Ciprofloxacin (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including Ciprofloxacin Injection, USP, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Overview
Ciprofloxacin (injection) is an antibiotic that is FDA approved for the treatment of urinary tract infections, lower respiratory infections,nosocomial pneumonia, skin infections, acute sinusitis,intra-abdominal infections and chronic bacterial prostatitis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash,diarrhea,nausea, vomiting and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Ciprofloxacin Injection, USP is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions and patient populations listed below when the intravenous administration offers a route of administration advantageous to the patient.
Urinary Tract Infections caused by Escherichia coli (including cases with secondary bacteremia), Klebsiella pneumoniae subspecies pneumoniae, Enterobacter cloacae, Serratia marcescens, Proteus mirabilis, Providencia rettgeri, Morganella morganii, Citrobacter diversus, Citrobacter freundii, Pseudomonas aeruginosa, methicillin-susceptible Staphylococcus epidermidis, Staphylococcus saprophyticus, or Enterococcus faecalis.
Lower Respiratory Infections caused by Escherichia coli, Klebsiella pneumoniae subspecies pneumoniae, Enterobacter cloacae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, or penicillin-susceptible Streptococcus pneumoniae.
- Also, Moraxella catarrhalis for the treatment of acute exacerbations of chronic bronchitis.
- Ciprofloxacin is not a drug of first choice in the treatment of presumed or confirmed pneumonia secondary to Streptococcus pneumoniae.
Nosocomial Pneumonia caused by Haemophilus influenzae or Klebsiella pneumoniae.
Skin and Skin Structure Infections caused by Escherichia coli, Klebsiella pneumoniae subspecies pneumoniae, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Morganella morganii, Citrobacter freundii, Pseudomonas aeruginosa, methicillin-susceptible Staphylococcus aureus, methicillin-susceptible Staphylococcus epidermidis, or Streptococcus pyogenes.
Bone and Joint Infections caused by Enterobacter cloacae, Serratia marcescens, or Pseudomonas aeruginosa.
Complicated Intra-Abdominal Infections (used in conjunction with metronidazole) caused by Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, or Bacteroides fragilis.
Acute Sinusitis caused by Haemophilus influenzae, penicillin-susceptible Streptococcus pneumoniae, or Moraxella catarrhalis.
Chronic Bacterial Prostatitis caused by Escherichia coli or Proteus mirabilis.
Empirical Therapy for Febrile Neutropenic Patients in combination with piperacillin sodium.
Inhalational anthrax (post-exposure): To reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
Dosage
- Ciprofloxacin Injection, USP should be administered to adults by intravenous infusion over a period of 60 minutes at dosages described in the Dosage Guidelines table. Slow infusion of a dilute solution into a larger vein will minimize patient discomfort and reduce the risk of venous irritation.
- The determination of dosage for any particular patient must take into consideration the severity and nature of the infection, the susceptibility of the causative microorganism, the integrity of the patient’s host-defense mechanisms, and the status of renal and hepatic function.
