Norfloxacin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
|fdaLIADAdult=[[file:DosageNor.png|thumb|none|500px]] | |fdaLIADAdult=[[file:DosageNor.png|thumb|none|500px]] | ||
=====UTI's===== | ======UTI's====== | ||
[[Klebsiella pneumoniae]], [[Proteus mirabilis]], [[Pseudomonas aeruginosa]], [[Staphylococcus epidermidis]], [[Staphylococcus saprophyticus]], [[Citrobacter freundii]], [[Enterobacter aerogenes]], [[Enterobacter cloacae]], [[Proteus vulgaris]], [[Staphylococcus aureus]], or [[Streptococcus agalactiae]]. | [[Klebsiella pneumoniae]], [[Proteus mirabilis]], [[Pseudomonas aeruginosa]], [[Staphylococcus epidermidis]], [[Staphylococcus saprophyticus]], [[Citrobacter freundii]], [[Enterobacter aerogenes]], [[Enterobacter cloacae]], [[Proteus vulgaris]], [[Staphylococcus aureus]], or [[Streptococcus agalactiae]]. | ||
=====Sexually Transmitted Diseases===== | ======Sexually Transmitted Diseases====== | ||
Uncomplicated urethral and cervical gonorrhea due to Neisseria gonorrhoeae. | Uncomplicated urethral and cervical gonorrhea due to [[Neisseria gonorrhoeae]]. | ||
=====Prostatitis===== | ======Prostatitis====== | ||
Prostatitis due to Escherichia coli. | Prostatitis due to [[Escherichia coli]]. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norfloxacin in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norfloxacin in adult patients. | ||
|offLabelAdultNoGuideSupport=*Cholera<ref name="pmid2193628">{{cite journal| author=Bhattacharya SK, Bhattacharya MK, Dutta P, Dutta D, De SP, Sikdar SN et al.| title=Double-blind, randomized, controlled clinical trial of norfloxacin for cholera. | journal=Antimicrob Agents Chemother | year= 1990 | volume= 34 | issue= 5 | pages= 939-40 | pmid=2193628 | doi= | pmc=PMC171728 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2193628 }} </ref> | |offLabelAdultNoGuideSupport=*Cholera<ref name="pmid2193628">{{cite journal| author=Bhattacharya SK, Bhattacharya MK, Dutta P, Dutta D, De SP, Sikdar SN et al.| title=Double-blind, randomized, controlled clinical trial of norfloxacin for cholera. | journal=Antimicrob Agents Chemother | year= 1990 | volume= 34 | issue= 5 | pages= 939-40 | pmid=2193628 | doi= | pmc=PMC171728 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2193628 }} </ref> | ||
*Immunodeficiency disorder - Infectious disease; Prophylaxis | *Immunodeficiency disorder - Infectious disease; Prophylaxis | ||
**Dosage: 400 q12h <ref name="pmid3538962">{{cite journal| author=Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ et al.| title=Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double-blind, placebo-controlled trial. | journal=Ann Intern Med | year= 1987 | volume= 106 | issue= 1 | pages= 1-7 | pmid=3538962 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3538962 }} </ref> | **Dosage: 400 q12h <ref name="pmid3538962">{{cite journal| author=Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ et al.| title=Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double-blind, placebo-controlled trial. | journal=Ann Intern Med | year= 1987 | volume= 106 | issue= 1 | pages= 1-7 | pmid=3538962 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3538962 }} </ref> | ||
*Malaria | |||
**Dosage: 400 mg q12h for 3 days <ref name="pmid2667420">{{cite journal| author=Sarma PS| title=Norfloxacin: a new drug in the treatment of falciparum malaria. | journal=Ann Intern Med | year= 1989 | volume= 111 | issue= 4 | pages= 336-7 | pmid=2667420 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2667420 }} </ref> | |||
*Salmonellosis | |||
*Dosage: 400 mg q12h for 7 days <ref name="pmid2259863">{{cite journal| author=Carlstedt G, Dahl P, Niklasson PM, Gullberg K, Banck G, Kahlmeter G| title=Norfloxacin treatment of salmonellosis does not shorten the carrier stage. | journal=Scand J Infect Dis | year= 1990 | volume= 22 | issue= 5 | pages= 553-6 | pmid=2259863 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2259863 }} </ref> | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norfloxacin in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norfloxacin in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Norfloxacin in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Norfloxacin in pediatric patients. | ||
Revision as of 15:59, 8 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Norfloxacin is an fluoroquinolone antibiotic that is FDA approved for the treatment of urinary tract infections (UTI), prostatitis and sexually transmited diseases (STD). Common adverse reactions include nausea, stomach cramps, dizziness and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)

UTI's
Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus saprophyticus, Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, Proteus vulgaris, Staphylococcus aureus, or Streptococcus agalactiae.
