Edoxaban
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
(A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN Edoxaban should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with Edoxaban 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used. B. PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If Edoxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. C. SPINAL/EPIDURAL HEMATOMA Epidural or spinal hematomas may occur in patients treated with Edoxaban who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. |
Overview
Edoxaban is a factor Xa inhibitor that is FDA approved for the prevention of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). Is also indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding and anemia in the treatment of NVAF (≥ 5%) and bleeding, rash, abnormal liver function tests and anemia in the treatment of DVT and PE (≥ 1%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Reduction in the Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
Edoxaban is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF).
- Limitation of Use for NVAF
Edoxaban should not be used in patients with CrCL > 95 mL/min because of an increased risk of ischemic stroke compared to warfarin.
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
Edoxaban is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant.
Dosage
The recommended dose of Edoxaban is 60 mg taken orally once daily. Assess creatinine clearance, as calculated using the Cockcroft-Gault equation*, before initiating therapy with Edoxaban. Do not use Edoxaban in patients with CrCL > 95 mL/min.
Reduce Edoxaban dose to 30 mg once daily in patients with CrCL 15 to 50 mL/min.
*Cockcroft-Gault CrCL = (140-age) x (weight in kg) x (0.85 if female) / (72 x creatinine in mg/dL).
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
The recommended dose of Edoxaban is 60 mg taken orally once daily following 5 to 10 days of initial therapy with a parenteral anticoagulant.
The recommended dose of Edoxaban is 30 mg once daily in patients with CrCL 15 to 50 mL/min, patients who weigh less than or equal to 60 kg, or patients who are taking certain concomitant P-gp inhibitor medications based on clinical study data in this indication.
- Administration Information
If a dose of Edoxaban is missed, the dose should be taken as soon as possible on the same day. Dosing should resume the next day according to the normal dosing schedule. The dose should not be doubled to make up for a missed dose.
Edoxaban can be taken without regard to food.
- Transition to or from Edoxaban
- Transition to Edoxaban

SAVAYSA: Edoxaban tosylate's Brand name
- Transition from Edoxaban
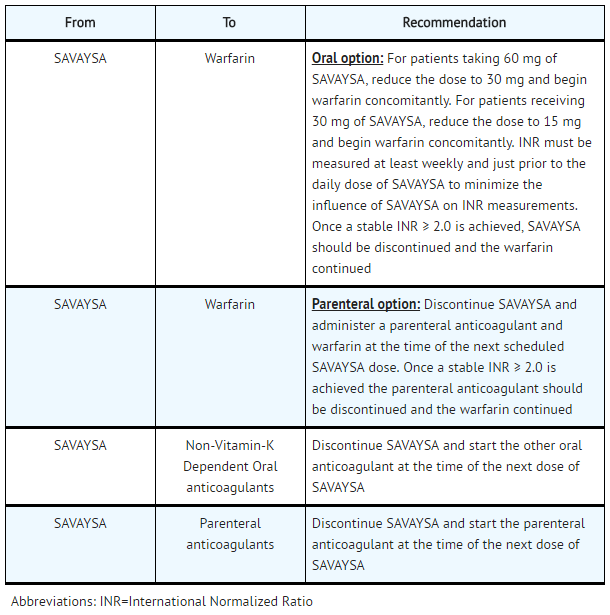
SAVAYSA: Edoxaban tosylate's Brand name
- Discontinuation for Surgery and Other Interventions
Discontinue Edoxaban at least 24 hours before invasive or surgical procedures because of the risk of bleeding.
If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention.
Edoxaban can be restarted after the surgical or other procedure as soon as adequate hemostasis has been established noting that the time to onset of pharmacodynamic effect is 1-2 hours. Administer a parenteral anticoagulant and then switch to oral Edoxaban, if oral medication cannot be taken during or after surgical intervention.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Total arthroplasty of knee - Postoperative deep vein thrombosis (Prophylaxis)[1]
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Edoxaban tosylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Edoxaban tosylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Edoxaban tosylate in pediatric patients.
Contraindications
Edoxaban is contraindicated in patients with active pathological bleeding.
Warnings
|
(A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN Edoxaban should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with Edoxaban 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used. B. PREMATURE DISCONTINUATION OF EDOXABAN TOSYLATE INCREASES THE RISK OF ISCHEMIC EVENTS Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If Edoxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance. C. SPINAL/EPIDURAL HEMATOMA Epidural or spinal hematomas may occur in patients treated with Edoxaban who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. |
Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min
Edoxaban should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study, NVAF patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with Edoxaban 60 mg daily compared to patients treated with warfarin. In these patients another anticoagulant should be used.
Increased Risk of Stroke with Discontinuation of Edoxaban in Patients with Nonvalvular Atrial Fibrillation
Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If Edoxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance.
Risk of Bleeding
Edoxaban increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss.
Discontinue Edoxaban in patients with active pathological bleeding.
Concomitant use of drugs affecting hemostasis may increase the risk of bleeding. These include aspirin and other antiplatelet agents, other antithrombotic agents, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors.
- Reversal of Anticoagulant Effect
There is no established way to reverse the anticoagulant effects of Edoxaban, which can be expected to persist for approximately 24 hours after the last dose. The anticoagulant effect of Edoxaban cannot be reliably monitored with standard laboratory testing. A specific reversal agent for edoxaban is not available. Hemodialysis does not significantly contribute to edoxaban clearance. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of Edoxaban. The use of prothrombin complex concentrates (PCC), or other procoagulant reversal agents such as activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) may be considered but has not been evaluated in clinical outcome studies. When PCCs are used, monitoring for anticoagulation effect of edoxaban using clotting test (PT, INR, or aPTT) or anti-FXa activity is not useful and is not recommended.
Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 12 hours after the last administration of Edoxaban. The next dose of Edoxaban should not be administered earlier than 2 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
Patients with Mechanical Heart Valves or Moderate to Severe Mitral Stenosis
The safety and efficacy of Edoxaban has not been studied in patients with mechanical heart valves or moderate to severe mitral stenosis. The use of Edoxaban is not recommended in these patients.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information.
- Increased risk of stroke with discontinuation of Edoxaban in patients with NVAF
- Spinal/epidural anesthesia or puncture
The most serious adverse reactions reported with Edoxaban were related to bleeding.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Edoxaban was evaluated in the ENGAGE AF-TIMI 48 and Hokusai VTE studies including 11,130 patients exposed to Edoxaban 60 mg and 7002 patients exposed to Edoxaban 30 mg once daily.
- The ENGAGE AF-TIMI 48 Study
In the ENGAGE AF-TIMI 48 study, the median study drug exposure for the Edoxaban and warfarin treatment groups was 2.5 years.
Bleeding was the most common reason for treatment discontinuation. Bleeding led to treatment discontinuation in 3.9% and 4.1% of patients in the Edoxaban 60 mg and warfarin treatment groups, respectively.
In the overall population, Major Bleeding was lower in the Edoxaban group compared to the warfarin group [HR 0.80 (0.70, 0.91), p<0.001]. TABLE 1 shows Major Bleeding events (percentage of patients with at least one bleeding event, per year) for the indicated population (CrCL ≤ 95 mL/min).
- Table 1: Adjudicated Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*

