is not expected to alter the metabolic clearance of coadministered drugs that are metabolized by these enzymes. Apixaban is not a significant inhibitor of P-gp.
- The effects of coadministered drugs on the pharmacokinetics of apixaban and associated dose recommendations are summarized in Figure 2.
- In dedicated studies conducted in healthy subjects, famotidine, atenolol, prasugrel, and enoxaparin did not meaningfully alter the pharmacokinetics of apixaban.
- In studies conducted in healthy subjects, apixaban did not meaningfully alter the pharmacokinetics of digoxin, naproxen, atenolol, prasugrel, or acetylsalicylic acid.
Specific Populations
- The effects of level of renal impairment, age, body weight, and level of hepatic impairment on the pharmacokinetics of apixaban are summarized in Figure 3.
- A study in healthy subjects comparing the pharmacokinetics in males and females showed no meaningful difference.
- The results across pharmacokinetic studies in normal subjects showed no differences in apixaban pharmacokinetics among White/Caucasian, Asian, and Black/African American subjects. No dose adjustment is required based on race/ethnicity.
- In subjects with ESRD, a 4-hour hemodialysis session with a dialysate flow rate of 500 mL/min and a blood flow rate in the range of 350 to 500 mL/min started 2 hours after administration of a single 5 mg dose of apixaban, the AUC of apixaban was 17% greater compared to those with normal renal function. The dialysis clearance of apixaban is approximately 18 mL/min resulting in a 14% decrease in exposure due to hemodialysis compared to off-dialysis period.
- Protein binding was similar (92%-94%) between healthy controls and the on-dialysis and off-dialysis periods.
Nonclinical Toxicology
- Carcinogenesis
- Apixaban was not carcinogenic when administered to mice and rats for up to 2 years. The systemic exposures (AUCs) of unbound apixaban in male and female mice at the highest doses tested (1500 and 3000 mg/kg/day) were 9 and 20 times, respectively, the human exposure of unbound drug at the MRHD of 10 mg/day. Systemic exposures of unbound apixaban in male and female rats at the highest dose tested (600 mg/kg/day) were 2 and 4 times, respectively, the human exposure.
- Mutagenesis
- Apixaban was neither mutagenic in the bacterial reverse mutation (Ames) assay, nor clastogenic in Chinese hamster ovary cells in vitro, in a 1-month in vivo/in vitro cytogenetics study in rat peripheral blood lymphocytes, or in a rat micronucleus study in vivo.
- Impairment of Fertility
- Apixaban had no effect on fertility in male or female rats when given at doses up to 600 mg/kg/day, a dose resulting in exposure levels that are 3 and 4 times, respectively, the human exposure.
- Apixaban administered to female rats at doses up to 1000 mg/kg/day from implantation through the end of lactation produced no adverse findings in male offspring (F1 generation) at doses up to 1000 mg/kg/day, a dose resulting in exposure that is 5 times the human exposure. Adverse effects in the F1-generation female offspring were limited to decreased mating and fertility indices at 1000 mg/kg/day.
Clinical Studies
Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
ARISTOTLE
- Evidence for the efficacy and safety of Eliquis was derived from ARISTOTLE, a multinational, double-blind study in patients with nonvalvular AF comparing the effects of Eliquis and warfarin on the risk of stroke and non-central nervous system (CNS) systemic embolism. In ARISTOTLE, patients were randomized to Eliquis 5 mg orally twice daily (or 2.5 mg twice daily in subjects with at least 2 of the following characteristics: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL) or to warfarin (targeted to an INR range of 2.0–3.0). Patients had to have one or more of the following additional risk factors for stroke:
- Prior stroke or transient ischemic attack (TIA)
- Prior systemic embolism
- Age ≥75 years
- Arterial hypertension requiring treatment
- Diabetes mellitus
- Heart failure ≥New York Heart Association Class 2
- Left ventricular ejection fraction ≤40%
- The primary objective of ARISTOTLE was to determine whether Eliquis 5 mg twice daily (or 2.5 mg twice daily) was effective (noninferior to warfarin) in reducing the risk of stroke (ischemic or hemorrhagic) and systemic embolism. Superiority of Eliquis to warfarin was also examined for the primary endpoint (rate of stroke and systemic embolism), major bleeding, and death from any cause.
- A total of 18,201 patients were randomized and followed on study treatment for a median of 89 weeks. Forty-three percent of patients were vitamin K antagonist (VKA) “naive,” defined as having received ≤30 consecutive days of treatment with warfarin or another VKA before entering the study. The mean age was 69 years and the mean CHADS2 score (a scale from 0 to 6 used to estimate risk of stroke, with higher scores predicting greater risk) was 2.1. The population was 65% male, 83% Caucasian, 14% Asian, and 1% Black. There was a history of stroke, TIA, or non-CNS systemic embolism in 19% of patients. Concomitant diseases of patients in this study included hypertension 88%, diabetes 25%, congestive heart failure (or left ventricular ejection fraction ≤40%) 35%, and prior myocardial infarction 14%. Patients treated with warfarin in ARISTOTLE had a mean percentage of time in therapeutic range (INR 2.0–3.0) of 62%.
- Eliquis was superior to warfarin for the primary endpoint of reducing the risk of stroke and systemic embolism (Table 5 and Figure 4). Superiority to warfarin was primarily attributable to a reduction in hemorrhagic stroke and ischemic strokes with hemorrhagic conversion compared to warfarin. Purely ischemic strokes occurred with similar rates on both drugs.
- Eliquis also showed significantly fewer major bleeds than warfarin.