Sexually Transmitted Diseases
Uncomplicated urethral and cervical gonorrhea due to Neisseria gonorrhoeae.
Prostatitis
Prostatitis due to Escherichia coli.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Norfloxacin in adult patients.
Non–Guideline-Supported Use
- Cholera[1]
- Immunodeficiency disorder - Infectious disease; Prophylaxis
- Dosage: 400 q12h [2]
- Malaria
- Dosage: 400 mg q12h for 3 days [3]
- Salmonellosis
- Dosage: 400 mg q12h for 7 days [4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Norfloxacin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Norfloxacin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Norfloxacin in pediatric patients.
Contraindications
Norfloxacin is contraindicated in persons with a history of hypersensitivity, tendinitis, or tendon rupture associated with the use of norfloxacin or any member of the quinolone group of antimicrobial agents.
Warnings
Tendinopathy and Tendon Rupture
Fluoroquinolones, including Norfloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Norfloxacin should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Exacerbation of Myasthenia Gravis
Fluoroquinolones, including Norfloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Post-marketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid Norfloxacin in patients with known history of myasthenia gravis.
Safety in Children, Adolescents, Nursing mothers, and during Pregnancy
THE SAFETY AND EFFICACY OF ORAL NORFLOXACIN IN PEDIATRIC PATIENTS, ADOLESCENTS (UNDER THE AGE OF 18), PREGNANT WOMEN, AND NURSING MOTHERS HAVE NOT BEEN ESTABLISHED. The oral administration of single doses of norfloxacin, 6 times2 the recommended human clinical dose (on a mg/kg basis), caused lameness in immature dogs. Histologic examination of the weight-bearing joints of these dogs revealed permanent lesions of the cartilage. Other quinolones also produced erosions of the cartilage in weight-bearing joints and other signs of arthropathy in immature animals of various species.
Central Nervous System Effects/Disorders
Convulsions have been reported in patients receiving norfloxacin. Convulsions, increased intracranial pressure (including pseudotumor cerebri), and toxic psychoses have been reported in patients receiving drugs in this class. Quinolones may also cause central nervous system (CNS) stimulation which may lead to tremors, restlessness, lightheadedness, confusion, and hallucinations. If these reactions occur in patients receiving norfloxacin, the drug should be discontinued and appropriate measures instituted. The effects of norfloxacin on brain function or on the electrical activity of the brain have not been tested. Therefore, until more information becomes available, norfloxacin, like all other quinolones, should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral arteriosclerosis, epilepsy, and other factors which predispose to seizures.
Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy, including Norfloxacin. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal edema or facial edema, dyspnea, urticaria and itching. Only a few patients had a history of hypersensitivity reactions. If an allergic reaction to norfloxacin occurs, discontinue the drug. Serious acute hypersensitivity reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, including intubation, should be administered as indicated.
Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including Norfloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- Fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome);
- Vasculitis; arthralgia; myalgia; serum sickness;
- Allergic pneumonitis;
- Interstitial nephritis; acute renal insufficiency or failure;
- Hepatitis; jaundice; acute hepatic necrosis or failure;
- Anemia, including hemolytic anemia and aplastic anemia; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity, and supportive measures should be instituted
Clostridium Difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Norfloxacin and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD.
Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Peripheral Neuropathy
Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including norfloxacin. Symptoms may occur soon after initiation of norfloxacin and may be irreversible. Norfloxacin should be discontinued immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness, or other alterations in sensations including light touch, pain, temperature, position sense and vibratory sensation.
