Tiagabine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 72: | Line 72: | ||
*This represents an estimated incidence of 0.0026 deaths per patient-year. This rate is within the range of estimates for the incidence of sudden and unexpected deaths in patients with epilepsy not receiving Tiagabine (ranging from 0.0005 for the general population with epilepsy, 0.003 to 0.004 for clinical trial populations similar to that in the clinical development program for Tiagabine to 0.005 for patients with refractory epilepsy). The estimated SUDEP rates in patients receiving Tiagabine are also similar to those observed in patients receiving other antiepilepsy drugs, chemically unrelated to Tiagabine that underwent clinical testing in similar populations at about the same time. This evidence suggests that the SUDEP rates reflect population rates, not a drug effect. | *This represents an estimated incidence of 0.0026 deaths per patient-year. This rate is within the range of estimates for the incidence of sudden and unexpected deaths in patients with epilepsy not receiving Tiagabine (ranging from 0.0005 for the general population with epilepsy, 0.003 to 0.004 for clinical trial populations similar to that in the clinical development program for Tiagabine to 0.005 for patients with refractory epilepsy). The estimated SUDEP rates in patients receiving Tiagabine are also similar to those observed in patients receiving other antiepilepsy drugs, chemically unrelated to Tiagabine that underwent clinical testing in similar populations at about the same time. This evidence suggests that the SUDEP rates reflect population rates, not a drug effect. | ||
|clinicalTrials=*The most commonly observed adverse events in placebo-controlled, parallel-group, add-on epilepsy trials associated with the use of Tiagabine in combination with other antiepilepsy drugs not seen at an equivalent frequency among placebo-treated patients were [[dizziness]]/[[light-headedness]], [[asthenia]]/[[lack of energy]], [[somnolence]], [[nausea]], [[nervousness]]/[[irritability]], [[tremor]], [[abdominal pain]], and thinking abnormal/difficulty with concentration or attention. | |clinicalTrials=*The most commonly observed adverse events in placebo-controlled, parallel-group, add-on epilepsy trials associated with the use of Tiagabine in combination with other antiepilepsy drugs not seen at an equivalent frequency among placebo-treated patients were [[dizziness]]/[[light-headedness]], [[asthenia]]/[[lack of energy]], [[somnolence]], [[nausea]], [[nervousness]]/[[irritability]], [[tremor]], [[abdominal pain]], and thinking abnormal/difficulty with concentration or attention. | ||
*Approximately 21% of the 2531 patients who received Tiagabine in clinical trials of epilepsy discontinued treatment because of an adverse event. The adverse events most commonly associated with discontinuation were [[dizziness]] (1.7%), [[somnolence]] (1.6%), [[depression]] (1.3%), [[confusion]] (1.1%), and [[asthenia]] (1.1%). | |||
Approximately 21% of the 2531 patients who received Tiagabine in clinical trials of epilepsy discontinued treatment because of an adverse event. The adverse events most commonly associated with discontinuation were [[dizziness]] (1.7%), [[somnolence]] (1.6%), [[depression]] (1.3%), [[confusion]] (1.1%), and [[asthenia]] (1.1%). | *In Studies 1 and 2 (U.S. studies), the double-blind, placebo-controlled, parallel-group, add-on studies, the proportion of patients who discontinued treatment because of adverse events was 11% for the group treated with Tiagabine and 6% for the placebo group. The most common adverse events considered the primary reason for discontinuation were [[confusion]] (1.2%), [[somnolence]] (1.0%), and [[ataxia]] (1.0%). | ||
In Studies 1 and 2 (U.S. studies), the double-blind, placebo-controlled, parallel-group, add-on studies, the proportion of patients who discontinued treatment because of adverse events was 11% for the group treated with Tiagabine and 6% for the placebo group. The most common adverse events considered the primary reason for discontinuation were [[confusion]] (1.2%), [[somnolence]] (1.0%), and [[ataxia]] (1.0%). | |||
===Adverse Event Incidence in Controlled Clinical Trials=== | ===Adverse Event Incidence in Controlled Clinical Trials=== | ||
Table 5 lists treatment-emergent signs and symptoms that occurred in at least 1% of patients treated with Tiagabine for [[epilepsy]] participating in parallel-group, placebo-controlled trials and were numerically more common in the Tiagabine group. In these studies, either Tiagabine or placebo was added to the patient’s current antiepilepsy drug therapy. Adverse events were usually mild or moderate in intensity. | *Table 5 lists treatment-emergent signs and symptoms that occurred in at least 1% of patients treated with Tiagabine for [[epilepsy]] participating in parallel-group, placebo-controlled trials and were numerically more common in the Tiagabine group. In these studies, either Tiagabine or placebo was added to the patient’s current antiepilepsy drug therapy. Adverse events were usually mild or moderate in intensity. | ||
*The prescriber should be aware that these figures, obtained when Tiagabine was added to concurrent antiepilepsy drug therapy, cannot be used to predict the frequency of adverse events in the course of usual medical practice when patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied. | |||
The prescriber should be aware that these figures, obtained when Tiagabine was added to concurrent antiepilepsy drug therapy, cannot be used to predict the frequency of adverse events in the course of usual medical practice when patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied. | |||
'''Table 5: Treatment-Emergent Adverse Event1 Incidence in Parallel-Group, Placebo-Controlled, Add-On Trials (events in at least 1% of patients treated with Tiagabine and numerically more frequent than in the placebo group)''' | '''Table 5: Treatment-Emergent Adverse Event1 Incidence in Parallel-Group, Placebo-Controlled, Add-On Trials (events in at least 1% of patients treated with Tiagabine and numerically more frequent than in the placebo group)''' | ||
[[file:Tiagabine AR1.png|none| | [[file:Tiagabine AR1.png|none|200px]] | ||
Patients in these add-on studies were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category. | *Patients in these add-on studies were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category. | ||
*Other events reported by 1% or more of patients treated with Tiagabine but equally or more frequent in the placebo group were: accidental injury, [[chest pain]], [[constipation]], [[flu syndrome]], [[rhinitis]], [[anorexia]], [[back pain]], [[dry mouth]], [[flatulence]], [[ecchymosis]], [[twitching]], [[fever]], [[amblyopia]], [[conjunctivitis]], [[urinary tract infection]], [[urinary frequency]], [[infection]], [[dyspepsia]], [[gastroenteritis]], [[nausea and vomiting]], [[myalgia]], [[diplopia]], [[headache]], [[anxiety]], [[acne]], [[sinusitis]], and [[incoordination]]. | |||
Other events reported by 1% or more of patients treated with Tiagabine but equally or more frequent in the placebo group were: accidental injury, [[chest pain]], [[constipation]], [[flu syndrome]], [[rhinitis]], [[anorexia]], [[back pain]], [[dry mouth]], [[flatulence]], [[ecchymosis]], [[twitching]], [[fever]], [[amblyopia]], [[conjunctivitis]], [[urinary tract infection]], [[urinary frequency]], [[infection]], [[dyspepsia]], [[gastroenteritis]], [[nausea and vomiting]], [[myalgia]], [[diplopia]], [[headache]], [[anxiety]], [[acne]], [[sinusitis]], and [[incoordination]]. | *Study 1 was a dose-response study including doses of 32 mg and 56 mg. Table 6 shows adverse events reported at a rate of ≥ 5% in at least one Tiagabine group and more frequent than in the placebo group. Among these events, depression, tremor, nervousness, difficulty with concentration/attention, and perhaps asthenia exhibited a positive relationship to dose. | ||