SAVAYSA: Edoxaban tosylate's Brand name
The most common site of a Major Bleeding event was the gastrointestinal (GI) tract. TABLE 2 shows the number of and the rate at which patients experienced GI bleeding in the Edoxaban 60 mg and warfarin treatment groups.
- Table 2: Gastrointestinal Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min*

SAVAYSA: Edoxaban tosylate's Brand name
The rate of anemia-related adverse events was greater with Edoxaban 60 mg than with warfarin (9.6% vs. 6.8%).
The comparative rates of Major Bleeding on Edoxaban and warfarin were generally consistent among subgroups (see FIGURE 1). Bleeding rates appeared higher in both treatment arms (Edoxaban and warfarin) in the following subgroups of patients: those receiving aspirin, those in the United States, those more than 75 years old and those with reduced renal function.
- Figure 1: Adjudicated Major Bleeding in the ENGAGE AF-TIMI 48* Study

SAVAYSA: Edoxaban tosylate's Brand name
- Other Adverse Reactions
The most common non-bleeding adverse reactions (≥ 1%) for Edoxaban 60 mg versus warfarin were rash (4.2% vs. 4.1%), and abnormal liver function tests (4.8% vs. 4.6%), respectively.
Interstitial Lung Disease (ILD) was reported as a serious adverse event on treatment for Edoxaban 60 mg and warfarin in 15 (0.2%) and 7 (0.1%) patients, respectively. Many of the cases in both treatment groups were confounded by the use of amiodarone, which has been associated with ILD, or by infectious pneumonia. In the overall study period, there were 5 and 0 fatal ILD cases in the Edoxaban 60 mg and warfarin groups, respectively.
- The Hokusai VTE Study
In the Hokusai VTE study, the duration of drug exposure for Edoxaban was ≤ 6 months for 1561 (37.9%) of patients, > 6 months for 2557 (62.1%) of patients and 12 months for 1661 (40.3%) of patients.
Bleeding was the most common reason for treatment discontinuation and occurred in 1.4% and 1.4% of patients in the Edoxaban and warfarin arms, respectively.
The primary safety endpoint was Clinically Relevant Bleeding, defined as the composite of Major and Clinically Relevant Non-Major (CRNM) Bleeding that occurred during or within three days of stopping study treatment. The incidence of Clinically Relevant Bleeding was lower in Edoxaban than warfarin [HR (95% CI): 0.81 (0.71, 0.94); p =0.004].
TABLE 3 shows the number of patients experiencing bleeding events in the Hokusai VTE Study.
- Table 3: Bleeding Events in the Hokusai VTE Study