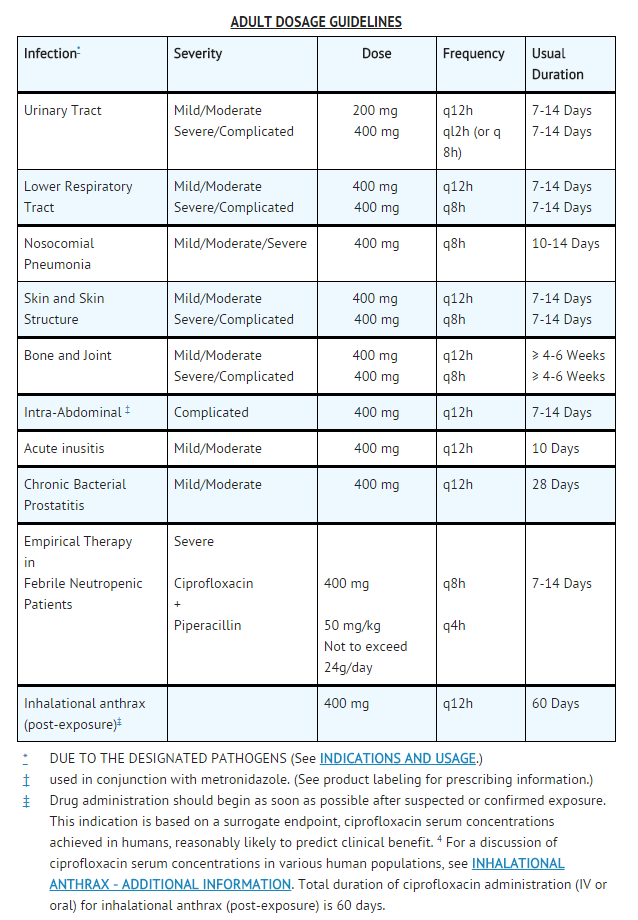
- Ciprofloxacin Injection, USP should be administered by intravenous infusion over a period of 60 minutes.
Conversion of I.V. to Oral Dosing in Adults
- Ciprofloxacin Tablets and ciprofloxacin Oral Suspension for oral administration are available. Parenteral therapy may be switched to oral ciprofloxacin when the condition warrants, at the discretion of the physician.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
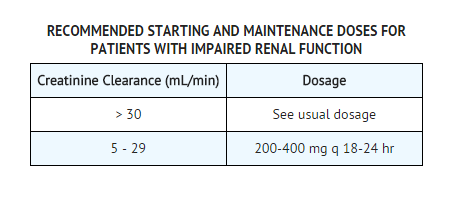
Adults with Impaired Renal Function
Ciprofloxacin is eliminated primarily by renal excretion; however, the drug is also metabolized and partially cleared through the biliary system of the liver and through the intestine. These alternative pathways of drug elimination appear to compensate for the reduced renal excretion in patients with renal impairment. Nonetheless, some modification of dosage is recommended for patients with severe renal dysfunction. The following table provides dosage guidelines for use in patients with renal impairment:
- When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance:
- Men: Creatinine clearance (mL/min) =

- Women: 0.85 x the value calculated for men.
- The serum creatinine should represent a steady state of renal function.
- For patients with changing renal function or for patients with renal impairment and hepatic insufficiency, careful monitoring is suggested.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ciprofloxacin (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ciprofloxacin (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Pediatric patients (1 to 17 years of age):
Complicated Urinary Tract Infections and Pyelonephritis due to Escherichia coli.
- NOTE: Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse events compared to controls, including events related to joints and/or surrounding tissues. Ciprofloxacin, like other fluoroquinolones, is associated with arthropathy and histopathological changes in weight-bearing joints of juvenile animals.
Inhalational anthrax (post-exposure):
- To reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
Dosage
- Ciprofloxacin Injection, USP should be administered as described in the Dosage Guidelines table. An increased incidence of adverse events compared to controls, including events related to joints and/or surrounding tissues, has been observed.
- Dosing and initial route of therapy (i.e., IV or oral) for complicated urinary tract infection or pyelonephritis should be determined by the severity of the infection. In the clinical trial, pediatric patients with moderate to severe infection were initiated on 6 to 10 mg/kg IV every 8 hours and allowed to switch to oral therapy (10 to 20 mg/kg every 12 hours), at the discretion of the physician.
