Low molecular weight heparin
Template:WikiDoc Cardiology News Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
In medicine, low molecular weight heparin (LMWH) is a class of medication used as an anticoagulant in diseases that feature thrombosis, as well as for prophylaxis in situations that lead to a high risk of thrombosis.[1]
Heparin is a naturally-occurring polysaccharide that inhibits coagulation, the process whereby thrombosis occurs (see Heparin: Mechanisms of action). Natural heparin consists of molecular chains of varying lengths, or molecular weights. Chains of molecular weight from 5000 to over 40,000 Daltons, making up polydisperse pharmacetical grade heparin.[2]
Heparin derived from natural sources, mainly porcine intestine or bovine lung, can be administered therapeutically to prevent thrombosis (see anticoagulation). However, the effects of natural, or unfractionated, heparin can be difficult to predict. After a standard dose of unfractionated heparin, coagulation parameters must be monitored very closely to prevent over- or under-anticoagulation.
Low molecular weight heparins (LMWHs), in contrast, consist of only short chains of polysaccharide. LMWHs are defined as heparin salts having an average molecular weight of less than 8000 Da and for which at least 60% of all chains have a molecular weight less than 8000 Da. These are obtained by various methods of fractionation or depolymerisation of polymeric heparin. They have a potency of greater than 70 units/mg of anti factor Xa activity and a ratio of anti factor Xa activity to anti thrombin activity of >1.5.[3]
Low molecular weight heparin products
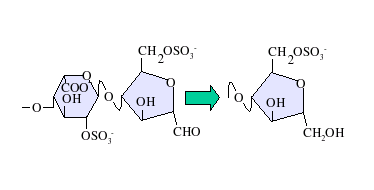
Various methods of heparin depolymerisation are used in the manufacture of low molecular weight heparin.[4] These are listed below:
- Oxidative depolymerisation with hydrogen peroxide. Used in the manufacture of ardeparin (Normiflo®)
- Deaminative cleavage with isoamyl nitrite. Used in the manufacture of certoparin (Sandoparin®)
- Alkaline beta-eliminative cleavage of the benzyl ester of heparin. Used in the manufacture of enoxaparin (Lovenox® and Clexane®)
- Oxidative depolymerisation with Cu2+ and hydrogen peroxide. Used in the manufacture of parnaparin (Fluxum®)
- Beta-eliminative cleavage by the heparinase enzyme. Used in the manufacture of tinzaparin (Innohep® and Logiparin®)
- Deaminative cleavage with nitrous acid. Used in the manufacture of dalteparin (Fragmin®), reviparin (Clivarin®) and nadroparin (Fraxiparin®)
Deaminative cleavage with nitrous acid results in the formation of an unnatural anhydromannose residue at the reducing terminal of the oligosaccharides produced. This can subsequently be converted to anhydromannitol using a suitable reducing agent as shown to the left.
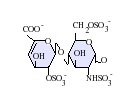
Likewise both chemical and enzymatic beta-elimination result in the formation of an unnatural unsaturated uronate residue(UA) at the non-reducing terminal, as shown to the left.
Differences between low molecular weight heparin products
Comparisons between LMWHs prepared by similar processes vary. For example a comparison of Dalteparin and Nadroparin suggests they are more similar than products produced by different processes.However comparison of enoxaparin and tinzaparin shows they are very different to each other with respect to chemical, physical and biological properties.
As might be expected products prepared by distinctly different processes are dissimilar in physical, chemical and biological properties.
see references.[5][6][7][8][9]
Differences from unfractionated heparin
Its differences with unfractioned heparin include:
- Average molecular weight: heparin is about 20000 Da and LMWH is about 3000 Da
- Once daily dosing, rather than a continuous infusion of unfractionated heparin
- No need for monitoring of the APTT coagulation parameter
- Possibly a smaller risk of bleeding
- Smaller risk of osteoporosis in long-term use
- Smaller risk of heparin-induced thrombocytopenia, a feared side-effect of heparin.
- The anticoagulant effects of heparin are typically reversible with protamine sulfate, while the effect on LMWH is limited
Clinical uses
Because it can be given subcutaneously and does not require APTT monitoring, LMWH permits outpatient treatment of conditions such as deep vein thrombosis or pulmonary embolism that previously mandated inpatient hospitalization for unfractionated heparin administration
The use of LMWH needs to be monitored closely in patients at extremes of weight or in patients with renal dysfunction. An anti-factor Xa activity may be useful for monitoring anticoagulation. Given its renal clearance, LMWH may not be feasible in patients who have end stage renal disease.
Use in venothromboembolic disease associated with cancer
The CLOT study, published in 2003, showed that in patients with malignancy and acute venous thromboembolism, dalteparin was more effective than coumarin in reducing the risk of recurrent embolic events.[10]
According to PROTECT study, the low-molecular-weight heparin dalteparin was found to be no better than unfractionated heparin in preventing proximal leg deep vein thrombosis among critically ill adults.[11]
References
- ↑ Weitz JI (1997). "Low-molecular-weight heparins". N Engl J Med. 337 (10): 688–98. PMID 9278467.
- ↑ Linhardt, R.J. Gunay, N. S. (1999). "Production and chemical processing of low molecular weight heparins". Sem. Thromb. Hem. 25 (3): 5–16.
- ↑ European Pharmacopedia Commission (1991). Pharmeuropa. 3: 161–165. Missing or empty
|title=(help) - ↑ Linhardt, R.J. Gunay, N. S. (1999). "Production and chemical processing of low molecular weight heparins". Sem. Thromb. Hem. 25 (3): 5–16.
- ↑ Green, D. Hirsh, J. Heit, J.; et al. (1991). "Low molecular weight heparin: A critical analysis of clinical trials". Pharmacol. Rev. 2: 45–50.
- ↑ Barrowcliffe, T. W. (1995). "Low molecular weight heparin(s)". Br. J. Haematol. 90: 1–7.
- ↑ Donayre C. E. (1996). "Current use of low molecular weight heparins". Semin. Vascul. Surg. 9: 362–371.
- ↑ Hunt, D. (1998). "Low molecular weight heparins in clinical practice". Southern Medical J. 91: 2–10.
- ↑ Fareed, J. Jeske, W. Hoppensteadt, D. Clarizio, R. Walenga, J. M. (1998). "Low molecular weight heparins: Pharmacologic profile and product differentiation". Am. J. Cardiol. 82: 3L–10L.
- ↑ Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M (2003). "Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer". N Engl J Med. 349 (2): 146–53. PMID 12853587.
- ↑ PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D; et al. (2011). "Dalteparin versus unfractionated heparin in critically ill patients". N Engl J Med. 364 (14): 1305–14. doi:10.1056/NEJMoa1014475. PMID 21417952. Review in: Ann Intern Med. 2011 Jul 19;155(2):JC1-7
External links
- Low+Molecular+Weight+Heparin at the US National Library of Medicine Medical Subject Headings (MeSH)