SAVAYSA: Edoxaban tosylate's Brand name
Patients with low body weight (≤ 60 kg), CrCL ≤ 50 mL/min, or concomitant use of select P-gp inhibitors were randomized to receive Edoxaban 30 mg or warfarin. As compared to all patients who received Edoxaban or warfarin in the 60 mg cohort, all patients who received Edoxaban or warfarin in the 30 mg cohort (n= 1452, 17.6% of the entire study population) were older (60.1 vs 54.9 years), more frequently female (66.5% vs 37.7%), more frequently of Asian race (46.0% vs 15.6%) and had more co-morbidities (e.g., history of bleeding, hypertension, diabetes, cardiovascular disease, cancer). Clinically relevant bleeding events occurred in 58/733 (7.9%) of the Edoxaban patients receiving 30 mg once daily and 92/719 (12.8%) of warfarin patients meeting the above criteria.
In the Hokusai VTE study, among all patients the most common bleeding adverse reactions (≥ 1%) are shown in TABLE 4.
- Table 4: Adverse Reactions Occurring in ≥ 1% of Patients Treated in Hokusai VTE

SAVAYSA: Edoxaban tosylate's Brand name
Postmarketing Experience
There is limited information regarding Edoxaban Postmarketing Experience in the drug label.
Drug Interactions
Co-administration of anticoagulants, antiplatelet drugs, and thrombolytics may increase the risk of bleeding. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with anticoagulants, aspirin, other platelet aggregation inhibitors, and/or NSAIDs.
Long-term concomitant treatment with Edoxaban and other anticoagulants is not recommended because of increased risk of bleeding. Short term co-administration may be needed for patients transitioning to or from Edoxaban.
In clinical studies with Edoxaban concomitant use of aspirin (low dose ≤ 100 mg/day) or thienopyridines, and NSAIDs was permitted and resulted in increased rates of Clinically Relevant Bleeding. Carefully monitor for bleeding in patients who require chronic treatment with low dose aspirin and/or NSAIDs.
- P-gp Inducers
Avoid the concomitant use of Edoxaban with rifampin.
- P-gp Inhibitors
- Treatment of NVAF
Based on clinical experience from the ENGAGE AF-TIMI 48 study, dose reduction in patients concomitantly receiving P-gp inhibitors resulted in edoxaban blood levels that were lower than in patients who were given the full dose. Consequently, no dose reduction is recommended for concomitant P-gp inhibitor use.
- Treatment of Deep Vein Thrombosis and Pulmonary Embolism
See Clinical Studies
Use in Specific Populations
Pregnancy
- Risk Summary
There are no adequate and well-controlled studies in pregnant women. Edoxaban should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Human Data
In the Hokusai VTE study there were 10 pregnancy cases reported in patients receiving Edoxaban with exposure in the first trimester and estimated duration of exposure for up to approximately 6 weeks. Among these there were 6 live births (4 full term, 2 pre-term), 1 first-trimester spontaneous abortion, and 3 cases of elective termination of pregnancy.
- Animal Data
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no teratogenic effects were seen when edoxaban was administered orally at doses up to 300 mg/kg/day, or 49 times the human dose of 60 mg/day normalized to body surface area. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal hemorrhage seen at this dose. In rabbits, no teratogenic effects were seen at doses up to 600 mg/kg/day (49 times the human exposure at a dose of 60 mg/day when based on AUC). Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal gallbladder at 600 mg/kg/day, and increased post-implantation loss, increased spontaneous abortion, and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day, which is equal to or greater than 20 times the human exposure.
In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of organogenesis and through lactation day 20 at doses up to 30 mg/kg/day, which is up to 3 times the human exposure when based on AUC. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Edoxaban in women who are pregnant.
Labor and Delivery
Safety and effectiveness of Edoxaban during labor and delivery have not been studied in clinical studies. The risks of bleeding should be balanced with the risk of thrombotic events when considering the use of Edoxaban in this setting.
Nursing Mothers
It is not known if edoxaban is excreted in human milk. Edoxaban was excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from Edoxaban, a decision should be made to discontinue nursing or the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Of the total patients in the ENGAGE AF-TIMI 48 study, 5182 (74%) were 65 years and older, while 2838 (41%) were 75 years and older. In Hokusai VTE, 1334 (32%) patients were 65 years and older, while 560 (14%) patients were 75 years and older. In clinical trials the efficacy and safety of Edoxaban in elderly (65 years or older) and younger patients were similar.
Gender
There is no FDA guidance on the use of Edoxaban with respect to specific gender populations.
Race
There is no FDA guidance on the use of Edoxaban with respect to specific racial populations.
Renal Impairment
Renal clearance accounts for approximately 50% of the total clearance of edoxaban. Consequently, edoxaban blood levels are increased in patients with poor renal function compared to those with higher renal function. Reduce Edoxaban dose to 30 mg once daily in patients with CrCL 15-50 mL/min. There are limited clinical data with Edoxaban in patients with CrCL < 15 mL/min; Edoxaban is therefore not recommended in these patients. Hemodialysis does not significantly contribute to Edoxaban clearance.
As renal function improves and edoxaban blood levels decrease, the risk for ischemic stroke increases in patients with NVAF.
Hepatic Impairment
The use of Edoxaban in patients with moderate or severe hepatic impairment (Child-Pugh B and C) is not recommended as these patients may have intrinsic coagulation abnormalities. No dose reduction is required in patients with mild hepatic impairment (Child-Pugh A).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Edoxaban in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Edoxaban in patients who are immunocompromised.
Low Body Weight Consideration for Patients treated for DVT and/or PE
Based on the clinical experience from the Hokusai VTE study, reduce Edoxaban dose to 30 mg in patients with body weight less than or equal to 60 kg.
Administration and Monitoring
Administration
There is limited information regarding Edoxaban Administration in the drug label.
Monitoring
There is limited information regarding Edoxaban Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Edoxaban and IV administrations.
Overdosage
A specific reversal agent for edoxaban is not available. Overdose of Edoxaban increases the risk of bleeding.
The following are not expected to reverse the anticoagulant effects of edoxaban: protamine sulfate, vitamin K, and tranexamic acid.
Hemodialysis does not significantly contribute to edoxaban clearance.
Pharmacology
Mechanism of Action
Edoxaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Edoxaban inhibits free FXa, and prothrombinase activity and inhibits thrombin-induced platelet aggregation. Inhibition of FXa in the coagulation cascade reduces thrombin generation and reduces thrombus formation.
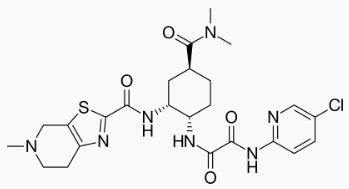
Structure
Edoxaban, a factor Xa inhibitor, is supplied as edoxaban tosylate monohydrate. The chemical name is N-(5-Chloropyridin-2-yl)-N′-[(1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl] oxamide mono (4-methylbenzenesulfonate) monohydrate. Edoxaban tosylate monohydrate has the empirical formula C24H30ClN7O4S•C7H8O3S•H2O representing a molecular weight of 738.27. The chemical structure of edoxaban tosylate monohydrate is:

It is a white to pale yellowish-white crystalline powder. The solubility of edoxaban tosylate (pKa 6.7) decreases with increasing pH. It is slightly soluble in water, pH 3 to 5 buffer, very slightly soluble at pH 6 to 7; and practically insoluble at pH 8 to 9.
Edoxaban is available for oral administration as a 60 mg, 30 mg, or 15 mg round shaped, film-coated tablet, debossed with product identification markings. Each 60 mg tablet contains 80.82 mg edoxaban tosylate monohydrate equivalent to 60 mg of edoxaban. Each 30 mg tablet contains 40.41 mg edoxaban tosylate monohydrate equivalent to 30 mg of edoxaban. Each 15 mg tablet contains 20.20 mg edoxaban tosylate monohydrate equivalent to 15 mg of edoxaban. The inactive ingredients are: mannitol, pregelatinized starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, talc, and carnauba wax. The color coatings contain hypromellose, titanium dioxide, talc, polyethylene glycol 8000, iron oxide yellow (60 mg tablets and 15 mg tablets), and iron oxide red (30 mg tablets and 15 mg tablets).
Pharmacodynamics
As a result of FXa inhibition, edoxaban prolongs clotting time tests such as prothrombin time (PT), and activated partial thromboplastin time (aPTT). Changes observed in PT, INR, and aPTT at the expected therapeutic dose, however, are small, subject to a high degree of variability and not useful in monitoring the anticoagulant effect of edoxaban. Following oral administration, peak pharmacodynamic effects are observed within 1-2 hours, which correspond with peak edoxaban concentrations (Cmax).
In a thorough QT study in healthy men and women aged 19-45 years, no QTc interval prolongation was observed with edoxaban (90 mg and 180 mg).
- Effect of PCCs on Pharmacodynamics of Edoxaban
There is no systematic evaluation of bleeding reversal by 4-factor prothrombin complex concentrate (PCC) products in patients who have received Edoxaban.
Effects of PCC (50 IU/kg) on the pharmacodynamics of edoxaban were studied in healthy subjects following a punch biopsy. Following administration of a single dose of edoxaban, endogenous thrombin potential (ETP) returned to pre-edoxaban baseline levels in 0.5 hours after the initiation of a 15 minute infusion of 50 IU/kg PCC, compared to more than 24 hours with placebo. Mean ETP levels continued to increase and exceeded pre-edoxaban baseline, reaching maximum elevations (~40% over pre-edoxaban levels) at 22 hours after initiating PCC dose, which was the last observation of ETP. The clinical relevance of this ETP increase is unknown.
- Pharmacodynamic Interactions
Co-administration of aspirin (100 mg or 325 mg) and edoxaban increased bleeding time relative to that seen with either drug alone.
Co-administration of naproxen (500 mg) and edoxaban increased bleeding time relative to that seen with either drug alone.
Pharmacokinetics
Edoxaban displays approximately dose-proportional pharmacokinetics for doses of 15 to 150 mg and 60 to 120 mg following single and repeat doses, respectively, in healthy subjects.
Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%. Food does not affect total systemic exposure to edoxaban. Edoxaban was administered with or without food in the ENGAGE AF-TIMI 48 and Hokusai VTE trials. No data are available regarding the bioavailability upon crushing and/or mixing of edoxaban tablets into food, liquids, or administration through feeding tubes.
Disposition is biphasic. The steady-state volume of distribution (Vdss) is 107 (19.9) L [mean (SD)]. In vitro plasma protein binding is approximately 55%. There is no clinically relevant accumulation of edoxaban (accumulation ratio 1.14) with once daily dosing.
Steady-state concentrations are achieved within 3 days.
Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4.
The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban.
- Elimination
Edoxaban is eliminated primarily as unchanged drug in the urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance. The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours.
- Specific Populations
- Hepatic Impairment
In a dedicated pharmacokinetic study, patients with mild or moderate hepatic impairment (classified as Child-Pugh A or Child-Pugh B) exhibited similar pharmacokinetics and pharmacodynamics to their matched healthy control group. There is no clinical experience with edoxaban in patients with severe hepatic impairment.
- Renal Impairment
In a dedicated pharmacokinetic study, total systemic exposure to edoxaban for subjects with CrCL > 50 to < 80 mL/min, CrCL 30 to 50 mL/min, CrCL < 30 mL/min, or undergoing peritoneal dialysis, were increased by 32%, 74%, 72%, and 93%, respectively, relative to subjects with CrCL ≥ 80 mL/min.
A 4-hour hemodialysis session reduced total edoxaban exposure by less than 7%.
- Age
In a population pharmacokinetic analysis, after taking renal function and body weight into account, age had no additional clinically significant effect on edoxaban pharmacokinetics.
- Weight
In a population pharmacokinetic analysis, total exposure in patients with median low body weight (55 kg) was increased by 13% as compared with patients with median high body weight (84 kg).
- Gender
In a population pharmacokinetic analysis, after accounting for body weight, gender had no additional clinically significant effect on edoxaban pharmacokinetics.
- Race
In a population pharmacokinetic analysis, edoxaban exposures in Asian patients and non-Asian patients were similar.
- Drug Interactions
- In vitro Drug Interactions Studies