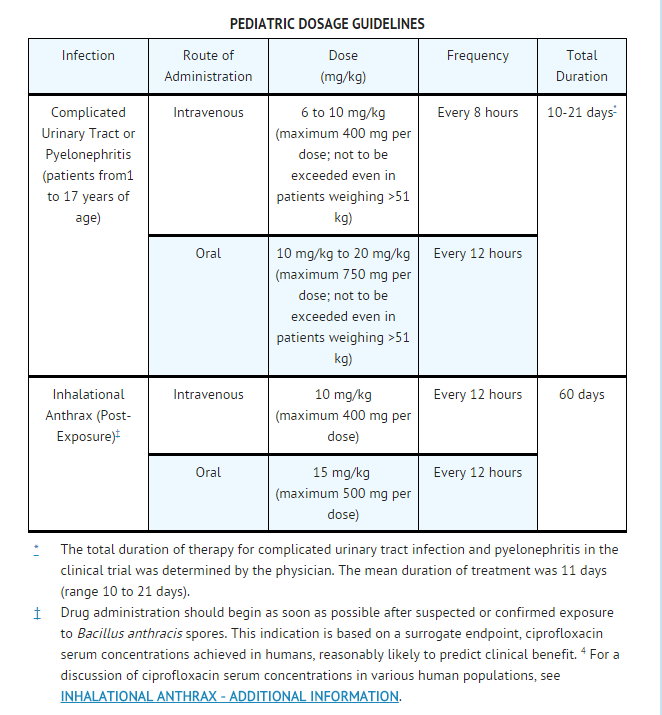
- Pediatric patients with moderate to severe renal insufficiency were excluded from the clinical trial of complicated urinary tract infection and pyelonephritis. No information is available on dosing adjustments necessary for pediatric patients with moderate to severe renal insufficiency (i.e., creatinine clearance of < 50 mL/min/1.73m2).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ciprofloxacin (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ciprofloxacin (injection) in pediatric patients.
Contraindications
- Ciprofloxacin is contraindicated in persons with a history of hypersensitivity to ciprofloxacin, any member of the quinolone class of antimicrobial agents, or any of the product components.
- Concomitant administration with tizanidine is contraindicated.
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including Ciprofloxacin Injection, USP, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Tendinopathy and Tendon Rupture
- Fluoroquinolones, including Ciprofloxacin injection, USP are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Ciprofloxacin Injection, USP should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Exacerbation of Myasthenia Gravis
- Fluoroquinolones, including Ciprofloxacin Injection, USP, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid Ciprofloxacin Injection, USP in patients with known history of myasthenia gravis.
Pregnant Women
- THE SAFETY AND EFFECTIVENESS OF CIPROFLOXACIN IN PREGNANT AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED.
Hypersensitivity Reactions
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Only a few patients had a history of hypersensitivity reactions. Serious anaphylactic reactions require immediate emergency treatment with epinephrine and other resuscitation measures, including oxygen, intravenous fluids, intravenous antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
- Other Serious and Sometimes Fatal Reactions
- Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including ciprofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- Fever, rash, or severe dermatologic reactions (for example, toxic epidermal necrolysis, Stevens-Johnson syndrome);
- Interstitial nephritis; acute renal insufficiency or failure;
- Hepatitis; jaundice; acute hepatic necrosis or failure;
- Anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
- The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted.
Theophylline
- SERIOUS AND FATAL REACTIONS HAVE BEEN REPORTED IN PATIENTS RECEIVING CONCURRENT ADMINISTRATION OF INTRAVENOUS CIPROFLOXACIN AND THEOPHYLLINE. These reactions have included cardiac arrest, seizure, status epilepticus, and respiratory failure. Although similar serious adverse events have been reported in patients receiving theophylline alone, the possibility that these reactions may be potentiated by ciprofloxacin cannot be eliminated. If concomitant use cannot be avoided, serum levels of theophylline should be monitored and dosage adjustments made as appropriate.
Adverse Reactions
Clinical Trials Experience
Adverse Reactions in Adult Patients
- During clinical investigations with oral and parenteral ciprofloxacin, 49,038 patients received courses of the drug. Most of the adverse events reported were described as only mild or moderate in severity, abated soon after the drug was discontinued, and required no treatment. Ciprofloxacin was discontinued because of an adverse event in 1.8% of intravenously treated patients.