Study 1 was a dose-response study including doses of 32 mg and 56 mg. Table 6 shows adverse events reported at a rate of ≥ 5% in at least one Tiagabine group and more frequent than in the placebo group. Among these events, depression, tremor, nervousness, difficulty with concentration/attention, and perhaps asthenia exhibited a positive relationship to dose. | |||
'''Table 6: Treatment-Emergent Adverse Event Incidence in Study 1† (events in at least 5% of patients treated with Tiagabine 32 or 56 mg and numerically more frequent than in the placebo group)''' | '''Table 6: Treatment-Emergent Adverse Event Incidence in Study 1† (events in at least 5% of patients treated with Tiagabine 32 or 56 mg and numerically more frequent than in the placebo group)''' | ||
| Line 96: | Line 91: | ||
[[file:Tiagabine AR2.png|none|300px]] | [[file:Tiagabine AR2.png|none|300px]] | ||
Patients in this study were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category. | *Patients in this study were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category. | ||
*The effects of Tiagabine in relation to those of placebo on the incidence of adverse events and the types of adverse events reported were independent of age, weight, and gender. Because only 10% of patients were non-Caucasian in parallel-group, placebo-controlled trials, there is insufficient data to support a statement regarding the distribution of adverse experience reports by race. | |||
The effects of Tiagabine in relation to those of placebo on the incidence of adverse events and the types of adverse events reported were independent of age, weight, and gender. Because only 10% of patients were non-Caucasian in parallel-group, placebo-controlled trials, there is insufficient data to support a statement regarding the distribution of adverse experience reports by race. | |||
===Other Adverse Events Observed During All Clinical Trials=== | ===Other Adverse Events Observed During All Clinical Trials=== | ||
Tiagabine has been administered to 2531 patients during all phase 2/3 clinical trials, only some of which were placebo-controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below. The frequencies presented represent the proportion of the 2531 patients exposed to Tiagabine who experienced events of the type cited on at least one occasion while receiving Tiagabine All reported events are included except those already listed above, events seen only three times or fewer (unless potentially important), events very unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to tiagabine. | *Tiagabine has been administered to 2531 patients during all phase 2/3 clinical trials, only some of which were placebo-controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below. The frequencies presented represent the proportion of the 2531 patients exposed to Tiagabine who experienced events of the type cited on at least one occasion while receiving Tiagabine All reported events are included except those already listed above, events seen only three times or fewer (unless potentially important), events very unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to tiagabine. | ||
*Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients. | |||
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients. | |||
====Body as a Whole==== | ====Body as a Whole==== | ||
Revision as of 17:20, 27 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tiagabine is an anticonvulsant, gamma aminobutyric acid Uptake Inhibitor that is FDA approved for the treatment of partial seizures in adults and children > 12 years. Common adverse reactions include pruritus, abdominal pain, nausea, asthenia, ataxia, confusion, disturbance in speech, dizziness, feeling nervous, insomnia, somnolence, tremor, unable to concentrate and pharyngitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General
- The blood level of tiagabine obtained after a given dose depends on whether the patient also is receiving a drug that induces the metabolism of tiagabine. The presence of an inducer means that the attained blood level will be substantially reduced. Dosing should take the presence of concomitant medications into account.
- Tiagabine is recommended as adjunctive therapy for the treatment of partial seizures in patients 12 years and older.
The following dosing recommendations apply to all patients taking GABITRIL:
- GABITRIL is given orally and should be taken with food.
- Do not use a loading dose of GABITRIL.
- Dose titration: Rapid escalation and/or large dose increments of GABITRIL should not be used.
- Missed dose(s): If the patient forgets to take the prescribed dose of GABITRIL at the scheduled time, the patient should not attempt to make up for the missed dose by increasing the next dose. If a patient has missed multiple doses, patient should refer back to his or her physician for possible re-titration as clinically indicated.
- Dosage adjustment of GABITRIL should be considered whenever a change in patient’s enzyme-inducing status occurs as a result of the addition, discontinuation, or dose change of the enzyme-inducing agent.
Induced Adults and Adolescents 12 Years or Older
- The following dosing recommendations apply to patients who are already taking enzyme-inducing antiepilepsy drugs (AEDs) (e.g., carbamazepine, phenytoin, primidone, and phenobarbital). Such patients are considered induced patients when administering GABITRIL.
- In adolescents 12 to 18 years old, GABITRIL should be initiated at 4 mg once daily. Modification of concomitant antiepilepsy drugs is not necessary, unless clinically indicated. The total daily dose of GABITRIL may be increased by 4 mg at the beginning of Week 2. Thereafter, the total daily dose may be increased by 4 to 8 mg at weekly intervals until clinical response is achieved or up to 32 mg/day. The total daily dose should be given in divided doses two to four times daily. Doses above 32 mg/day have been tolerated in a small number of adolescent patients for a relatively short duration.
- In adults, GABITRIL should be initiated at 4 mg once daily. Modification of concomitant antiepilepsy drugs is not necessary, unless clinically indicated. The total daily dose of GABITRIL may be increased by 4 to 8 mg at weekly intervals until clinical response is achieved or, up to 56 mg/day. The total daily dose should be given in divided doses two to four times daily. Doses above 56 mg/day have not been systematically evaluated in adequate and well-controlled clinical trials.
Experience is limited in patients taking total daily doses above 32 mg/day using twice daily dosing. A typical dosing titration regimen for patients taking enzyme-inducing AEDs (induced patients) is provided in Table 7
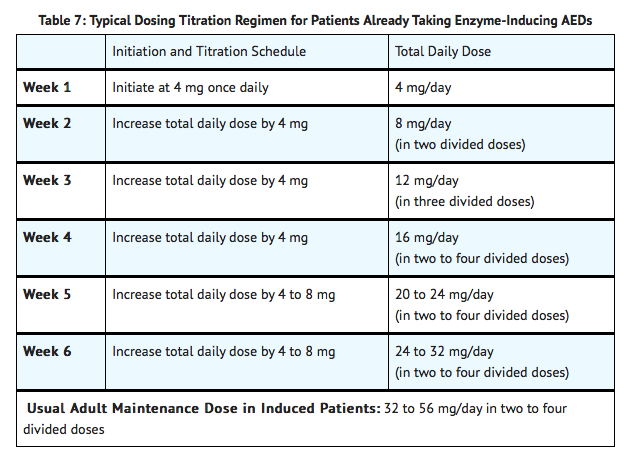
Non-Induced Adults and Adolescents 12 Years or Older
- The following dosing recommendations apply to patients who are taking only non-enzyme-inducing AEDs. Such patients are considered non-induced patients:
- Following a given dose of GABITRIL, the estimated plasma concentration in the non-induced patients is more than twice that in patients receiving enzyme-inducing agents. Use in non-induced patients requires lower doses of GABITRIL. These patients may also require a slower titration of GABITRIL compared to that of induced patients
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tiagabine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tiagabine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Tiagabine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tiagabine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tiagabine in pediatric patients.