Syphilis Treatment
Norfloxacin has not been shown to be effective in the treatment of syphilis. Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with norfloxacin should have a follow-up serologic test for syphilis after three months.
Adverse Reactions
Clinical Trials Experience
SIngle-Dose Studies
In clinical trials involving 82 healthy subjects and 228 patients with gonorrhea, treated with a single dose of norfloxacin, 6.5% reported drug-related adverse experiences. However, the following incidence figures were calculated without reference to drug relationship.
- The most common adverse experiences (>1.0%) were: dizziness (2.6%), nausea (2.6%), headache (2.0%), and abdominal cramping (1.6%).
- Additional reactions (0.3%-1.0%) were: anorexia, diarrhea, hyperhidrosis, asthenia, anal/rectal pain, constipation, dyspepsia, flatulence, tingling of the fingers, and vomiting.
- Laboratory adverse changes considered drug-related were reported in 4.5% of patients/subjects. These laboratory changes were: increased AST (SGOT) (1.6%), decreased WBC (1.3%), decreased platelet count (1.0%), increased urine protein (1.0%), decreased hematocrit and hemoglobin (0.6%), and increased eosinophils (0.6%).
Multiple-Dose Studies
In clinical trials involving 52 healthy subjects and 1980 patients with urinary tract infections or prostatitis treated with multiple doses of norfloxacin, 3.6% reported drug-related adverse experiences. However, the incidence figures below were calculated without reference to drug relationship.
- The most common adverse experiences (>1.0%) were: nausea (4.2%), headache (2.8%), dizziness (1.7%), and asthenia (1.3%).
- Additional reactions (0.3%-1.0%) were: abdominal pain, back pain, constipation, diarrhea, dry mouth, dyspepsia/heartburn, fever, flatulence, hyperhidrosis, loose stools, pruritus, rash, somnolence, and vomiting.
- Less frequent reactions (0.1%-0.2%) included: abdominal swelling, allergies, anorexia, anxiety, bitter taste, blurred vision, bursitis, chest pain, chills, depression, dysmenorrhea, edema, erythema, foot or hand swelling, insomnia, mouth ulcer, myocardial infarction, palpitation, pruritus ani, renal colic, sleep disturbances, and urticaria.
- Abnormal laboratory values observed in these patients/subjects were: eosinophilia (1.5%), elevation of ALT (SGPT) (1.4%), decreased WBC and/or neutrophil count (1.4%), elevation of AST (SGOT) (1.4%), and increased alkaline phosphatase (1.1%). Those occurring less frequently included increased BUN, increased LDH, increased serum creatinine, decreased hematocrit, and glycosuria.
Postmarketing Experience
The most frequently reported adverse reaction in post-marketing experience is rash. CNS effects characterized as generalized seizures, myoclonus and tremors have been reported with Norfloxacin. Visual disturbances have been reported with drugs in this class.
The following additional adverse reactions have been reported since the drug was marketed: Hypersensitivity Reactions
Hypersensitivity Reactions
Dermatological Effects
- Toxic epidermal necrolysis
- Stevens-Johnson syndrome
- Erythema multiforme
- Exfoliative dermatitis
- Photosensitivity/phototoxicity reactions
- Leukocytoclastic vasculitis
- Drug rash with eosinophilia and systemic symptoms (DRESS syndrome).
Gastrointestinal Effects
- Pseudomembranous colitis
- Hepatitis
- Jaundice
- Elevated liver function tests
- Pancreatitis (rare)
- Stomatitis
Hepatic Effects
Cardiovascular Effects
- Prolonged QTc interval and ventricular arrhythmia including torsades de pointes.
Renal Effects
Nervous System/Psychiatric Effects
Musculoskeletal
- Tendinitis
- Tendon rupture
- Exacerbation of myasthenia gravis
- Elevated creatine kinase (CK)
- Muscle spasms
Hematologic Effects
Special Senses
Other adverse events reported with quinolones include: agranulocytosis, albuminuria, candiduria, crystalluria, cylindruria, dysphagia, elevation of blood glucose, elevation of serum cholesterol, elevation of serum potassium, elevation of serum triglycerides, hematuria, hepatic necrosis, symptomatic hypoglycemia, nystagmus, postural hypotension, prolongation of prothrombin time, and vaginal candidiasis.