- The most frequently reported drug related events, from clinical trials of all formulations, all dosages, all drug-therapy durations, and for all indications of ciprofloxacin therapy were nausea (2.5%), diarrhea (1.6%), liver function tests abnormal (1.3%), vomiting (1.0%), and rash (1.0%).
- In clinical trials the following events were reported, regardless of drug relationship, in greater than 1% of patients treated with intravenous ciprofloxacin: nausea, diarrhea, central nervous system disturbance, local IV site reactions, liver function tests abnormal, eosinophilia, headache, restlessness, and rash. Many of these events were described as only mild or moderate in severity, abated soon after the drug was discontinued, and required no treatment. Local IV site reactions are more frequent if the infusion time is 30 minutes or less. These may appear as local skin reactions, which resolve rapidly upon completion of the infusion. Subsequent intravenous administration is not contraindicated unless the reactions recur or worsen.
- Additional medically important events, without regard to drug relationship or route of administration, that occurred in 1% or less of ciprofloxacin patients are listed below:
- BODY AS A WHOLE: abdominal pain/discomfort, foot pain, pain, pain in extremities
- CARDIOVASCULAR: cardiovascular collapse, cardiopulmonary arrest, myocardial infarction, arrhythmia, tachycardia, palpitation, cerebral thrombosis, syncope, cardiac murmur, hypertension, hypotension, angina pectoris, atrial flutter, ventricular ectopy, (thrombo)-phlebitis, vasodilation, migraine
- CENTRAL NERVOUS SYSTEM: convulsive seizures (including status epilepticus), grand mal convulsion, paranoia, toxic psychosis, depression (potentially culminatin]g in self-injurious behavior, such as suicidal ideations/thoughts and attempted or completed suicide), dysphasia, phobia, depersonalization, manic reaction, unresponsiveness, ataxia, confusion, hallucinations, dizziness, lightheadedness, paresthesia, anxiety, tremor, insomnia, nightmares, weakness, drowsiness, irritability, malaise, lethargy, abnormal gait.
- GASTROINTESTINAL: ileus, jaundice, gastrointestinal bleeding, C. difficile associated diarrhea, pseudomembranous colitis, pancreatitis, hepatic necrosis, intestinal perforation, dyspepsia, epigastric pain, constipation, oral ulceration, oral candidiasis, mouth dryness, anorexia, dysphagia, flatulence, hepatitis, painful oral mucosa
- HEMIC/LYMPHATIC: agranulocytosis, prolongation of prothrombin time, lymphadenopathy, petechia
- MUSCULOSKELETAL: arthralgia, jaw, arm or back pain, joint stiffness, neck and chest pain, achiness, flare up of gout, myasthenia gravis, muscle weakness
- RENAL/UROGENITAL: renal failure, interstitial nephritis, nephritis, hemorrhagic cystitis, renal calculi, frequent urination, acidosis, urethral bleeding, polyuria, urinary retention, gynecomastia, candiduria, vaginitis, breast pain. Crystalluria, cylindruria, hematuria and albuminuria have also been reported.
- RESPIRATORY: respiratory arrest, pulmonary embolism, dyspnea, laryngeal or pulmonary edema, respiratory distress, pleural effusion, hemoptysis, epistaxis, hiccough, bronchospasm
- SKIN/HYPERSENSITIVITY: allergic reactions, anaphylactic reactions including life-threatening anaphylactic shock, erythema multiforme/Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, vasculitis, angioedema, edema of the lips, face, neck, conjunctivae, hands or lower extremities, purpura, fever, chills, flushing, pruritus, urticaria, cutaneous candidiasis, vesicles, increased perspiration, hyperpigmentation, erythema nodosum, thrombophlebitis, burning, paresthesia, erythema, swelling, photosensitivity/phototoxicity reaction.