Contraindications
- Tiagabine is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
Warnings
- Seizures in Patients Without Epilepsy: Post-marketing reports have shown that Tiagabine use has been associated with new onset seizures and status epilepticus in patients without epilepsy. Dose may be an important predisposing factor in the development of seizures, although seizures have been reported in patients taking daily doses of Tiagabine as low as 4 mg/day. In most cases, patients were using concomitant medications (antidepressants, antipsychotics, stimulants, narcotics) that are thought to lower the seizure threshold. Some seizures occurred near the time of a dose increase, even after periods of prior stable dosing.
- The Tiagabine dosing recommendations in current labeling for treatment of epilepsy were based on use in patients with partial seizures 12 years of age and older, most of whom were taking enzyme-inducing antiepileptic drugs (AEDs; e.g., carbamazepine, phenytoin, primidone and phenobarbital) which lower plasma levels of Tiagabine by inducing its metabolism. Use of Tiagabine without enzyme-inducing antiepileptic drugs results in blood levels about twice those attained in the studies on which current dosing recommendations are based.
- Safety and effectiveness of Tiagabine have not been established for any indication other than as adjunctive therapy for partial seizures in adults and children 12 years and older.
- In nonepileptic patients who develop seizures while on Tiagabine treatment, Tiagabine should be discontinued and patients should be evaluated for an underlying seizure disorder.
- Seizures and status epilepticus are known to occur with Tiagabine overdosage.
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including Tiagabine, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs use for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 4 shows absolute and relative risk by indication for all evaluated AEDs
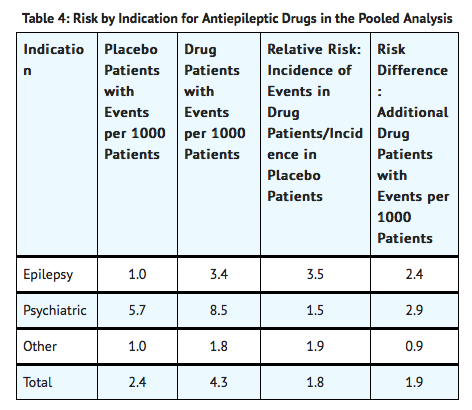
- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
- Anyone considering prescribing Tiagabine or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Withdrawal Seizures
- As a rule, antiepilepsy drugs should not be abruptly discontinued because of the possibility of increasing seizure frequency. In a placebo-controlled, double-blind, dose-response study (Study 1 described in CLINICAL STUDIES) designed, in part, to investigate the capacity of Tiagabine to induce withdrawal seizures, study drug was tapered over a 4-week period after 16 weeks of treatment. Patients’ seizure frequency during this 4-week withdrawal period was compared to their baseline seizure frequency (before study drug). For each partial seizure type, for all partial seizure types combined, and for secondarily generalized tonic-clonic seizures, more patients experienced increases in their seizure frequencies during the withdrawal period in the three Tiagabine groups than in the placebo group. The increase in seizure frequency was not affected by dose. Tiagabine should be withdrawn gradually to minimize the potential of increased seizure frequency, unless safety concerns require a more rapid withdrawal.
Cognitive/Neuropsychiatric Adverse Events
- Adverse events most often associated with the use of Tiagabine were related to the central nervous system. The most significant of these can be classified into 2 general categories: 1) impaired concentration, speech or language problems, and confusion (effects on thought processes); and 2) somnolence and fatigue (effects on level of consciousness). The majority of these events were mild to moderate. In controlled clinical trials, these events led to discontinuation of treatment with Tiagabine in 6% (31 of 494) of patients compared to 2% (5 of 275) of the placebo-treated patients. A total of 1.6% (8 of 494) of the Tiagabine treated patients in the controlled trials were hospitalized secondary to the occurrence of these events compared to 0% of the placebo treated patients. Some of these events were dose related and usually began during initial titration.
- Patients with a history of spike and wave discharges on EEG have been reported to have exacerbations of their EEG abnormalities associated with these cognitive/neuropsychiatric events. This raises the possibility that these clinical events may, in some cases, be a manifestation of underlying seizure activity. In the documented cases of spike and wave discharges on EEG with cognitive/neuropsychiatric events, patients usually continued tiagabine, but required dosage adjustment.
- Additionally, there have been postmarketing reports of patients who have experienced cognitive/neuropsychiatric symptoms, some accompanied by EEG abnormalities such as generalized spike and wave activity, that have been reported as nonconvulsant status epilepticus. Some reports describe recovery following reduction of dose or discontinuation of Tiagabine.
Status Epilepticus
- In the three double-blind, placebo-controlled, parallel-group studies (Studies 1, 2, and 3), the incidence of any type of status epilepticus (simple, complex, or generalized tonic-clonic) in patients receiving Tiagabine was 0.8% (4 of 494 patients) versus 0.7% (2 of 275 patients) receiving placebo. Among the patients treated with Tiagabine across all epilepsy studies (controlled and uncontrolled), 5% had some form of status epilepticus. Of the 5%, 57% of patients experienced complex partial status epilepticus. A critical risk factor for status epilepticus was the presence of a previous history; 33% of patients with a history of status epilepticus had recurrence during Tiagabine treatment. Because adequate information about the incidence of status epilepticus in a similar population of patients with epilepsy who have not received treatment with Tiagabine is not available, it is impossible to state whether or not treatment with Tiagabine is associated with a higher or lower rate of status epilepticus than would be expected to occur in a similar population not treated with Tiagabine.
Sudden Unexpected Death In Epilepsy (SUDEP)
- There have been as many as 10 cases of sudden unexpected deaths during the clinical development of tiagabine among 2531 patients with epilepsy (3831 patient-years of exposure).
- This represents an estimated incidence of 0.0026 deaths per patient-year. This rate is within the range of estimates for the incidence of sudden and unexpected deaths in patients with epilepsy not receiving Tiagabine (ranging from 0.0005 for the general population with epilepsy, 0.003 to 0.004 for clinical trial populations similar to that in the clinical development program for Tiagabine to 0.005 for patients with refractory epilepsy). The estimated SUDEP rates in patients receiving Tiagabine are also similar to those observed in patients receiving other antiepilepsy drugs, chemically unrelated to Tiagabine that underwent clinical testing in similar populations at about the same time. This evidence suggests that the SUDEP rates reflect population rates, not a drug effect.
