Palonosetron: Difference between revisions
m (Protected "Palonosetron": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Rabin Bista (talk | contribs) No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
|IUPAC_name=(3'' | | Verifiedfields = changed | ||
|image=Palonosetron. | | Watchedfields = changed | ||
| | | verifiedrevid = 464197120 | ||
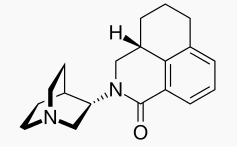
| IUPAC_name = (3''aS'')-2-[(3''S'')-1-Azabicyclo[2.2.2]oct-3-yl]-2,3,3''a'',4,5,6-hexahydro-1''H''-benz[''de'']isoquinolin-1-one | |||
| | | image = Palonosetron Wiki Str.png | ||
| | | width = 200 | ||
| | |||
| | <!--Clinical data--> | ||
| | | tradename = | ||
| | | Drugs.com = {{drugs.com|monograph|palonosetron-hydrochloride}} | ||
| | | MedlinePlus = a610002 | ||
|bioavailability= | | licence_US = Palonosetron | ||
|protein_bound=62% | | pregnancy_AU = B1 | ||
|metabolism=[[Liver|Hepatic]], 50% (mostly [[CYP2D6]]-mediated, [[CYP3A4]] and [[CYP1A2]] also involved) | | pregnancy_US = B | ||
|elimination_half-life=Approximately 40 hours | | legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | ||
|excretion=[[Kidney|Renal]], 80% (of which 49% unchanged) | | legal_UK = <!-- GSL / P / POM / CD --> | ||
| | | legal_US = Rx-only | ||
| | | routes_of_administration = [[Intravenous therapy|Intravenous]], oral | ||
| | |||
| | <!--Pharmacokinetic data--> | ||
| | | bioavailability = 97% (oral) | ||
| | | protein_bound = 62% | ||
| | | metabolism = [[Liver|Hepatic]], 50% (mostly [[CYP2D6]]-mediated, [[CYP3A4]] and [[CYP1A2]] also involved) | ||
| | | elimination_half-life = Approximately 40 hours | ||
| excretion = [[Kidney|Renal]], 80% (of which 49% unchanged); fecal (5 to 8%) | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 135729-61-2 | |||
| ATC_prefix = A04 | |||
| ATC_suffix = AA05 | |||
| PubChem = 148211 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00377 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 85161 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4892289 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 5D06587D6R | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1189679 | |||
<!--Chemical data--> | |||
| C=19 | H=24 | N=2 | O=1 | |||
| molecular_weight = 296.407 g/mol | |||
| smiles = O=C5N([C@H]2C1CCN(CC1)C2)C[C@@H]4c3c5cccc3CCC4 | |||
| InChI = 1/C19H24N2O/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20/h2,4,6,13,15,17H,1,3,5,7-12H2/t15-,17-/m1/s1 | |||
| InChIKey = CPZBLNMUGSZIPR-NVXWUHKLBP | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H24N2O/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20/h2,4,6,13,15,17H,1,3,5,7-12H2/t15-,17-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = CPZBLNMUGSZIPR-NVXWUHKLSA-N | |||
}} | }} | ||
'''Palonosetron''' ([[International Nonproprietary Name|INN]], trade name '''Aloxi''') is a [[5-HT3 antagonist|5-HT<sub>3</sub> antagonist]] used in the prevention and treatment of [[chemotherapy | __NOTOC__ | ||
{{SI}} | |||
{{CMG}} | |||
== Overview == | |||
'''Palonosetron''' ([[International Nonproprietary Name|INN]], trade name '''Aloxi''') is a [[5-HT3 antagonist|5-HT<sub>3</sub> antagonist]] used in the prevention and treatment of [[chemotherapy-induced nausea and vomiting]] (CINV). It is used for the control of delayed CINV—nausea and vomiting and there are tentative data to suggest that it may be better than [[granisetron]].<ref>{{cite journal|last=Billio|first=A|author2=Morello, E |author3=Clarke, MJ |title=Serotonin receptor antagonists for highly emetogenic chemotherapy in adults.|journal=The Cochrane database of systematic reviews|date=Jan 20, 2010|issue=1|pages=CD006272|pmid=20091591|doi=10.1002/14651858.CD006272.pub2}}</ref> | |||
