Prasugrel
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Adeel Jamil, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: BLEEDING RISK
See full prescribing information for complete Boxed Warning.
* Effient can cause significant, sometimes fatal, bleeding (5.1, 5.2, 6.1).
|
Overview
Prasugrel is a P2Y12 platelet inhibitor, Platelet aggregation inhibitor that is FDA approved for the treatment of Acute Coronary Syndrome. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, hyperlipidemia, backache, headache, epistaxis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Coronary Syndrome
- Dosing Information
- Initial loading dosage: 60 mg PO
- Maintaining dosage: 10 mg PO qd
- Incombination with: aspirin 75 mg-325 mg
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prasugrel in adult patients.
Non–Guideline-Supported Use
Prophylaxis treatment of Thrombosis of Acute coronary syndrome
- Dosing Information
- Recommended: 10 mg/day[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Prasugrel FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prasugrel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Prasugrel in pediatric patients.
Contraindications
- Active Bleeding
- Effient is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage [see Warnings and Precautions and Adverse Reactions ].
- Prior Transient Ischemic Attack or Stroke
- Effient is contraindicated in patients with a history of prior transient ischemic attack (TIA) or stroke. In TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel), patients with a history of TIA or ischemic stroke (>3 months prior to enrollment) had a higher rate of stroke on Effient (6.5%; of which 4.2% were thrombotic stroke and 2.3% were intracranial hemorrhage [[[ICH]]]) than on clopidogrel (1.2%; all thrombotic). In patients without such a history, the incidence of stroke was 0.9% (0.2% ICH) and 1.0% (0.3% ICH) with Effient and clopidogrel, respectively. Patients with a history of ischemic stroke within 3 months of screening and patients with a history of hemorrhagic stroke at any time were excluded from TRITON-TIMI 38. Patients who experience a stroke or TIA while on Effient generally should have therapy discontinued [see Adverse Reactions and Clinical Studies].
- Effient is contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to prasugrel or any component of the product [see Adverse Reactions].
Warnings
|
WARNING: BLEEDING RISK
See full prescribing information for complete Boxed Warning.
* Effient can cause significant, sometimes fatal, bleeding (5.1, 5.2, 6.1).
|
General Risk of Bleeding==
clopidogrel, including Effient, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL) bleeding events were more common on Effient than on clopidogrel [see Adverse Reactions]. The bleeding risk is highest initially, as shown in Figure 1 (events through 450 days; inset shows events through 7 days).

Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding. Do not use Effient in patients with active bleeding, prior TIA or stroke [see Contraindications]. Other risk factors for bleeding are:
- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of Effient is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered [see Adverse Reactions, Use in Specific Populations, Clinical Pharmacology, and Clinical Studies].
- CABG or other surgical procedure [see Warnings and Precautions].
- Body weight <60 kg. Consider a lower (5-mg) maintenance dose [see Dosage and Administration, Adverse Reactions, and Use in Specific Populations].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment) [see Adverse Reactions and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of non-steroidal anti-inflammatory drugs NSAIDs, and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38 [see Drug Interaction, and Clinical Studies].
clopidogrel inhibit platelet aggregation for the lifetime of the platelet (7-10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel's active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.
Coronary Artery Bypass Graft Surgery-Related Bleeding
The risk of bleeding is increased in patients receiving Effient who undergo CABG. If possible, Effient should be discontinued at least 7 days prior to CABG. Of the 437 patients who underwent CABG during TRITON-TIMI 38, the rates of CABG-related TIMI Major or Minor bleeding were 14.1% in the Effient group and 4.5% in the clopidogrel group [see Adverse Reactions]. The higher risk for bleeding events in patients treated with Effient persisted up to 7 days from the most recent dose of study drug. For patients receiving a thienopyridine within 3 days prior to CABG, the frequencies of TIMI Major or Minor bleeding were 26.7% (12 of 45 patients) in the Effient group, compared with 5.0% (3 of 60 patients) in the clopidogrel group. For patients who received their last dose of thienopyridine within 4 to 7 days prior to CABG, the frequencies decreased to 11.3% (9 of 80 patients) in the prasugrel group and 3.4% (3 of 89 patients) in the clopidogrel group. Do not start Effient in patients likely to undergo urgent CABG. CABG-related bleeding may be treated with transfusion of blood products, including packed red blood cells and platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.