Adverse Reactions
Clinical Trials Experience
- The most commonly observed adverse events in placebo-controlled, parallel-group, add-on epilepsy trials associated with the use of Tiagabine in combination with other antiepilepsy drugs not seen at an equivalent frequency among placebo-treated patients were dizziness/light-headedness, asthenia/lack of energy, somnolence, nausea, nervousness/irritability, tremor, abdominal pain, and thinking abnormal/difficulty with concentration or attention.
- Approximately 21% of the 2531 patients who received Tiagabine in clinical trials of epilepsy discontinued treatment because of an adverse event. The adverse events most commonly associated with discontinuation were dizziness (1.7%), somnolence (1.6%), depression (1.3%), confusion (1.1%), and asthenia (1.1%).
- In Studies 1 and 2 (U.S. studies), the double-blind, placebo-controlled, parallel-group, add-on studies, the proportion of patients who discontinued treatment because of adverse events was 11% for the group treated with Tiagabine and 6% for the placebo group. The most common adverse events considered the primary reason for discontinuation were confusion (1.2%), somnolence (1.0%), and ataxia (1.0%).
Adverse Event Incidence in Controlled Clinical Trials
- Table 5 lists treatment-emergent signs and symptoms that occurred in at least 1% of patients treated with Tiagabine for epilepsy participating in parallel-group, placebo-controlled trials and were numerically more common in the Tiagabine group. In these studies, either Tiagabine or placebo was added to the patient’s current antiepilepsy drug therapy. Adverse events were usually mild or moderate in intensity.
- The prescriber should be aware that these figures, obtained when Tiagabine was added to concurrent antiepilepsy drug therapy, cannot be used to predict the frequency of adverse events in the course of usual medical practice when patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied.
Table 5: Treatment-Emergent Adverse Event1 Incidence in Parallel-Group, Placebo-Controlled, Add-On Trials (events in at least 1% of patients treated with Tiagabine and numerically more frequent than in the placebo group)
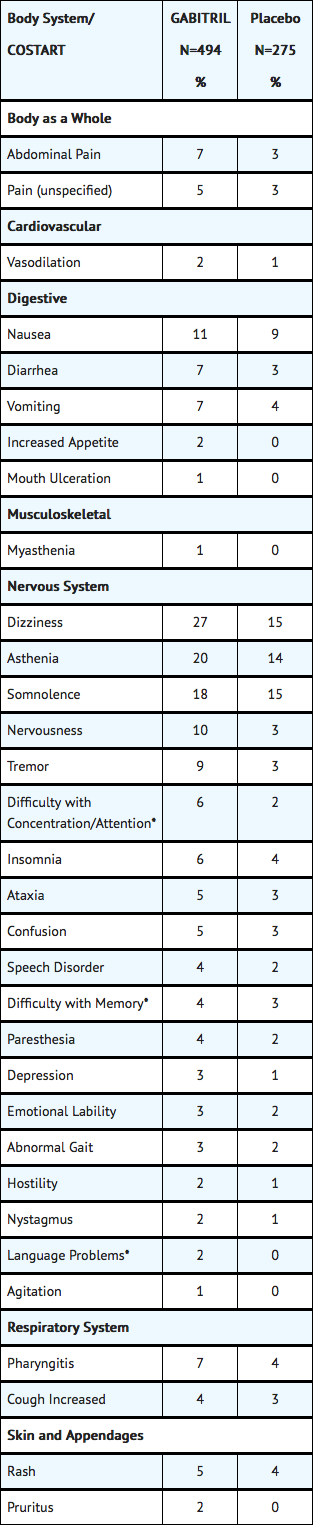
- Patients in these add-on studies were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category.
- Other events reported by 1% or more of patients treated with Tiagabine but equally or more frequent in the placebo group were: accidental injury, chest pain, constipation, flu syndrome, rhinitis, anorexia, back pain, dry mouth, flatulence, ecchymosis, twitching, fever, amblyopia, conjunctivitis, urinary tract infection, urinary frequency, infection, dyspepsia, gastroenteritis, nausea and vomiting, myalgia, diplopia, headache, anxiety, acne, sinusitis, and incoordination.
- Study 1 was a dose-response study including doses of 32 mg and 56 mg. Table 6 shows adverse events reported at a rate of ≥ 5% in at least one Tiagabine group and more frequent than in the placebo group. Among these events, depression, tremor, nervousness, difficulty with concentration/attention, and perhaps asthenia exhibited a positive relationship to dose.
Table 6: Treatment-Emergent Adverse Event Incidence in Study 1† (events in at least 5% of patients treated with Tiagabine 32 or 56 mg and numerically more frequent than in the placebo group)
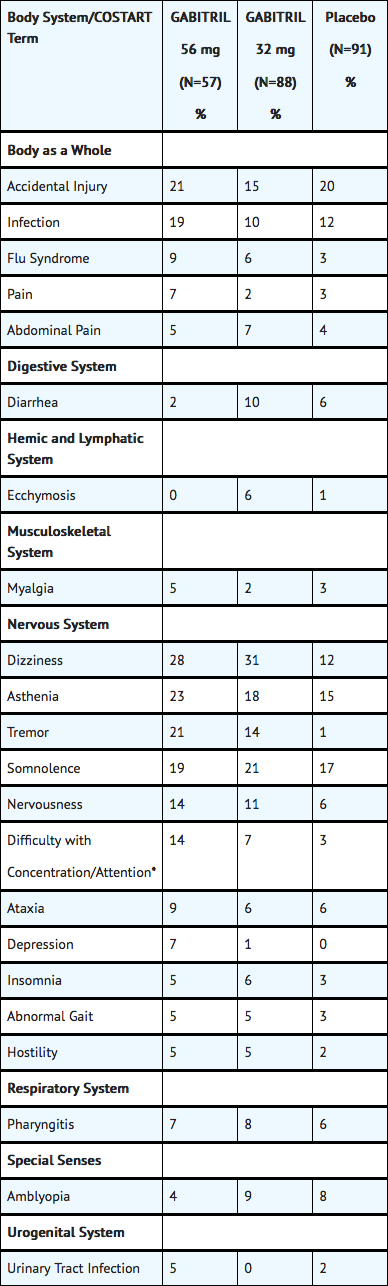
- Patients in this study were receiving one to three concomitant enzyme-inducing antiepilepsy drugs in addition to Tiagabine or placebo. Patients may have reported multiple adverse experiences; thus, patients may be included in more than one category.
- The effects of Tiagabine in relation to those of placebo on the incidence of adverse events and the types of adverse events reported were independent of age, weight, and gender. Because only 10% of patients were non-Caucasian in parallel-group, placebo-controlled trials, there is insufficient data to support a statement regarding the distribution of adverse experience reports by race.
Other Adverse Events Observed During All Clinical Trials
- Tiagabine has been administered to 2531 patients during all phase 2/3 clinical trials, only some of which were placebo-controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below. The frequencies presented represent the proportion of the 2531 patients exposed to Tiagabine who experienced events of the type cited on at least one occasion while receiving Tiagabine All reported events are included except those already listed above, events seen only three times or fewer (unless potentially important), events very unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to tiagabine.
- Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Body as a Whole
- Frequent: Allergic reaction, chest pain, chills, cyst, neck pain, and malaise.
- Infrequent: Abscess, cellulitis, facial edema, halitosis, hernia, neck rigidity, neoplasm, pelvic pain, photosensitivity reaction, sepsis, sudden death, and suicide attempt.
Cardiovascular System
- Frequent: Hypertension, palpitation, syncope, and tachycardia.
- Infrequent: Angina pectoris, cerebral ischemia, electrocardiogram abnormal, hemorrhage, hypotension, myocardial infarct, pallor, peripheral vascular disorder, phlebitis, postural hypotension, and thrombophlebitis.
Digestive System
- Frequent: Gingivitis and stomatitis.
- Infrequent: Abnormal stools, cholecystitis, cholelithiasis, dysphagia, eructation, esophagitis, fecal incontinence, gastritis, gastrointestinal hemorrhage, glossitis, gum hyperplasia, hepatomegaly, increased salivation, liver function tests abnormal, melena, periodontal abscess, rectal hemorrhage, thirst, tooth caries, and ulcerative stomatitis.
Endocrine System
- Infrequent: Goiter and hypothyroidism.
Hemic and Lymphatic System
- Frequent: Lymphadenopathy.
- Infrequent: Anemia, erythrocytes abnormal, leukopenia, petechia, and thrombocytopenia.
Metabolic and Nutritional
- Frequent: Edema, peripheral edema, weight gain, and weight loss.
- Infrequent: Dehydration, hypercholesteremia, hyperglycemia, hyperlipemia, hypoglycemia, hypokalemia, and hyponatremia.
Musculoskeletal System
- Frequent: Arthralgia.
- Infrequent: Arthritis, arthrosis, bursitis, generalized spasm, and tendinous contracture.
Nervous System
- Frequent: Depersonalization, dysarthria, euphoria, hallucination, hyperkinesia, hypertonia, hypesthesia, hypokinesia, hypotonia, migraine, myoclonus, paranoid reaction, personality disorder, reflexes decreased, stupor, twitching, and vertigo.
- Infrequent: Abnormal dreams, apathy, choreoathetosis, circumoral paresthesia, CNS neoplasm, coma, delusions, dry mouth, dystonia, encephalopathy, hemiplegia, leg cramps, libido increased, libido decreased, movement disorder, neuritis, neurosis, paralysis, peripheral neuritis, psychosis, reflexes increased, and urinary retention.
Respiratory System
- Frequent: Bronchitis, dyspnea, epistaxis, and pneumonia.
- lnfrequent: Apnea, asthma, hemoptysis, hiccups, hyperventilation, laryngitis, respiratory disorder, and voice alteration.
Skin and Appendages
- Frequent: Alopecia, dry skin, and sweating.
- Infrequent: Contact dermatitis, eczema, exfoliative dermatitis, furunculosis, herpes simplex, herpes zoster, hirsutism, maculopapular rash, psoriasis, skin benign neoplasm, skin carcinoma, skin discolorations, skin nodules, skin ulcer, subcutaneous nodule, urticaria, and vesiculobullous rash.
Special Senses
- Frequent: Abnormal vision, ear pain, otitis media, and tinnitus.
- Infrequent: Blepharitis, blindness, deafness, eye pain, hyperacusis, keratoconjunctivitis, otitis externa, parosmia, photophobia, taste loss, taste perversion, and visual field defect.
Urogenital System
- Frequent: Dysmenorrhea, dysuria, metrorrhagia, urinary incontinence, and vaginitis.
- Infrequent: Abortion, amenorrhea, breast enlargement, breast pain, cystitis, fibrocystic breast, hematuria, impotence, kidney failure, menorrhagia, nocturia, papanicolaou smear suspicious, polyuria, pyelonephritis, salpingitis, urethritis, urinary urgency, and vaginal hemorrhage.
Postmarketing Experience
There is limited information regarding Tiagabine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tiagabine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Tiagabine has been shown to have adverse effects on embryo-fetal development, including teratogenic effects, when administered to pregnant rats and rabbits at doses greater than the human therapeutic dose.
- An increased incidence of malformed fetuses (various craniofacial, appendicular, and visceral defects) and decreased fetal weights were observed following oral administration of 100 mg/kg/day to pregnant rats during the period of organogenesis. This dose is approximately 16 times the maximum recommended human dose (MRHD) of 56 mg/day, based on body surface area (mg/m2). Maternal toxicity (transient weight loss/reduced maternal weight gain during gestation) was associated with this dose, but there is no evidence to suggest that the teratogenic effects were secondary to the maternal effects. No adverse maternal or embryo-fetal effects were seen at a dose of 20 mg/kg/day (3 times the MRHD on a mg/m2 basis).
- Decreased maternal weight gain, increased resorption of embryos and increased incidences of fetal variations, but not malformations, were observed when pregnant rabbits were given 25 mg/kg/day (8 times the MRHD on a mg/m2 basis) during organogenesis. The no effect level for maternal and embryo-fetal toxicity in rabbits was 5 mg/kg/day (equivalent to the MRHD on a mg/m2 basis).
- When female rats were given tiagabine 100 mg/kg/day during late gestation and throughout parturition and lactation, decreased maternal weight gain during gestation, an increase in stillbirths, and decreased postnatal offspring viability and growth were found. There are no adequate and well-controlled studies in pregnant women. Tiagabine should be used during pregnancy only if clearly needed.
- To provide additional information regarding the effects of in utero exposure to Tiagabine physicians are advised to recommend that pregnant patients taking Tiagabine enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website. Decreased maternal weight gain, increased resorption of embryos and increased incidences of fetal variations, but not malformations, were observed when pregnant rabbits were given 25 mg/kg/day (8 times the MRHD on a mg/m2 basis) during organogenesis. The no effect level for maternal and embryo-fetal toxicity in rabbits was 5 mg/kg/day (equivalent to the MRHD on a mg/m2 basis).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tiagabine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tiagabine during labor and delivery.
Nursing Mothers
- Studies in rats have shown that tiagabine HCl and/or its metabolites are excreted in the milk of that species. Levels of excretion of tiagabine and/or its metabolites in human milk have not been determined and effects on the nursing infant are unknown. Tiagabine should be used in women who are nursing only if the benefits clearly outweigh the risks.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 12 have not been established. The pharmacokinetics of tiagabine were evaluated in pediatric patients age 3 to 10 years (
Geriatic Use
- Because few patients over the age of 65 (approximately 20) were exposed to Tiagabine during its clinical evaluation, no specific statements about the safety or effectiveness of Tiagabine in this age group could be made.
Gender
There is no FDA guidance on the use of Tiagabine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tiagabine with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of total and unbound tiagabine were similar in subjects with normal renal function (creatinine clearance >80 mL/min) and in subjects with mild (creatinine clearance 40 to 80 mL/min), moderate (creatinine clearance 20 to 39 mL/min), or severe (creatinine clearance 5 to 19 mL/min) renal impairment. The pharmacokinetics of total and unbound tiagabine were also unaffected in subjects with renal failure requiring hemodialysis.