In vitro studies indicate that edoxaban does not inhibit the major cytochrome P450 enzymes (CYP1A2, 2A6, 2B6, 2C8/9, 2C19, 2D6, 2E1, or 3A4) and does not induce CYP1A2, CYP3A4 or the P-gp transporter (MDR1). In vitro data also indicate that edoxaban does not inhibit the following transporters at clinically relevant concentrations: P-gp, the organic anion transporters OAT1 or OAT3; the organic cation transporters OCT1 or OCT2; or the organic ion transporting polypeptides OATP1B1 or OATP1B3. Edoxaban is a substrate of P-gp transporter.
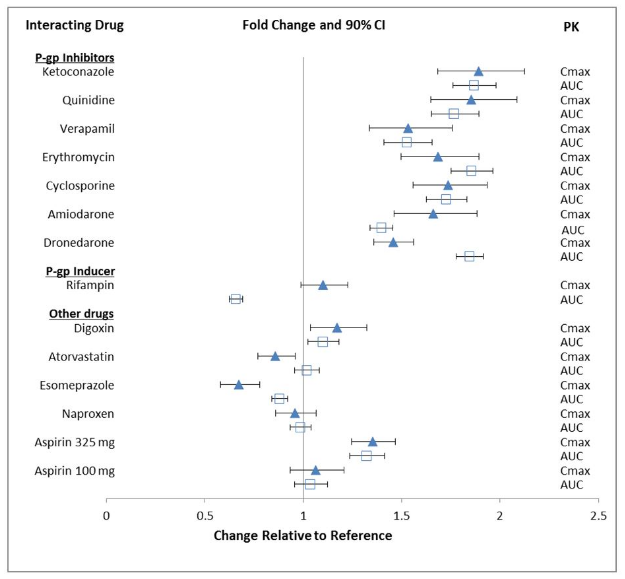
- Impact of Other Drugs on Edoxaban
The effect of co-administered amiodarone, cyclosporine, dronedarone, erythromycin, ketoconazole, quinidine, verapamil, and rifampin on edoxaban exposure is shown in FIGURE 2.
- Figure 2: Summary of Drug Interaction Study Results
- Impact of Edoxaban on Other Drugs
Edoxaban increased the Cmax of concomitantly administered digoxin by 28%; however, the AUC was not affected. Edoxaban had no effect on the Cmax and AUC of quinidine.
Edoxaban decreased the Cmax and AUC of concomitantly administered verapamil by 14% and 16%, respectively.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Edoxaban was not carcinogenic when administered daily to mice and rats by oral gavage for up to 104 weeks. The highest dose tested (500 mg/kg/day) in male and female mice was 3 and 6 times, respectively, the human exposure (AUC) at the human dose of 60 mg/day, and the highest doses tested in male (600/400 mg/kg/day) and female (200 mg/kg/day) rats were 8 and 14 times, respectively, the human exposure at the human dose of 60 mg/day.
Edoxaban and its human-specific metabolite, M-4, were genotoxic in in vitro chromosomal aberration tests but were not genotoxic in the in vitro bacterial reverse mutation (Ames test), in in vitro human lymphocytes micronucleus test, in in vivo rat bone marrow micronucleus test, in in vivo rat liver micronucleus test, and in in vivo unscheduled DNA synthesis tests.
Edoxaban showed no effects on fertility and early embryonic development in rats at doses of up to 1000 mg/kg/day (162 times the human dose of 60 mg/day normalized to body surface area).
Clinical Studies
Nonvalvular Atrial Fibrillation
- The ENGAGE AF-TIMI 48 Study
The ENGAGE AF-TIMI 48 study was a multi-national, double-blind, non-inferiority study comparing the efficacy and safety of two Edoxaban treatment arms (60 mg and 30 mg) to warfarin (titrated to INR 2.0 to 3.0) in reducing the risk of stroke and systemic embolic events in patients with NVAF. The non-inferiority margin (degree of inferiority of Edoxaban to warfarin that was to be ruled out) was set at 38%, reflecting the substantial effect of warfarin in reducing strokes. The primary analysis included both ischemic and hemorrhagic strokes.
To enter the study, patients had to have one or more of the following additional risk factors for stroke:
- a prior stroke (ischemic or unknown type), transient ischemic attack (TIA) or non-CNS systemic embolism, or
- 2 or more of the following risk factors:
- age ≥ 75 years,
- hypertension,
- heart failure, or
- diabetes mellitus
A total of 21,105 patients were randomized and followed for a median of 2.8 years and treated for a median of 2.5 years. Patients in the Edoxaban treatment arms had their dose halved (60 mg halved to 30 mg or 30 mg halved to 15 mg) if one or more of the following clinical factors were present: CrCL ≤ 50 mL/min, low body weight (≤ 60 kg) or concomitant use of specific P-gp inhibitors (verapamil, quinidine, dronedarone). Patients on antiretroviral therapy (ritonavir, nelfinavir, indinavir, saquinavir) as well as cyclosporine were excluded from the study. Approximately 25% of patients in all treatment groups received a reduced dose at baseline, and an additional 7% were dose-reduced during the study. The most common reason for dose reduction was a CrCL ≤ 50 mL/min at randomization (19% of patients).
Patients were well balanced with respect to demographic and baseline characteristics. The percentages of patients age ≥ 75 years and ≥ 80 years were approximately 40% and 17%, respectively. The majority of patients were Caucasian (81%) and male (62%). Approximately 40% of patients had not taken a Vitamin K Antagonist (VKA) (i.e., never took a VKA or had not taken a VKA for more than 2 months).
The mean patient body weight was 84 kg (185 lbs) and 10% of patients had a body weight of ≤ 60 kg. Concomitant diseases of patients in this study included hypertension (94%), congestive heart failure (58%), and prior stroke or transient ischemic attack (28%). At baseline, approximately 30% of patients were on aspirin and approximately 2% of patients were taking a thienopyridine.
Patients randomized to the warfarin arm achieved a mean TTR (time in therapeutic range, INR 2.0 to 3.0) of 65% during the course of the study.
The primary endpoint of the study was the occurrence of first stroke (either ischemic or hemorrhagic) or of a systemic embolic event (SEE) that occurred during treatment or within 3 days from the last dose taken. In the overall results of the study, shown in TABLE 5, both treatment arms of Edoxaban were non-inferior to warfarin for the primary efficacy endpoint of stroke or SEE. However, the 30 mg (15 mg dose-reduced) treatment arm was numerically less effective than warfarin for the primary endpoint, and was also markedly inferior in reducing the rate of ischemic stroke. Based on the planned superiority analysis (ITT, which required p < 0.01 for success), statistical superiority of the 60 mg (30 mg dose-reduced) treatment arm compared to warfarin was not established in the total study population, but there was a favorable trend [HR (99% CI): 0.87 (0.71, 1.07)].
- Table 5: Strokes and Systemic Embolic Events in the ENGAGE AF-TIMI 48 Study (mITT, on Treatmenta)