Discontinuation of Effient
Discontinue clopidogrel, including Effient, for active bleeding, elective surgery, stroke, or TIA. The optimal duration of thienopyridine therapy is unknown. In patients who are managed with PCI and stent placement, premature discontinuation of any antiplatelet medication, including clopidogrel, conveys an increased risk of stent thrombosis, myocardial infarction, and death. Patients who require premature discontinuation of a thienopyridine will be at increased risk for cardiac events. Lapses in therapy should be avoided, and if clopidogrel must be temporarily discontinued because of an adverse event(s), they should be restarted as soon as possible [see Contraindications and Warnings and Precautions].
Thrombotic Thrombocytopenic Purpura
Thrombotic thrombocytopenic purpura (TTP) has been reported with the use of Effient. TTP can occur after a brief exposure (<2 weeks). TTP is a serious condition that can be fatal and requires urgent treatment, including plasmapheresis (plasma exchange). TTP is characterized by thrombocytopenia, microangiopathic hemolytic anemia (schistocytes [[[Red blood cell|fragment red blood cells]]] seen on peripheral smear), neurological findings, renal dysfunction, and fever [see Adverse Reactions].
Hypersensitivity Including Angioedema
Hypersensitivity including angioedema has been reported in patients receiving Effient, including patients with a history of hypersensitivity reaction to other clopidogrel [see Contraindications and Adverse Reactions].
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are also discussed elsewhere in the labeling:
- Bleeding [see Boxed Warning and Warnings and Precautions]
- Thrombotic thrombocytopenic purpura [see Warnings and Precautions]
Safety in patients with ACS undergoing PCI was evaluated in a clopidogrel-controlled study, TRITON-TIMI 38, in which 6741 patients were treated with Effient (60-mg loading dose and 10-mg once daily) for a median of 14.5 months (5802 patients were treated for over 6 months; 4136 patients were treated for more than 1 year). The population treated with Effient was 27 to 96 years of age, 25% female, and 92% Caucasian. All patients in the TRITON-TIMI 38 study were to receive aspirin. The dose of clopidogrel in this study was a 300-mg loading dose and 75-mg once daily. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials cannot be directly compared with the rates observed in other clinical trials of another drug and may not reflect the rates observed in practice.
- Drug Discontinuation
- The rate of study drug discontinuation because of adverse reactions was 7.2% for Effient and 6.3% for clopidogrel. Bleeding was the most common adverse reaction leading to study drug discontinuation for both drugs (2.5% for Effient and 1.4% for clopidogrel).
- Bleeding
- Bleeding Unrelated to CABG Surgery - In TRITON-TIMI 38, overall rates of TIMI Major or Minor bleeding adverse reactions unrelated to coronary artery bypass graft surgery (CABG) were significantly higher on Effient than on clopidogrel, as shown in Table 1.

Figure 1 demonstrates non-CABG related TIMI Major or Minor bleeding. The bleeding rate is highest initially, as shown in Figure 1 (inset: Days 0 to 7) [see Warnings and Precautions]. Bleeding by Weight and Age - In TRITON-TIMI 38, non-CABG-related TIMI Major or Minor bleeding rates in patients with the risk factors of age ≥75 years and weight <60 kg are shown in Table 2.

Bleeding Related to CABG - In TRITON-TIMI 38, 437 patients who received a thienopyridine underwent CABG during the course of the study. The rate of CABG-related TIMI Major or Minor bleeding was 14.1% for the Effient group and 4.5% in the clopidogrel group (see Table 3). The higher risk for bleeding adverse reactions in patients treated with Effient persisted up to 7 days from the most recent dose of study drug.

Bleeding Reported as Adverse Reactions - Hemorrhagic events reported as adverse reactions in TRITON-TIMI 38 were, for Effient and clopidogrel, respectively: epistaxis (6.2%, 3.3%), gastrointestinal hemorrhage (1.5%, 1.0%), hemoptysis (0.6%, 0.5%), subcutaneous hematoma (0.5%, 0.2%), post-procedural hemorrhage (0.5%, 0.2%), retroperitoneal hemorrhage (0.3%, 0.2%), pericardial effusion/hemorrhage/tamponade (0.3%, 0.2%), and retinal hemorrhage (0.0%, 0.1%).
During TRITON-TIMI 38, newly-diagnosed malignancies were reported in 1.6% and 1.2% of patients treated with prasugrel and clopidogrel, respectively. The sites contributing to the differences were primarily colon and lung. In another Phase 3 clinical study of ACS patients not undergoing PCI, in which data for malignancies were prospectively collected, newly-diagnosed malignancies were reported in 1.8% and 1.7% of patients treated with prasugrel and clopidogrel, respectively. The site of malignancies was balanced between treatment groups except for colorectal malignancies. The rates of colorectal malignancies were 0.3% prasugrel, 0.1% clopidogrel and most were detected during investigation of GI bleed or anemia. It is unclear if these observations are causally-related, are the result of increased detection because of bleeding, or are random occurrences.