Hepatic Impairment
- In patients with moderate hepatic impairment (Child-Pugh Class B), clearance of unbound tiagabine was reduced by about 60%. Patients with impaired liver function may require reduced initial and maintenance doses of tiagabine and/or longer dosing intervals compared to patients with normal hepatic function
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tiagabine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tiagabine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tiagabine Administration in the drug label.
Monitoring
There is limited information regarding Tiagabine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tiagabine and IV administrations.
Overdosage
Human Overdose Experience
- Human experience of acute overdose with Tiagabine is limited. Eleven patients in clinical trials took single doses of Tiagabine up to 800 mg. All patients fully recovered, usually within one day. The most common symptoms reported after overdose included somnolence, impaired consciousness, agitation, confusion, speech difficulty, hostility, depression, weakness, and myoclonus. One patient who ingested a single dose of 400 mg experienced generalized tonic-clonic status epilepticus, which responded to intravenous phenobarbital.
- From post-marketing experience, there have been no reports of fatal overdoses involving Tiagabine alone (doses up to 720 mg), although a number of patients required intubation and ventilatory support as part of the management of their status epilepticus. Overdoses involving multiple drugs, including Tiagabine have resulted in fatal outcomes. Symptoms most often accompanying Tiagabine overdose, alone or in combination with other drugs, have included: seizures including status epilepticus in patients with and without underlying seizure disorders, nonconvulsive status epilepticus, coma, ataxia, confusion, somnolence, drowsiness, impaired speech, agitation, lethargy, myoclonus, spike wave stupor, tremors, disorientation, vomiting, hostility, and temporary paralysis. Respiratory depression was seen in a number of patients, including children, in the context of seizures.
Management of Overdose
- There is no specific antidote for overdose with Tiagabine If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. General supportive care of the patient is indicated including monitoring of vital signs and observation of clinical status of the patient. Since tiagabine is mostly metabolized by the liver and is highly protein bound, dialysis is unlikely to be beneficial. A Certified Poison Control Center should be consulted for up to date information on the management of overdose with Tiagabine.
Pharmacology
| |
Tiagabine
| |
| Systematic (IUPAC) name | |
| (R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-enyl] piperidine-3-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 375.55 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 96% |
| Metabolism | Hepatic (CYP450 system) |
| Half life | 7-9 hours |
| Excretion | Fecal and renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- The precise mechanism by which tiagabine exerts its antiseizure effect is unknown, although it is believed to be related to its ability, documented in in vitro experiments, to enhance the activity of gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. These experiments have shown that tiagabine binds to recognition sites associated with the GABA uptake carrier. It is thought that, by this action, tiagabine blocks GABA uptake into presynaptic neurons, permitting more GABA to be available for receptor binding on the surfaces of post-synaptic cells. Inhibition of GABA uptake has been shown for synaptosomes, neuronal cell cultures, and glial cell cultures. In rat-derived hippocampal slices, tiagabine has been shown to prolong GABA-mediated inhibitory post-synaptic potentials. Tiagabine increases the amount of GABA available in the extracellular space of the globus pallidus, ventral palladum, and substantia nigra in rats at the ED50 and ED85 doses for inhibition of pentylenetetrazol (PTZ)-induced tonic seizures. This suggests that tiagabine prevents the propagation of neural impulses that contribute to seizures by a GABA-ergic action.
- Tiagabine has shown efficacy in several animal models of seizures. It is effective against the tonic phase of subcutaneous PTZ-induced seizures in mice and rats, seizures induced by the proconvulsant DMCM in mice, audiogenic seizures in genetically epilepsy-prone rats (GEPR), and amygdala-kindled seizures in rats. Tiagabine has little efficacy against maximal electroshock seizures in rats and is only partially effective against subcutaneous PTZ-induced clonic seizures in mice, picrotoxin-induced tonic seizures in the mouse, bicuculline-induced seizures in the rat, and photic seizures in photosensitive baboons. Tiagabine produces a biphasic dose-response curve against PTZ- and DMCM-induced convulsions, with attenuated effectiveness at higher doses.
- Based on in vitro binding studies, tiagabine does not significantly inhibit the uptake of dopamine, norepinephrine, serotonin, glutamate, or choline and shows little or no binding to dopamine D1 and D2, muscarinic, serotonin 5HT1A, 5HT2, and 5HT3, beta-1 and beta-2 adrenergic, alpha-1 and alpha-2 adrenergic, histamine H2 and H3, adenosine A1 and adenosine A2, opiate µ and opiate K1, NMDA glutamate, and GABAA receptors at 100 µM. It also lacks significant affinity for sodium or calcium channels. Tiagabine binds to histamine H1, serotonin 5HT1B, benzodiazepine, and chloride channel receptors at concentrations 20 to 400 times those inhibiting the uptake of GABA.
Structure
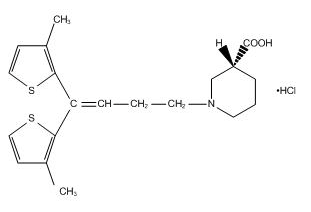
- Its chemical name is (-)-(R)-1-4,4-Bis(3-methyl-2-thienyl)-3-buteny)nipecotic acid hydrochloride, its molecular formula is C20H25NO2S2 HCl, and its molecular weight is 412.0. Tiagabine HCl is a white to off-white, odorless, crystalline powder. It is insoluble in heptane, sparingly soluble in water, and soluble in aqueous base. The structural formula is:

Pharmacodynamics
There is limited information regarding Tiagabine Pharmacodynamics in the drug label.
Pharmacokinetics
- Tiagabine is well absorbed, with food slowing absorption rate but not altering the extent of absorption. The elimination half-life of tiagabine is 7 to 9 hours in normal volunteers. In epilepsy clinical trials, most patients were receiving hepatic enzyme-inducing agents (e.g., carbamazepine, phenytoin, primidone, and phenobarbital). The pharmacokinetic profile in induced patients is significantly different from the non-induced population. The systemic clearance of tiagabine in induced patients is approximately 60% greater resulting in considerably lower plasma concentrations and an elimination half-life of 2 to 5 hours. Given this difference in clearance, the systemic exposure after a dose of 32 mg/day in an induced population is expected to be comparable to the systemic exposure after a dose of 12 mg/day in a non-induced population. Similarly, the systemic exposure after a dose of 56 mg/day in an induced population is expected to be comparable to the systemic exposure after a dose of 22 mg/day in a non-induced population.
Absorption and Distribution
- Absorption of tiagabine is rapid, with peak plasma concentrations occurring at approximately 45 minutes following an oral dose in the fasting state. Tiagabine is nearly completely absorbed (>95%), with an absolute oral bioavailability of about 90%. A high fat meal decreases the rate (mean Tmax was prolonged to 2.5 hours, and mean Cmax was reduced by about 40%) but not the extent (AUC) of tiagabine absorption. In all clinical trials, tiagabine was given with meals. The pharmacokinetics of tiagabine are linear over the single dose range of 2 to 24 mg. Following multiple dosing, steady state is achieved within 2 days.