- SPECIAL SENSES: decreased visual acuity, blurred vision, disturbed vision (flashing lights, change in color perception, overbrightness of lights, diplopia), eye pain, anosmia, hearing loss, tinnitus, nystagmus, chromatopsia, a bad taste.
- In several instances, nausea, vomiting, tremor, irritability, or palpitation were judged by investigators to be related to elevated serum levels of theophylline possibly as a result of drug interaction with ciprofloxacin.
- In randomized, double-blind controlled clinical trials comparing ciprofloxacin (I.V. and I.V./P.O. sequential) with intravenous beta-lactam control antibiotics, the CNS adverse event profile of ciprofloxacin was comparable to that of the control drugs.
Adverse Reactions in Pediatric Patients
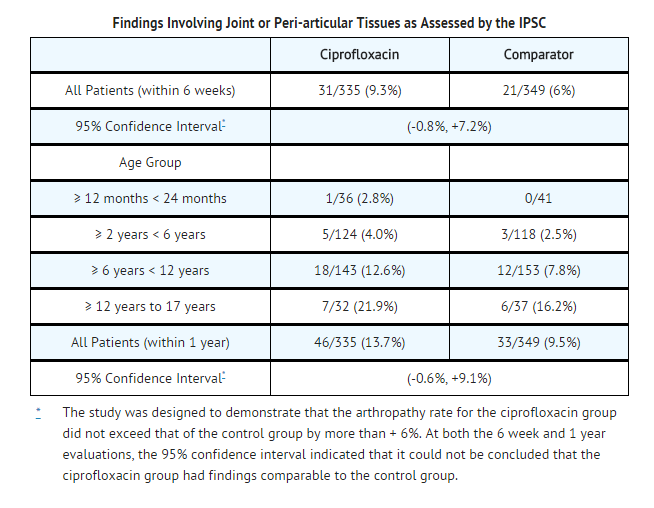
- Ciprofloxacin, administered IV and /or orally, was compared to a cephalosporin for treatment of complicated urinary tract infections (cUTI) or pyelonephritis in pediatric patients 1 to 17 years of age (mean age of 6 ± 4 years). The trial was conducted in the US, Canada, Argentina, Peru, Costa Rica, Mexico, South Africa, and Germany. The duration of therapy was 10 to 21 days (mean duration of treatment was 11 days with a range of 1 to 88 days). The primary objective of the study was to assess musculoskeletal and neurological safety within 6 weeks of therapy and through one year of follow-up in the 335 ciprofloxacin- and 349 comparator-treated patients enrolled.
- An Independent Pediatric Safety Committee (IPSC) reviewed all cases of musculoskeletal adverse events as well as all patients with an abnormal gait or abnormal joint exam (baseline or treatment-emergent). These events were evaluated in a comprehensive fashion and included such conditions as arthralgia, abnormal gait, abnormal joint exam, joint sprains, leg pain, back pain, arthrosis, bone pain, pain, myalgia, arm pain, and decreased range of motion in a joint. The affected joints included: knee, elbow, ankle, hip, wrist, and shoulder. Within 6 weeks of treatment initiation, the rates of these events were 9.3% (31/335) in the ciprofloxacin-treated group versus 6.0% (21/349) in comparator-treated patients. The majority of these events were mild or moderate in intensity. All musculoskeletal events occurring by 6 weeks resolved (clinical resolution of signs and symptoms), usually within 30 days of end of treatment. Radiological evaluations were not routinely used to confirm resolution of the events. The events occurred more frequently in ciprofloxacin-treated patients than control patients, regardless of whether they received IV or oral therapy. Ciprofloxacin-treated patients were more likely to report more than one event and on more than one occasion compared to control patients. These events occurred in all age groups and the rates were consistently higher in the ciprofloxacin group compared to the control group. At the end of 1 year, the rate of these events reported at any time during that period was 13.7% (46/335) in the ciprofloxacin-treated group versus 9.5% (33/349) comparator-treated patients.