Other Adverse Events
In TRITON-TIMI 38, common and other important non-hemorrhagic adverse events were, for Effient and clopidogrel, respectively: severe thrombocytopenia (0.06%, 0.04%), anemia (2.2%, 2.0%), abnormal hepatic function (0.22%, 0.27%), allergic reactions (0.36%, 0.36%), and angioedema (0.06%, 0.04%). Table 4 summarizes the adverse events reported by at least 2.5% of patients.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of Effient. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders — Thrombocytopenia, Thrombotic thrombocytopenic purpura (TTP) [see Warnings and Precautions and Patient Counseling Information (17)]
- Immune system disorders — Hypersensitivity reactions including anaphylaxis [see Contraindications]
Drug Interactions
- Coadministration of Effient and warfarin increases the risk of bleeding [see Warnings and Precautions and Clinical Pharmacology].
- Coadministration of Effient and NSAIDs (used chronically) may increase the risk of bleeding [see Warnings and Precautions].
- Other Concomitant Medications
- Effient can be administered with drugs that are inducers or inhibitors of cytochrome P450 enzymes [see Clinical Pharmacology].
- Effient can be administered with aspirin (75-mg to 325-mg per day), heparin, GPIIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including proton pump inhibitors and H2 blockers [see Clinical Pharmacology].
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Pregnancy Category B - There are no adequate and well-controlled studies of Effient use in pregnant women. Reproductive and developmental toxicology studies in rats and rabbits at doses of up to 30 times the recommended therapeutic exposures in humans (based on plasma exposures to the major circulating human metabolite) revealed no evidence of fetal harm; however, animal studies are not always predictive of a human response. Effient should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
In embryo fetal developmental toxicology studies, pregnant rats and rabbits received prasugrel at maternally toxic oral doses equivalent to more than 40 times the human exposure. A slight decrease in pup body weight was observed; but, there were no structural malformations in either species. In prenatal and postnatal rat studies, maternal treatment with prasugrel had no effect on the behavioral or reproductive development of the offspring at doses greater than 150 times the human exposure [see Nonclinical Toxicology].
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Prasugrel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Prasugrel during labor and delivery.
Nursing Mothers
It is not known whether Effient is excreted in human milk; however, metabolites of Effient were found in rat milk. Because many drugs are excreted in human milk, prasugrel should be used during nursing only if the potential benefit to the mother justifies the potential risk to the nursing infant.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established [see Clinical Pharmacology].
Geriatic Use
In TRITON-TIMI 38, 38.5% of patients were ≥65 years of age and 13.2% were ≥75 years of age. The risk of bleeding increased with advancing age in both treatment groups, although the relative risk of bleeding (Effient compared with clopidogrel) was similar across age groups. Patients ≥75 years of age who received Effient 10-mg had an increased risk of fatal bleeding events (1.0%) compared to patients who received clopidogrel (0.1%). In patients ≥75 years of age, symptomatic intracranial hemorrhage occurred in 7 patients (0.8%) who received Effient and in 3 patients (0.3%) who received clopidogrel. Because of the risk of bleeding, and because effectiveness is uncertain in patients ≥75 years of age [see Clinical Studies], use of Effient is generally not recommended in these patients, except in high-risk situations (diabetes and past history of myocardial infarction) where its effect appears to be greater and its use may be considered [see Warnings and Precautions, Clinical Pharmacology, and Clinical Studies].
Gender
There is no FDA guidance on the use of Prasugrel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Prasugrel with respect to specific racial populations.
Renal Impairment
No dosage adjustment is necessary for patients with renal impairment. There is limited experience in patients with end-stage renal disease, but such patients are generally at higher risk of bleeding [see Warnings and Precautions and Clinical Pharmacology].
Hepatic Impairment
No dosage adjustment is necessary in patients with mild to moderate hepatic impairment (Child-Pugh Class A and B). The pharmacokinetics and pharmacodynamics of prasugrel in patients with severe hepatic disease have not been studied, but such patients are generally at higher risk of bleeding [see Warnings and Precautions and Clinical Pharmacology].
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Prasugrel in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Prasugrel in patients who are immunocompromised.
Low Body Weight
In TRITON-TIMI 38, 4.6% of patients treated with Effient had body weight <60 kg. Individuals with body weight <60 kg had an increased risk of bleeding and an increased exposure to the active metabolite of prasugrel [see Dosage and Administration, Warnings and Precautions, and Clinical Pharmacology]. Consider lowering the maintenance dose to 5-mg in patients <60 kg. The effectiveness and safety of the 5-mg dose have not been prospectively studied [see Dosage and Administration and Clinical Pharmacology].