- Tiagabine is 96% bound to human plasma proteins, mainly to serum albumin and α1-acid glycoprotein over the concentration range of 10 ng/mL to 10,000 ng/mL. While the relationship between tiagabine plasma concentrations and clinical response is not currently understood, trough plasma concentrations observed in controlled clinical trials at doses from 30 to 56 mg/day ranged from <1 ng/mL to 234 ng/mL.
Metabolism and Elimination
- Although the metabolism of tiagabine has not been fully elucidated, in vivo and in vitro studies suggest that at least two metabolic pathways for tiagabine have been identified in humans: 1) thiophene ring oxidation leading to the formation of 5-oxo-tiagabine; and 2) glucuronidation. The 5-oxo-tiagabine metabolite does not contribute to the pharmacologic activity of tiagabine.
- Based on in vitro data, tiagabine is likely to be metabolized primarily by the 3A isoform subfamily of hepatic cytochrome P450 (CYP3A), although contributions to the metabolism of tiagabine from CYP1A2, CYP2D6 or CYP2C19 have not been excluded.
- Approximately 2% of an oral dose of tiagabine is excreted unchanged, with 25% and 63% of the remaining dose excreted into the urine and feces, respectively, primarily as metabolites, at least 2 of which have not been identified. The mean systemic plasma clearance is 109 mL/min (CV = 23%) and the average elimination half-life for tiagabine in healthy subjects ranged from 7 to 9 hours. The elimination half-life decreased by 50 to 65% in hepatic enzyme-induced patients with epilepsy compared to uninduced patients with epilepsy.
- A diurnal effect on the pharmacokinetics of tiagabine was observed. Mean steady-state Cminvalues were 40% lower in the evening than in the morning. Tiagabine steady-state AUC values were also found to be 15% lower following the evening tiagabine dose compared to the AUC following the morning dose.
Nonclinical Toxicology
There is limited information regarding Tiagabine Nonclinical Toxicology in the drug label.
Clinical Studies
- The effectiveness of Tiagabine as adjunctive therapy (added to other antiepilepsy drugs) was examined in three multi-center, double-blind, placebo-controlled, parallel-group, clinical trials in 769 patients with refractory partial seizures who were taking at least one hepatic enzyme-inducing antiepilepsy drug (AED), and two placebo-controlled cross-over studies in 90 patients. In the parallel-group trials, patients had a history of at least six complex partial seizures (Study 1 and Study 2, U.S. studies), or six partial seizures of any type (Study 3, European study), occurring alone or in combination with any other seizure type within the 8-week period preceding the first study visit in spite of receiving one or more AEDs at therapeutic concentrations.
- In the first two studies, the primary protocol-specified outcome measure was the median reduction from baseline in the 4-week complex partial seizure (CPS) rates during treatment. In the third study, the protocol-specified primary outcome measure was the proportion of patients achieving a 50% or greater reduction from baseline in the 4-week seizure rate of all partial seizures during treatment. The results given below include data for complex partial seizures and all partial seizures for the intent-to-treat population (all patients who received at least one dose of treatment and at least one seizure evaluation) in each study.
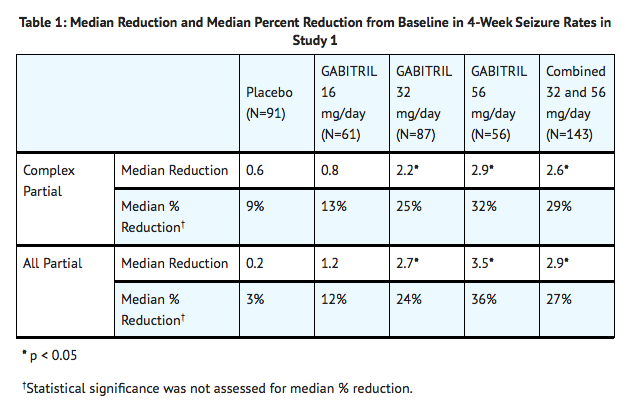
- Study 1 was a double-blind, placebo-controlled, parallel-group trial comparing Tiagabine 16 mg/day, Tiagabine 32 mg/day, Tiagabine 56 mg/day, and placebo. Study drug was given as a four times a day regimen. After a prospective Baseline Phase of 12 weeks, patients were randomized to one of the four treatment groups described above. The 16-week Treatment Phase consisted of a 4-week Titration Period, followed by a 12-week Fixed-Dose Period, during which concomitant AED doses were held constant. The primary outcome was assessed for the combined 32 and 56 mg/day groups compared to placebo.
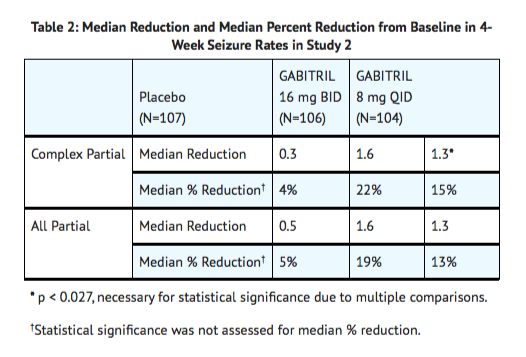
- Study 2 was a double-blind, placebo-controlled, parallel-group trial consisting of an 8-week Baseline Phase and a 12-week Treatment Phase, the first 4 weeks of which constituted a Titration Period and the last 8 weeks a Fixed-Dose Period. This study compared Tiagabine 16 mg BID and 8 mg QID to placebo. The protocol-specified primary outcome measure was assessed separately for each group treated with Tiagabine.
The following tables display the results of the analyses of these two trials.
- Figures 1 to 4 present the proportion of patients (X-axis) whose percent reduction from baseline in the all partial seizure rate was at least as great as that indicated on the Y axis in the three placebo-controlled adjunctive studies (Studies 1, 2, and 3). A positive value on the Y axis indicates an improvement from baseline (i.e., a decrease in seizure rate), while a negative value indicates a worsening from baseline (i.e., an increase in seizure rate). Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo.
- Figure 1 indicates that the proportion of patients achieving any particular level of reduction in seizure rate was consistently higher for the combined Tiagabine 32 mg and 56 mg groups compared to the placebo group in Study 1. For example, Figure 1 indicates that approximately 24% of patients treated with Tiagabine experienced a 50% or greater reduction, compared to 4% in the placebo group.

- Figure 2 also displays the results for Study 1, which was a dose-response study, by treatment group, without combining Tiagabine dosage groups. Figure 2 indicates a dose-response relationship across the three Tiagabine groups. The proportion of patients achieving any particular level of reduction in all partial seizure rates was consistently higher as the dose of Tiagabine was increased. For example, Figure 2 indicates that approximately 4% of patients in the placebo group experienced a 50% or greater reduction in all partial seizure rate, compared to approximately 10% of the Tiagabine 16 mg/day group, 21% of the Tiagabine 32 mg/day group, and 30% of the Tiagabine 56 mg/day group.