- An adolescent female discontinued ciprofloxacin for wrist pain that developed during treatment. An MRI performed 4 weeks later showed a tear in the right ulnar fibrocartilage. A diagnosis of overuse syndrome secondary to sports activity was made, but a contribution from ciprofloxacin cannot be excluded. The patient recovered by 4 months without surgical intervention.
- The incidence rates of neurological events within 6 weeks of treatment initiation were 3% (9/335) in the ciprofloxacin group versus 2% (7/349) in the comparator group and included dizziness, nervousness, [[]insomnia]], and somnolence.
- In this trial, the overall incidence rates of adverse events regardless of relationship to study drug and within 6 weeks of treatment initiation were 41% (138/335) in the ciprofloxacin group versus 31% (109/349) in the comparator group. The most frequent events were gastrointestinal: 15% (50/335) of ciprofloxacin patients compared to 9% (31/349) of comparator patients. Serious adverse events were seen in 7.5% (25/335) of ciprofloxacin-treated patients compared to 5.7% (20/349) of control patients.
- Discontinuation of drug due to an adverse event was observed in 3% (10/335) of ciprofloxacin-treated patients versus 1.4% (5/349) of comparator patients. Other adverse events that occurred in at least 1% of ciprofloxacin patients were diarrhea 4.8%, vomiting 4.8%, abdominal pain 3.3%, accidental injury 3.0%, rhinitis 3.0%, dyspepsia 2.7%, nausea 2.7%, fever 2.1%, asthma 1.8% and rash 1.8%.
- In addition to the events reported in pediatric patients in clinical trials, it should be expected that events reported in adults during clinical trials or post-marketing experience may also occur in pediatric patients.
In addition to the events reported in pediatric patients in clinical trials, it should be expected that events reported in adults during clinical trials or post-marketing experience may also occur in pediatric patients.
Postmarketing Experience
- The following adverse events have been reported from worldwide marketing experience with fluoroquinolones, including ciprofloxacin. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these events in labeling are typically based on one or more of the following factors: (1) seriousness of the event, (2) frequency of the reporting, or (3) strength of causal connection to the drug.
- Acute generalized exanthematous pustulosis (AGEP),Agitation, agranulocytosis, albuminuria, anosmia, candiduria, cholesterol elevation (serum), confusion, constipation, delirium, dyspepsia, dysphagia, erythema multiforme, exfoliative dermatitis, fixed eruption, flatulence, glucose elevation (blood), hemolytic anemia, hepatic failure (including fatal cases), hepatic necrosis, hyperesthesia, hypertonia, hypesthesia], hypotension (postural), International Normalized Ratio (INR) increased (in patients treated with Vitamin K antagonists) jaundice, marrow depression (life threatening), methemoglobinemia, moniliasis (oral, gastrointestinal, vaginal), myalgia, myasthenia, myasthenia gravis, myoclonus, nystagmus, pancreatitis, pancytopenia (life threatening or fatal outcome), peripheral neuropathy that may be irreversable, phenytoin alteration (serum), photosensitivity/phototoxicity reaction, polyneuropathy, potassium elevation (serum), prothrombin time prolongation or decrease, pseudomembranous colitis (The onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment.), psychosis (toxic), QT prolongation, renal calculi, serum sickness like reaction, Stevens-Johnson syndrome, taste loss, tendinitis, tendon rupture, torsade de pointes, toxic epidermal necrolysis (Lyell’s Syndrome), triglyceride elevation (serum), twitching, vaginal candidiasis, vasculitis and ventricular arrhythmia.
- Adverse events were also reported by persons who received ciprofloxacin for anthrax post-exposure prophylaxis following the anthrax bioterror attacks of October 2001
Drug Interactions
Tizanidine
- In a pharmacokinetic study, systemic exposure of tizanidine (4 mg single dose) was significantly increased (Cmax 7-fold, AUC 10-fold) when the drug was given concomitantly with ciprofloxacin (500 mg bid for 3 days). The hypotensive and sedative effects of tizanidine were also potentiated. Concomitant administration of tizanidine and ciprofloxacin is contraindicated.