SAVAYSA: Edoxaban tosylate's Brand name
FIGURE 3 is a plot of the time from randomization to the occurrence of the first primary endpoint in all patients randomized to 60 mg Edoxaban or warfarin.
- Figure 3: Kaplan-Meier Cumulative Event Rate Estimates for Primary Endpoint (first occurrence of stroke or SEE) (mITT*)

SAVAYSA: Edoxaban tosylate's Brand name
The incidence rate of the primary endpoint of stroke or SEE in patients (N=1776) treated with the 30 mg reduced dose of Edoxaban because of a CrCL level ≤ 50 mL/min, low body weight ≤ 60 kg, or the concomitant use of a P-gp inhibitor drug, was 1.79% per year. Patients with any of these characteristics who were randomized to receive warfarin had an incidence rate of the primary endpoint of 2.21% per year [HR (95% CI): 0.81 (0.58, 1.13)].
In all randomized patients during the overall study period, the rates of CV death with Edoxaban and warfarin were 2.74% per year vs. 3.17% per year, respectively [HR (95% CI): 0.86 (0.77, 0.97)].
The results in the ENGAGE AF-TIMI 48 study for the primary efficacy endpoint for most major subgroups are displayed in FIGURE 4.
- Figure 4: ENGAGE AF-TIMI 48 Study: Primary Efficacy Endpoint by Subgroups (ITT Analysis Set)