Administration and Monitoring
Administration
Initiate Effient treatment as a single 60-mg oral loading dose and then continue at 10-mg orally once daily. Patients taking Effient should also take aspirin (75-mg to 325-mg) daily [see Drug Interaction and Clinical Pharmacology]. Effient may be administered with or without food [see Clinical Pharmacology and Clinical Studies].
Dosing in Low Weight Patients
Compared to patients weighing ≥60 kg, patients weighing <60 kg have an increased exposure to the active metabolite of prasugrel and an increased risk of bleeding on a 10-mg once daily maintenance dose. Consider lowering the maintenance dose to 5-mg in patients <60 kg. The effectiveness and safety of the 5-mg dose have not been prospectively studied [see Warnings and Precautions, Adverse Reactions, and Clinical Pharmacology].
Monitoring
There is limited information regarding Prasugrel Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Prasugrel and IV administrations.
Overdosage
- Signs and Symptoms
- Platelet inhibition by prasugrel is rapid and irreversible, lasting for the life of the platelet, and is unlikely to be increased in the event of an overdose. In rats, lethality was observed after administration of 2000 mg/kg. Symptoms of acute toxicity in dogs included emesis, increased serum alkaline phosphatase, and hepatocellular atrophy. Symptoms of acute toxicity in rats included mydriasis, irregular respiration, decreased locomotor activity, ptosis, staggering gait, and lacrimation.
- Recommendations about Specific Treatment
- Platelet transfusion may restore clotting ability. The prasugrel active metabolite is not likely to be removed by dialysis.
Pharmacology
Mechanism of Action
Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
Structure
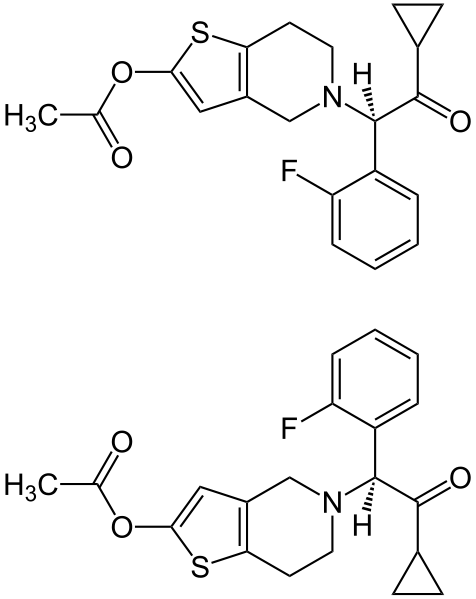
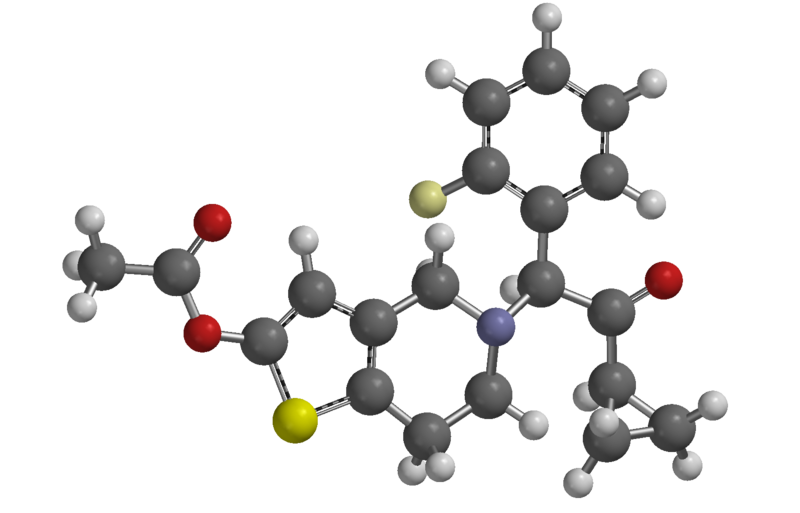
Effient contains prasugrel, a thienopyridine class inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP receptor. Effient is formulated as the hydrochloride salt, a racemate, which is chemically designated as 5-[(1RS)-2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate hydrochloride. Prasugrel hydrochloride has the empirical formula C20H20FNO3S•HCl representing a molecular weight of 409.90. The chemical structure of prasugrel hydrochloride is:

Prasugrel hydrochloride is a white to practically white solid. It is soluble at pH 2, slightly soluble at pH 3 to 4, and practically insoluble at pH 6 to 7.5. It also dissolves freely in methanol and is slightly soluble in 1- and 2-propanol and acetone. It is practically insoluble in diethyl ether and ethyl acetate. Effient is available for oral administration as 5-mg or 10-mg elongated hexagonal, film-coated, non-scored tablets, debossed on each side. Each yellow 5-mg tablet is manufactured with 5.49 mg prasugrel hydrochloride, equivalent to 5-mg prasugrel and each beige 10-mg tablet with 10.98 mg prasugrel hydrochloride, equivalent to 10-mg of prasugrel.