Theophylline
- As with some other quinolones, concurrent administration of ciprofloxacin with theophylline may lead to elevated serum concentrations of theophylline and prolongation of its elimination half-life. This may result in increased risk of theophylline-related adverse reactions.If concomitant use cannot be avoided, serum levels of theophylline should be monitored and dosage adjustments made as appropriate.
Other Xanthine Derivatives
- Some quinolones, including ciprofloxacin, have also been shown to interfere with the metabolism of caffeine. This may lead to reduced clearance of caffeine and prolongation of its serum half-life. On concurrent administration of ciprofloxacin and caffeine or pentoxifylline containing products, elevated serum concentrations of these xanthine derivatives were reported.
Cyclosporine
- Some quinolones, including ciprofloxacin, have been associated with transient elevations in serum creatinine in patients receiving cyclosporine concomitantly.
Phenytoin
- Altered serum levels of phenytoin (increased and decreased) have been reported in patients receiving concomitant ciprofloxacin. To avoid the loss of seizure control associated with decreased phenytoin levels, and to prevent phenytoin overdose-related undesirable effects when ciprofloxacin is discontinued in patients receiving both agents, monitoring of phenytoin therapy, including phenytoin serum concentration measurements, is recommended during and shortly after co-administration of CIPRO IV with phenytoin.
Oral Antidiabetic Agents
- Hypoglycemia has been reported when ciprofloxacin and oral antidiabetic agents, mainly sulfonylureas (for example, glyburide, glimepiride), were co-administered, presumably by intensifying the action of the oral antidiabetic agent (see ADVERSE REACTIONS). The concomitant administration of ciprofloxacin with glyburide has, on rare occasions, resulted in severe hypoglycemia. Fatalities have been reported.
Metronidazole
- The serum concentrations of ciprofloxacin and metronidazole were not altered when these two drugs were given concomitantly.
Oral Anti-Coagulants
- Simultaneous administration of ciprofloxacin with an oral anticoagulant may augment the effect of the anticoagulant. The risk may vary with the underlying infection, age and general status of the patient so that the contribution of ciprofloxacin to the increase in INR (international normalized ratio) is difficult to assess. Prothrombin time and INR should be monitored frequently during and shortly after co-administration of ciprofloxacin with an oral anticoagulant (for example, warfarin)
Probenecid
- Probenecid interferes with renal tubular secretion of ciprofloxacin and produces an increase in the level of ciprofloxacin in the serum. This should be considered if patients are receiving both drugs concomitantly.
Methotrxate
- Renal tubular transport of methotrexate may be inhibited by concomitant administration of ciprofloxacin potentially leading to increased plasma levels of methotrexate. This might increase the risk of methotrexate associated toxic reactions. Therefore, patients under methotrexate therapy should be carefully monitored when concomitant ciprofloxacin therapy is indicated.
Duloxetine
- In clinical studies it was demonstrated that concomitant use of duloxetine with strong inhibitors of the CYP450 1A2 isozyme such as fluvoxamine, may result in an a 5-fold increase of in mean AUC and a 2.5-fold increase in mean Cmax of duloxetine. Although no clinical data are available on a possible interaction with ciprofloxacin, similar effects can be expected upon concomitant administration.
NSAIDs
- Non-steroidal anti-inflammatory drugs (but not acetyl salicylic acid) in combination of very high doses of quinolones have been shown to provoke convulsions in pre-clinical studies.
Ropinirole
- In a study conducted in 12 patients with Parkinson’s disease who were administered 6 mg ropinirole once daily with 500 mg ciprofloxacin twice-daily, the mean Cmax and mean AUC of ropinirole were increased by 60% and 84%, respectively. Monitoring for ropinirole-related side effects and appropriate dose adjustment of ropinirole is recommended during and shortly after co-administration with ciprofloxacin.