Palonosetron is administered [[intravenous therapy|intravenously]], as a single dose, 30 minutes before chemotherapy | Palonosetron is administered [[intravenous therapy|intravenously]], as a single dose, 30 minutes before chemotherapy,<ref name="pmid17106506">{{cite journal |author=De Leon A |title=Palonosetron (Aloxi): a second-generation 5-HT(3) receptor antagonist for chemotherapy-induced nausea and vomiting |journal=Proceedings (Baylor University. Medical Center) |volume=19 |issue=4 |pages=413–6 |year=2006 |pmid=17106506 |doi= |pmc=1618755}}</ref> or as a single oral capsule one hour before chemotherapy.<ref name=Medscape>{{cite web |url=http://www.medscape.com/viewarticle/580032 |title=FDA Approvals: Nplate, Aloxi, Vidaza |author=Waknine, Yael |date=September 4, 2008 |accessdate=2008-09-04 |publisher=[[Medscape]]}} Freely available with registration.</ref> The oral formulation was approved on August 22, 2008 for prevention of acute CINV alone, as a large clinical trial did not show oral administration to be as effective as intravenous use against delayed CINV.<ref name=Medscape/> | ||
==See also== | |||
* [[5-HT3 receptor antagonist:drug discovery and development|5-HT<sub>3</sub> receptor antagonist: Drug discovery and development]] | |||
==References== | ==References== | ||
{{Reflist}} | {{Reflist|2}} | ||
{{5-HT3 antagonists}} | {{5-HT3 antagonists}} | ||
{{Serotonergics}} | |||
[[Category:Drug]] | |||
[[Category:5-HT3 antagonists]] | |||
[[Category:Quinuclidines]] | |||
[[Category:Isoquinolines]] | |||
[[Category:Lactams]] | |||
{{gastrointestinal-drug-stub}} | |||
Latest revision as of 13:54, 9 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610002 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Intravenous, oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97% (oral) |
| Protein binding | 62% |
| Metabolism | Hepatic, 50% (mostly CYP2D6-mediated, CYP3A4 and CYP1A2 also involved) |
| Elimination half-life | Approximately 40 hours |
| Excretion | Renal, 80% (of which 49% unchanged); fecal (5 to 8%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H24N2O |
| Molar mass | 296.407 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Palonosetron |
|
Articles |
|---|
|
Most recent articles on Palonosetron Most cited articles on Palonosetron |
|
Media |
|
Powerpoint slides on Palonosetron |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Palonosetron at Clinical Trials.gov Clinical Trials on Palonosetron at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Palonosetron
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Palonosetron Discussion groups on Palonosetron Patient Handouts on Palonosetron Directions to Hospitals Treating Palonosetron Risk calculators and risk factors for Palonosetron
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Palonosetron |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Palonosetron (INN, trade name Aloxi) is a 5-HT3 antagonist used in the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV). It is used for the control of delayed CINV—nausea and vomiting and there are tentative data to suggest that it may be better than granisetron.[1]
Palonosetron is administered intravenously, as a single dose, 30 minutes before chemotherapy,[2] or as a single oral capsule one hour before chemotherapy.[3] The oral formulation was approved on August 22, 2008 for prevention of acute CINV alone, as a large clinical trial did not show oral administration to be as effective as intravenous use against delayed CINV.[3]
See also
References
- ↑ Billio, A; Morello, E; Clarke, MJ (Jan 20, 2010). "Serotonin receptor antagonists for highly emetogenic chemotherapy in adults". The Cochrane database of systematic reviews (1): CD006272. doi:10.1002/14651858.CD006272.pub2. PMID 20091591.
- ↑ De Leon A (2006). "Palonosetron (Aloxi): a second-generation 5-HT(3) receptor antagonist for chemotherapy-induced nausea and vomiting". Proceedings (Baylor University. Medical Center). 19 (4): 413–6. PMC 1618755. PMID 17106506.
- ↑ 3.0 3.1 Waknine, Yael (September 4, 2008). "FDA Approvals: Nplate, Aloxi, Vidaza". Medscape. Retrieved 2008-09-04. Freely available with registration.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- 5-HT3 antagonists
- Quinuclidines
- Isoquinolines
- Lactams