SAVAYSA: Edoxaban tosylate's Brand name
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and most of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
The results of the ENGAGE AF-TIMI 48 study show a strong relationship between the blood levels of edoxaban and its effectiveness in reducing the rate of ischemic stroke. There was a 64% increase in the ischemic stroke rate in patients in the 30 mg treatment arm (including patients with dose reduced to 15 mg) compared to the 60 mg treatment arm (including patients with dose reduced to 30 mg). Approximately half of the Edoxaban dose is eliminated by the kidney, and edoxaban blood levels are lower in patients with better renal function, averaging about 30% less in patients with CrCL of > 80 mL/min, and 40% less in patients with CrCL > 95 mL/min when compared to patients with a CrCL of > 50 to ≤ 80 mL/min. Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Edoxaban compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.
TABLE 6 shows the results for the study primary efficacy endpoint of first stroke or SEE as well as the effects on ischemic and hemorrhagic stroke in the pre-randomization CrCL subgroups for Edoxaban 60 mg (including 30 mg dose-reduced) and warfarin. There was a decreased rate of ischemic stroke with Edoxaban 60 mg compared to warfarin in patients with CrCL > 50 to ≤ 80 mL/min [HR (95% CI): 0.63 (0.44, 0.89)]. In patients with CrCL > 80 to ≤ 95 mL/min the results for ischemic stroke slightly favor warfarin with a confidence interval that crosses 1.0 [HR (95% CI): 1.11 (0.58, 2.12)]. The rate of ischemic stroke was higher relative to warfarin in the patients with CrCL > 95 mL/min [HR (95% CI): 2.16 (1.17, 3.97)]. Pharmacokinetic data indicate that patients with CrCL > 95 mL/min had lower plasma edoxaban levels, along with a lower rate of bleeding relative to warfarin than patients with CrCL ≤ 95 mL/min. Consequently, Edoxaban should not be used in patients with CrCL > 95 mL/min.
In patients with CrCL ≤ 95 mL/min, the Edoxaban 60 mg (30 mg dose-reduced) treatment arm reduced the risk of Stroke or SEE when compared to warfarin [HR (95% CI): 0.68 (0.55, 0.84)].
In the indicated population (CrCL ≤ 95 mL/min), during the overall study period, the rates of CV death with Edoxaban and warfarin were 2.95% per year vs. 3.59% per year, respectively [HR (95% CI): 0.82 (0.72, 0.93)].
- Table 6: Primary Endpoint, Ischemic and Hemorrhagic Stroke Results in as a Function of Baseline Creatinine Clearance (mITT Population, On Treatment)