Drug Interactions
- Quinolones, including norfloxacin, have been shown in vitro to inhibit CYP1A2. Concomitant use with drugs metabolized by CYP1A2 (e.g., caffeine, clozapine, ropinirole, tacrine, theophylline, tizanidine) may result in increased substrate drug concentrations when given in usual doses. Patients taking any of these drugs concomitantly with norfloxacin should be carefully monitored.
- Elevated plasma levels of theophylline have been reported with concomitant quinolone use. There have been reports of theophylline-related side effects in patients on concomitant therapy with norfloxacin and theophylline. Therefore, monitoring of theophylline plasma levels should be considered and dosage of theophylline adjusted as required.
- Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with norfloxacin. Therefore, cyclosporine serum levels should be monitored and appropriate cyclosporine dosage adjustments made when these drugs are used concomitantly.
- Quinolones, including norfloxacin, may enhance the effects of oral anticoagulants, including warfarin or its derivatives or similar agents. When these products are administered concomitantly, prothrombin time or other suitable coagulation tests should be closely monitored.
- The concomitant administration of quinolones including norfloxacin with glyburide (a sulfonylurea agent) has, on rare occasions, resulted in severe hypoglycemia. Therefore, monitoring of blood glucose is recommended when these agents are co-administered.
- Diminished urinary excretion of norfloxacin has been reported during the concomitant administration of probenecid and norfloxacin.
- The concomitant use of nitrofurantoin is not recommended since nitrofurantoin may antagonize the antibacterial effect of NOROXIN in the urinary tract.
Multivitamins, or other products containing iron or zinc, antacids or sucralfate, should not be administered concomitantly with, or within 2 hours of, the administration of norfloxacin, because they may interfere with absorption resulting in lower serum and urine levels of norfloxacin.
Videx® (Didanosine) chewable/buffered tablets or the pediatric powder for oral solution should not be administered concomitantly with, or within 2 hours of, the administration of norfloxacin, because these products may interfere with absorption resulting in lower serum and urine levels of norfloxacin.
Some quinolones have also been shown to interfere with the metabolism of caffeine. This may lead to reduced clearance of caffeine and a prolongation of the plasma half-life that may lead to accumulation of caffeine in plasma when products containing caffeine are consumed while taking norfloxacin.
The concomitant administration of a non-steroidal anti-inflammatory drug (NSAID) with a quinolone, including norfloxacin, may increase the risk of CNS stimulation and convulsive seizures. Therefore, NOROXIN should be used with caution in individuals receiving NSAIDS concomitantly.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Norfloxacin has been shown to produce embryonic loss in monkeys when given in doses 10 times2 the maximum daily total human dose (on a mg/kg basis). At this dose, peak plasma levels obtained in monkeys were approximately 2 times those obtained in humans. There has been no evidence of a teratogenic effect in any of the animal species tested (rat, rabbit, mouse, monkey) at 6-50 times2 the maximum daily human dose (on a mg/kg basis). There are, however, no adequate and well-controlled studies in pregnant women. Norfloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Norfloxacin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Norfloxacin during labor and delivery.
Nursing Mothers
It is not known whether norfloxacin is excreted in human milk. When a 200-mg dose of NOROXIN was administered to nursing mothers, norfloxacin was not detected in human milk. However, because the dose studied was low, because other drugs in this class are secreted in human milk, and because of the potential for serious adverse reactions from norfloxacin in nursing infants, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of oral norfloxacin in pediatric patients and adolescents below the age of 18 years have not been established. Norfloxacin causes arthropathy in juvenile animals of several animal species.
Geriatic Use
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as Norfloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing Norfloxacin to elderly patients, especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue Norfloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur.