Original Formulation. During manufacture and storage, partial conversion from prasugrel hydrochloride to prasugrel free base may occur. Other ingredients include mannitol, hypromellose, croscarmellose sodium, microcrystalline cellulose, and vegetable magnesium stearate. The color coatings contain lactose, hypromellose, titanium dioxide, triacetin, iron oxide yellow, and iron oxide red (only in Effient 10-mg tablet).
Revised Formulation Other ingredients include mannitol, hypromellose, low-substituted hydroxypropyl cellulose, microcrystalline cellulose, sucrose stearate, and glyceryl behenate. The color coatings contain lactose, hypromellose, titanium dioxide, triacetin, iron oxide yellow, and iron oxide red (only in Effient 10-mg tablet).
Pharmacodynamics
Prasugrel produces inhibition of platelet aggregation to 20 μM or 5 μM ADP, as measured by light transmission aggregometry. Following a 60-mg loading dose of Effient, approximately 90% of patients had at least 50% inhibition of platelet aggregation by 1 hour. Maximum platelet inhibition was about 80% (see Figure 2). Mean steady-state inhibition of platelet aggregation was about 70% following 3 to 5 days of dosing at 10-mg daily after a 60-mg loading dose of Effient.

Platelet aggregation gradually returns to baseline values over 5-9 days after discontinuation of prasugrel, this time course being a reflection of new platelet production rather than pharmacokinetics of prasugrel. Discontinuing clopidogrel 75-mg and initiating a prasugrel 10-mg maintenance dose with or without a prasugrel 60-mg loading dose results in a decrease of 14 percentage points in maximum platelet aggregation (MPA) by Day 7. This decrease in MPA is not greater than that typically produced by a 10-mg maintenance dose of prasugrel alone. The relationship between inhibition of platelet aggregation and clinical activity has not been established. 5-mg in Low Body Weight Patients - In patients with stable coronary artery disease, mean platelet inhibition in subjects <60 kg taking 5-mg prasugrel was similar to that of subjects ≥60 kg taking 10-mg prasugrel. The relationship between inhibition of platelet aggregation and clinical activity has not been established.
Pharmacokinetics
Prasugrel is a prodrug and is rapidly metabolized to a pharmacologically active metabolite and inactive metabolites. The active metabolite has an elimination half-life of about 7 hours (range 2-15 hours). Healthy subjects, patients with stable atherosclerosis, and patients undergoing PCI show similar pharmacokinetics.
Absorption and Binding - Following oral administration, ≥79% of the dose is absorbed. The absorption and metabolism are rapid, with peak plasma concentrations (Cmax) of the active metabolite occurring approximately 30 minutes after dosing. The active metabolite's exposure (AUC) increases slightly more than proportionally over the dose range of 5 to 60-mg. Repeated daily doses of 10-mg do not lead to accumulation of the active metabolite. In a study of healthy subjects given a single 15-mg dose, the AUC of the active metabolite was unaffected by a high fat, high calorie meal, but Cmax was decreased by 49% and Tmax was increased from 0.5 to 1.5 hours. Effient can be administered without regard to food. The active metabolite is bound about 98% to human serum albumin.
Metabolism and Elimination - Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel's active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins. Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites.
Nonclinical Toxicology
Carcinogenesis - No compound-related tumors were observed in a 2-year rat study with prasugrel at oral doses up to 100 mg/kg/day (>100 times the recommended therapeutic exposures in humans (based on plasma exposures to the major circulating human metabolite). There was an increased incidence of tumors (Hepatocellular adenoma
Clinical Studies
There is limited information regarding Prasugrel Clinical Studies in the drug label.
How Supplied
There is limited information regarding Prasugrel How Supplied in the drug label.
Storage
There is limited information regarding Prasugrel Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Prasugrel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Prasugrel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Prasugrel Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Prasugrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Prasugrel Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Prasugrel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG et al. (2012) Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 367 (14):1297-309. DOI:10.1056/NEJMoa1205512 PMID: 22920930