SAVAYSA: Edoxaban tosylate's Brand name
- Transition to Other Anticoagulants in the ENGAGE AF-TIMI 48 Study
In the ENGAGE AF-TIMI 48 study, the schemes for transitioning from study medication to open-label warfarin at the end of study were associated with similar rates of stroke and systemic embolism in the Edoxaban 60 mg and warfarin groups. In the Edoxaban 60 mg group 7 (0.2%) of 4529 patients had a stroke or SEE compared to 7 (0.2%) of 4506 patients in the warfarin arm.
Treatment of Deep Vein Thrombosis and Pulmonary Embolism
- The Hokusai VTE Study
Edoxaban for the treatment of patients with deep vein thrombosis (DVT) and pulmonary embolism (PE) was studied in a multi-national, double-blind study (Hokusai VTE) which compared the efficacy and safety of Edoxaban 60 mg orally once daily to warfarin (titrated to INR 2.0 to 3.0) in patients with acute symptomatic venous thromboembolism (VTE) (DVT or PE with or without DVT). All patients had VTE confirmed by appropriate diagnostic imaging at baseline and received initial heparin therapy with low molecular weight heparin (LMWH) or unfractionated heparin for at least 5 days [median LMWH/heparin treatment in the Edoxaban 60 mg group was 7 days, and in the warfarin group it was 8.0 days] and until INR (sham or real) was ≥ 2.0 on two measurements. Blinded drug treatment in the warfarin arm was started concurrently with initial heparin therapy and in the Edoxaban arm after discontinuation of initial heparin. Patients randomized to Edoxaban received 30 mg once daily if they met one or more of the following criteria: CrCL 30 to 50 mL/min, body weight ≤ 60 kg, or concomitant use of specific P-gp inhibitors (verapamil and quinidine or the short-term concomitant administration of azithromycin, clarithromycin, erythromycin, oral itraconazole or oral ketoconazole). The edoxaban dosage regimen was to be returned to the regular dosage of 60 mg once daily at any time the subject is not taking the concomitant medication provided no other criteria for dose reduction are met. Other P-gp inhibitors were not permitted in the study. Patients on antiretroviral therapy (ritonavir, nelfinavir, indinavir, saquinavir) as well as cyclosporine were excluded from the Hokusai VTE study. The concomitant use of these drugs with Edoxaban has not been studied in patients. The treatment duration was from 3 months up to 12 months, determined by investigator based on patient clinical features. Patients were excluded if they required thrombectomy, insertion of a caval filter, use of a fibrinolytic agent, or use of other P-gp inhibitors, had a creatinine clearance < 30 mL/min, significant liver disease, or active bleeding. The primary efficacy outcome was symptomatic VTE, defined as the composite of recurrent DVT, new non-fatal symptomatic PE, and fatal PE during the 12-month study period.
A total of 8292 patients were randomized to receive Edoxaban or warfarin and were followed for a mean treatment duration of 252 days for Edoxaban and 250 days for warfarin. The mean age was approximately 56 years. The population was 57% male, 70% Caucasian, 21% Asian, and about 4% Black. The presenting diagnosis was PE (with or without DVT) in 40.7% and DVT only in 59.3% of patients. At baseline, 27.6% of patients had temporary risk factors only (e.g., trauma, surgery, immobilization, estrogen therapy). Overall 9.4% had a history of cancer, 17.3% of the patients had an age ≥ 75 years and/or a body weight ≤ 50 kg, and/or a CrCL < 50 mL/min, and 31.4% of patients had NT-ProBNP ≥ 500 pg/mL.
Aspirin was taken as on treatment concomitant antithrombotic medication by approximately 9% of patients in both groups.
In the warfarin group, the median TTR (time in therapeutic range, INR 2.0 to 3.0) was 65.6%.
A total of 8240 patients (n= 4118 for Edoxaban and n = 4122 for warfarin) received study drug and were included in the modified intent-to-treat (mITT) population. Edoxaban was demonstrated to be non inferior to warfarin for the primary endpoint of recurrent VTE [HR (95% CI): 0.89 (0.70, 1.13)] (TABLE 7, FIGURE 5).
- Table 7: Primary Composite Efficacy Endpoint Results in Hokusai VTE (mITT Overall Study Period)

SAVAYSA: Edoxaban tosylate's Brand name
- Figure 5: Kaplan-Meier Cumulative Event Rate Estimates for Adjudicated Recurrent VTE (mITT analysis – on treatment)

SAVAYSA: Edoxaban tosylate's Brand name
How Supplied
Edoxaban tosylate is supplied as:
- 60 mg, yellow round shaped, film-coated tablets, debossed with DSC L60 on one side
- 30 mg, pink round shaped, film-coated tablets, debossed with DSC L30 on one side
- 15 mg, orange round shaped, film-coated tablets, debossed with DSC L15 on one side

Storage
Store at 20-25°C (68-77°F); excursions permitted to 15°-30°C (59°-86°F).
Keep out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Edoxaban |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Edoxaban |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (MEDICATION GUIDE).
Advise patients of the following:
- they may bleed more easily, may bleed longer, or bruise more easily when treated with Edoxaban
- to report any unusual bleeding immediately to their healthcare provider
- to take Edoxaban exactly as prescribed
- to not discontinue Edoxaban without talking to the healthcare provider who prescribed it
- to inform their healthcare providers that they are taking Edoxaban before any surgery, medical, or dental procedure is scheduled
- to inform their healthcare providers and dentists if they plan to take, or are taking any prescription medications, over-the-counter drugs or herbal products
- to inform their healthcare provider immediately if they become pregnant or intend to become pregnant or are breastfeeding or intend to breastfeed during treatment with Edoxaban
- that if a dose is missed, take Edoxaban as soon as possible the same day, and resume the normal dosing schedule the following day. The dose should not be doubled to make up for a missing dose
- that if they are having neuraxial anesthesia or spinal puncture, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately.

Precautions with Alcohol
Alcohol-Edoxaban interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
SAVAYSA™
Look-Alike Drug Names
There is limited information regarding Edoxaban Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T; et al. (2014). "Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial". Thromb Res. 134 (6): 1198–204. doi:10.1016/j.thromres.2014.09.011. PMID 25294589.