Of the 340 subjects in one large clinical study of Norfloxacin for treatment of urinary tract infections, 103 patients were 65 and older, 77 of whom were 70 and older; no overall differences in safety and effectiveness were evident between these subjects and younger subjects. In clinical practice, no difference in the type of reported adverse experiences have been observed between the elderly and younger patients except for a possible increased risk of tendon rupture in elderly patients receiving concomitant corticosteroids. In addition, increased risk for other adverse experiences in some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. A pharmacokinetic study of Norfloxacin in elderly volunteers (65 to 75 years of age with normal renal function for their age) was carried out.
In general, elderly patients may be more susceptible to drug-associated effects of the QTc interval. Therefore, precaution should be taken when using NOROXIN concomitantly with drugs that can result in prolongation of the QTc interval (e.g., class IA or class III antiarrhythmics) or in patients with risk factors for torsades de pointes (e.g., known QTc prolongation, uncorrected hypokalemia).
Gender
There is no FDA guidance on the use of Norfloxacin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Norfloxacin with respect to specific racial populations.
Renal Impairment
Norfloxacin may be used for the treatment of urinary tract infections in patients with renal insufficiency. In patients with a creatinine clearance rate of 30 mL/min/1.73 m2 or less, the recommended dosage is one 400-mg tablet once daily for the duration given above. At this dosage, the urinary concentration exceeds the MICs for most urinary pathogens susceptible to norfloxacin, even when the creatinine clearance is less than 10 mL/min/1.73 m2.
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.

Hepatic Impairment
There is no FDA guidance on the use of Norfloxacin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Norfloxacin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Norfloxacin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Tablet, film coated
Monitoring
- The concomitant administration of quinolones including norfloxacin with glyburide (a sulfonylurea agent) has, on rare occasions, resulted in severe hypoglycemia. Therefore, monitoring of blood glucose is recommended when these agents are co-administered.
- Elevated plasma levels of theophylline have been reported with concomitant quinolone use. There have been reports of theophylline-related side effects in patients on concomitant therapy with norfloxacin and theophylline. Therefore, monitoring of theophylline plasma levels should be considered and dosage of theophylline adjusted as required.
IV Compatibility
There is limited information regarding the compatibility of Norfloxacin and IV administrations.
Overdosage
No significant lethality was observed in male and female mice and rats at single oral doses up to 4 g/kg. In the event of acute overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given symptomatic and supportive treatment. Adequate hydration must be maintained.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Noroxin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a687006 |
| Pregnancy category |
|
| Routes of administration | Oral,ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30 to 40% |
| Protein binding | 10 to 15% |
| Metabolism | Hepatic |
| Elimination half-life | 3 to 4 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H18FN3O3 |
| Molar mass | 319.331 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
Norfloxacin inhibits bacterial deoxyribonucleic acid synthesis and is bactericidal. At the molecular level, three specific events are attributed to norfloxacin in E. coli cells:
- Inhibition of the ATP-dependent DNA supercoiling reaction catalyzed by DNA gyrase.
- Inhibition of the relaxation of supercoiled DNA.
- Promotion of double-stranded DNA breakage.
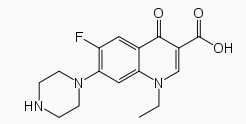
The fluorine atom at the 6 position provides increased potency against gram-negative organisms, and the piperazine moiety at the 7 position is responsible for antipseudomonal activity.
Structure
Norfloxacin, a fluoroquinolone, is 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C16H18FN3O3 and the structural formula is:

Pharmacodynamics
There is limited information regarding Norfloxacin Pharmacodynamics in the drug label.
Pharmacokinetics
In fasting healthy volunteers, at least 30-40% of an oral dose of NOROXIN is absorbed. Absorption is rapid following single doses of 200 mg, 400 mg and 800 mg. At the respective doses, mean peak serum and plasma concentrations of 0.8, 1.5 and 2.4 μg/mL are attained approximately one hour after dosing. The presence of food and/or dairy products may decrease absorption. The effective half-life of norfloxacin in serum and plasma is 3-4 hours. Steady-state concentrations of norfloxacin will be attained within two days of dosing.