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
- All-cause death was assessed using a sequential testing strategy that allowed testing for superiority if effects on earlier endpoints (stroke plus systemic embolus and major bleeding) were demonstrated. Eliquis treatment resulted in a significantly lower rate of all-cause death (p = 0.046) than did treatment with warfarin, primarily because of a reduction in cardiovascular death, particularly stroke deaths. Non-vascular death rates were similar in the treatment arms.
- In ARISTOTLE, the results for the primary efficacy endpoint were generally consistent across most major subgroups including weight, CHADS2 score (a scale from 0 to 6 used to predict risk of stroke in patients with AF, with higher scores predicting greater risk), prior warfarin use, level of renal impairment, geographic region, Eliquis dose, type of AF, and aspirin use at randomization (Figure 5).
- At the end of the ARISTOTLE study, warfarin patients who completed the study were generally maintained on a VKA with no interruption of anticoagulation. Eliquis patients who completed the study were generally switched to a VKA with a 2-day period of coadministration of Eliquis and VKA, so that some patients may not have been adequately anticoagulated after stopping Eliquis until attaining a stable and therapeutic INR. During the 30 days following the end of the study, there were 21 stroke or systemic embolism events in the 6791 patients (0.3%) in the Eliquis arm compared to 5 in the 6569 patients (0.1%) in the warfarin arm.
AVERROES
- In AVERROES, patients with nonvalvular atrial fibrillation thought not to be candidates for warfarin therapy were randomized to treatment with Eliquis 5 mg orally twice daily (or 2.5 mg twice daily in selected patients) or aspirin 81 to 324 mg once daily. The primary objective of the study was to determine if Eliquis was superior to aspirin for preventing the composite outcome of stroke or systemic embolism. AVERROES was stopped early on the basis of a prespecified interim analysis showing a significant reduction in stroke and systemic embolism for Eliquis compared to aspirin that was associated with a modest increase in major bleeding (Table 6).
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- The clinical evidence for the effectiveness of Eliquis is derived from the ADVANCE-1, ADVANCE-2, and ADVANCE-3 clinical trials in adult patients undergoing elective hip (ADVANCE-3) or knee (ADVANCE-2 and ADVANCE-1) replacement surgery. A total of 11,659 patients were randomized in 3 double-blind, multi-national studies. Included in this total were 1866 patients age 75 or older, 1161 patients with low body weight (≤60 kg), 2528 patients with Body Mass Index ≥33 kg/m2, and 625 patients with severe or moderate renal impairment.
- In the ADVANCE-3 study, 5407 patients undergoing elective hip replacement surgery were randomized to receive either Eliquis 2.5 mg orally twice daily or enoxaparin 40 mg subcutaneously once daily. The first dose of Eliquis was given 12 to 24 hours post surgery, whereas enoxaparin was started 9 to 15 hours prior to surgery. Treatment duration was 32 to 38 days.
- In patients undergoing elective knee replacement surgery, Eliquis 2.5 mg orally twice daily was compared to enoxaparin 40 mg subcutaneously once daily (ADVANCE-2, N=3057) or enoxaparin 30 mg subcutaneously every 12 hours (ADVANCE-1, N=3195). In the ADVANCE-2 study, the first dose of Eliquis was given 12 to 24 hours post surgery, whereas enoxaparin was started 9 to 15 hours prior to surgery. In the ADVANCE-1 study, both Eliquis and enoxaparin were initiated 12 to 24 hours post surgery. Treatment duration in both ADVANCE-2 and ADVANCE-1 was 10 to 14 days.
- In all 3 studies, the primary endpoint was a composite of adjudicated asymptomatic and symptomatic DVT, nonfatal PE, and all-cause death at the end of the double-blind intended treatment period. In ADVANCE-3 and ADVANCE-2, the primary endpoint was tested for noninferiority, then superiority, of Eliquis to enoxaparin. In ADVANCE-1, the primary endpoint was tested for noninferiority of Eliquis to enoxaparin.
- The efficacy data are provided in Tables 7 and 8.

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
- The efficacy profile of Eliquis was generally consistent across subgroups of interest for this indication (e.g., age, gender, race, body weight, renal impairment).
How Supplied

Storage
There is limited information regarding Apixaban Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Apixaban |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Apixaban |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Apixaban interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Eliquis®[2]
Look-Alike Drug Names
- N/A[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Agnelli, Giancarlo (2013-02-21). "Apixaban for extended treatment of venous thromboembolism". The New England Journal of Medicine. 368 (8): 699–708. doi:10.1056/NEJMoa1207541. ISSN 1533-4406. PMID 23216615. Unknown parameter
|coauthors=ignored (help) - ↑ "Eliquis (apixaban) tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Apixaban |Pill Name=Eliquis_NDC_00030894.jpg |Drug Name=Eliquis |Pill Ingred=apixaban[apixaban]|+sep=; |Pill Imprint=894;5 |Pill Dosage=5 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=10 |Pill Scoring=1 |Pill Image= |Drug Author=E.R. Squibb & Sons, L.L.C. |NDC=00030894
}}
{{#subobject:
|Label Page=Apixaban |Label Name=Apixaban17.png
}}
{{#subobject:
|Label Page=Apixaban |Label Name=Apixaban18.png
}}
{{#subobject:
|Label Page=Apixaban |Label Name=Apixaban19.png
}}
{{#subobject:
|Label Page=Apixaban |Label Name=Apixaban20.png
}}