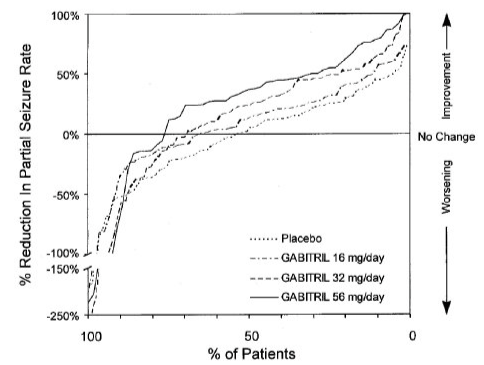
- Figure 3 indicates that the proportion of patients achieving any particular level of reduction in partial seizure rate was consistently greater in patients taking Tiagabine than in those taking placebo in Study 2. (Study 2 compared placebo to Tiagabine 32 mg/day; one of the Tiagabine groups received 8 mg QID, while the other Tiagabine group received 16 mg BID). For example, Figure 3 indicates that approximately 7% of patients in the placebo group experienced a 50% or greater reduction in their partial seizure rate, compared to approximately 23% of patients in the Tiagabine 8 mg QID group and 28% of patients in the Tiagabine 16 mg BID group.
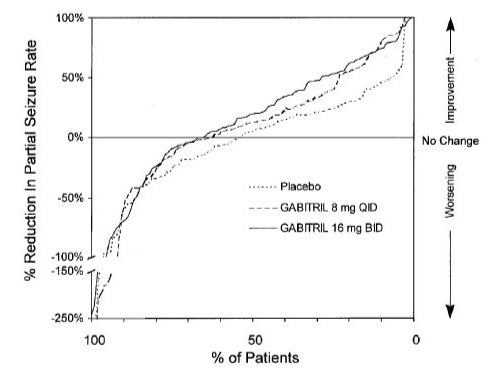
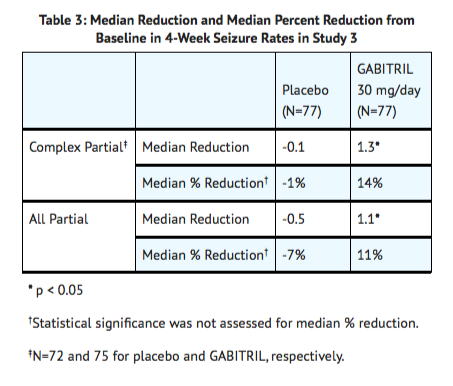
- Study 3 was a double-blind, placebo-controlled, parallel-group trial that compared Tiagabine 10 mg TID (N=77) with placebo (N=77). In this trial, patients were followed prospectively during a 12-week Baseline Phase and then randomized to receive study drug during an 18-week Treatment Phase. During the first 6 weeks of treatment (Titration Period), patients were titrated to 30 mg/day, after which they were maintained on this dose during the 12-week Fixed-Dose Period. The protocol-specified primary outcome measure (proportion of patients who achieved at least a 50% reduction from baseline in partial seizure rate) did not reach statistical significance. However, analyses of the median reduction from baseline in 4-week partial seizure rate (the analyses presented above for Study 1 and Study 2) were performed and showed a statistically significant improvement compared to placebo in all partial and complex partial seizure rates (Table 3):
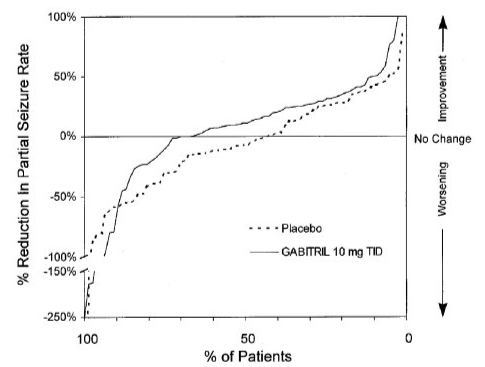
- Figure 4 indicates that the proportion of patients achieving any particular level of reduction in seizure activity was consistently higher in those taking Tiagabine than those taking placebo in Study 3. For example, Figure 4 indicates that approximately 5% of patients in the placebo group experienced a 50% or greater reduction in their partial seizure rate compared to approximately 10% of patients in the Tiagabine group.
- The two other placebo-controlled trials that examined the effectiveness of Tiagabine were small cross-over trials (N=46 and 44). Both trials included an open Screening Phase during which patients were titrated to an optimal dose and then treated with this dose for an additional 4 weeks. After this Open Phase, patients were randomized to one of two blinded treatment sequences (Tiagabine followed by placebo or placebo followed by Tiagabine). The Double-Blind Phase consisted of two Treatment Periods, each lasting 7 weeks (with a 3 week washout between periods). The outcome measures were median with-in patient differences between placebo and Tiagabine Treatment Periods in 4-week complex partial and all partial seizure rates. The reductions in seizure rates were statistically significant in both studies.
How Supplied
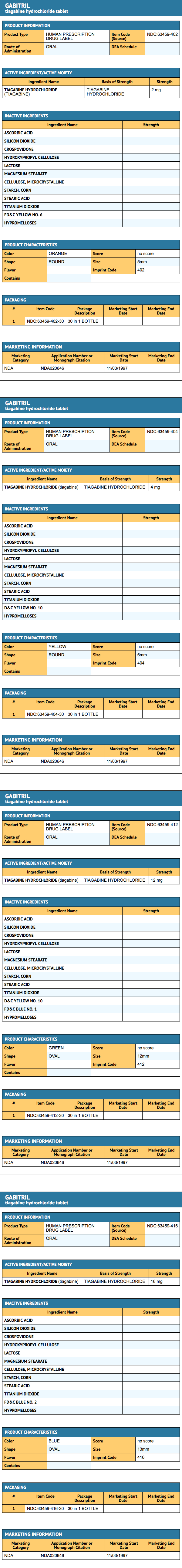
GABITRIL tablets are available in four dosage strengths.
- 2 mg orange-peach, round tablets, debossed with Cephalon Imprint on one side and 402 on the opposite side, are available in bottles of 30 (NDC 63459-402-30).
- 4 mg yellow, round tablets, debossed with Cephalon Imprint on one side and 404 on the opposite side, are available in bottles of 30 (NDC 63459-404-30).
- 12 mg green, ovaloid tablets, debossed with Cephalon Imprint on one side and 412 on the opposite side, are available in bottles of 30 (NDC 63459-412-30).
- 16 mg blue, ovaloid tablets, debossed with Cephalon Imprint on one side and 416 on the opposite side, are available in bottles of 30 (NDC 63459-416-30).
Storage
- Store tablets at controlled room temperature, between 20-25°C (68-77°F).
Images
Drug Images
{{#ask: Page Name::Tiagabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Tiagabine P2mg.png


{{#ask: Label Page::Tiagabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tiagabine Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Tiagabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Tiagabine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.