In healthy elderly volunteers (65-75 years of age with normal renal function for their age), norfloxacin is eliminated more slowly because of their slightly decreased renal function. Following a single 400-mg dose of norfloxacin, the mean (± SD) AUC and Cmax of 9.8 (2.83) μg•hr/mL and 2.02 (0.77) μg/mL, respectively, were observed in healthy elderly volunteers. The extent of systemic exposure was slightly higher than that seen in younger adults (AUC 6.4 μg•hr/mL and Cmax 1.5 μg/mL). Drug absorption appears unaffected. However, the effective half-life of norfloxacin in these elderly subjects is 4 hours.
There is no information on accumulation of norfloxacin with repeated administration in elderly patients. However, no dosage adjustment is required based on age alone. In elderly patients with reduced renal function, the dosage should be adjusted as for other patients with renal impairment.
The disposition of norfloxacin in patients with creatinine clearance rates greater than 30 mL/min/1.73 m2 is similar to that in healthy volunteers. In patients with creatinine clearance rates equal to or less than 30 mL/min/1.73 m2, the renal elimination of norfloxacin decreases so that the effective serum half-life is 6.5 hours. In these patients, alteration of dosage is necessary. Drug absorption appears unaffected by decreasing renal function.
Norfloxacin is eliminated through metabolism, biliary excretion, and renal excretion. After a single 400-mg dose of NOROXIN, mean antimicrobial activities equivalent to 278, 773, and 82 μg of norfloxacin/g of feces were obtained at 12, 24, and 48 hours, respectively. Renal excretion occurs by both glomerular filtration and tubular secretion as evidenced by the high rate of renal clearance (approximately 275 mL/min). Within 24 hours of drug administration, 26 to 32% of the administered dose is recovered in the urine as norfloxacin with an additional 5-8% being recovered in the urine as six active metabolites of lesser antimicrobial potency. Only a small percentage (less than 1%) of the dose is recovered thereafter. Fecal recovery accounts for another 30% of the administered dose. In elderly subjects (average creatinine clearance 91 mL/min/1.73 m2) approximately 22% of the administered dose was recovered in urine and renal clearance averaged 154 mL/min.
Two to three hours after a single 400-mg dose, urinary concentrations of 200 μg/mL or more are attained in the urine. In healthy volunteers, mean urinary concentrations of norfloxacin remain above 30 μg/mL for at least 12 hours following a 400-mg dose. The urinary pH may affect the solubility of norfloxacin. Norfloxacin is least soluble at urinary pH of 7.5 with greater solubility occurring at pHs above and below this value. The serum protein binding of norfloxacin is between 10 and 15%.
The following are mean concentrations of norfloxacin in various fluids and tissues measured 1 to 4 hours post-dose after two 400-mg doses, unless otherwise indicated:
Nonclinical Toxicology
There is limited information regarding Norfloxacin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Norfloxacin Clinical Studies in the drug label.
How Supplied
Tablets NOROXIN 400 mg are white to off-white, oval shaped, film-coated tablets, coded 705 on one side and plain on the other. They are supplied as follows: NDC 0006-0705-20 unit of use bottles of 20.
Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
Images
Drug Images
{{#ask: Page Name::Norfloxacin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Norfloxacin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Norfloxacin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Norfloxacin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Norfloxacin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bhattacharya SK, Bhattacharya MK, Dutta P, Dutta D, De SP, Sikdar SN; et al. (1990). "Double-blind, randomized, controlled clinical trial of norfloxacin for cholera". Antimicrob Agents Chemother. 34 (5): 939–40. PMC 171728. PMID 2193628.
- ↑ Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ; et al. (1987). "Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double-blind, placebo-controlled trial". Ann Intern Med. 106 (1): 1–7. PMID 3538962.
- ↑ Sarma PS (1989). "Norfloxacin: a new drug in the treatment of falciparum malaria". Ann Intern Med. 111 (4): 336–7. PMID 2667420.
- ↑ Carlstedt G, Dahl P, Niklasson PM, Gullberg K, Banck G, Kahlmeter G (1990). "Norfloxacin treatment of salmonellosis does not shorten the carrier stage". Scand J Infect Dis. 22 (5): 553–6. PMID 2259863.
{{#subobject:
|Label Page=Norfloxacin |Label Name=Paquete.png
}}
- Pages with script errors
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- CS1 maint: PMC format
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to watched fields