Lidocaine
- In a study conducted in 9 healthy volunteers, concomitant use of 1.5 mg/kg IV lidocaine with 500 mg ciprofloxacin twice daily, resulted in an increase of lidocaine Cmax and AUC by 12% and 26%, respectively. Although lidocaine treatment was well tolerated at this elevated exposure, a possible interaction with ciprofloxacin and an increase in side effects related to lidocaine may occur upon concomitant administration.
Clozapine
- Following concomitant administration of 250 mg ciprofloxacin with 304 mg clozapine for 7 days, serum concentrations of clozapine and N-desmethylclozapine were increased by 29% and 31%, respectively. Careful monitoring of clozapine associated adverse effects and and appropriate adjustment of clozapine dosage during and shortly after co-administration with ciprofloxacin are advised.
Sildenafil
- Following concomitant administration of a single oral dose of 50 mg sildenafil with 500 mg ciprofloxacin to healthy subjects, the mean Cmax and mean AUC of sildenafil were both increased approximately two-fold. Therefore, sildenafil should be used with caution when co-administered with ciprofloxacin.
Drugs known to prolong QT interval
- Precaution should be taken when using ciprofloxacin concomitantly with drugs known to prolong the QT interval (for example, class IA or III antiarrhythmics, tricyclic antidepressants, macrolides, antipsychotics) as ciprofloxacin may have an additive effect on the QT interval.
Piperacillin Sodium
- Following infusion of 400 mg IV ciprofloxacin every eight hours in combination with 50 mg/kg IV piperacillin sodium every four hours, mean serum ciprofloxacin concentrations were 3.02 mcg/mL 1/2 hour and 1.18 mcg/mL between 6-8 hours after the end of infusion.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects. Pregnancy Category C
- There are no adequate and well-controlled studies in pregnant women. An expert review of published data on experiences with ciprofloxacin use during pregnancy by TERIS - the Teratogen Information System -concluded that therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk (quantity and quality of data=fair), but the data are insufficient to state that there is no risk.
- A controlled prospective observational study followed 200 women exposed to fluoroquinolones (52.5% exposed to ciprofloxacin and 68% first trimester exposures) during gestation.8 In utero exposure to fluoroquinolones during embryogenesis was not associated with increased risk of major malformations. The reported rates of major congenital malformations were 2.2% for the fluoroquinolone group and 2.6% for the control group (background incidence of major malformations is 1-5%). Rates of spontaneous abortions, prematurity and low birth weight did not differ between the groups and there were no clinically significant musculoskeletal dysfunctions up to one year of age in the ciprofloxacin exposed children.
- Another prospective follow-up study reported on 549 pregnancies with fluoroquinolone exposure (93% first trimester exposures).9 There were 70 ciprofloxacin exposures, all within the first trimester. The malformation rates among live-born babies exposed to ciprofloxacin and to fluoroquinolones overall were both within background incidence ranges. No specific patterns of congenital abnormalities were found. The study did not reveal any clear adverse reactions due to in utero exposure to ciprofloxacin.
- No differences in the rates of prematurity, spontaneous abortions, or birth weight were seen in women exposed to ciprofloxacin during pregnancy.7,8 However, these small postmarketing epidemiology studies, of which most experience is from short term, first trimester exposure, are insufficient to evaluate the risk for less common defects or to permit reliable and definitive conclusions regarding the safety of ciprofloxacin in pregnant women and their developing fetuses. Ciprofloxacin should not be used during pregnancy unless the potential benefit justifies the potential risk to both fetus and mother.
- Reproduction studies have been performed in rats and mice using oral doses up to 100 mg/kg (0.6 and 0.3 times the maximum daily human dose based upon body surface area, respectively) and have revealed no evidence of harm to the fetus due to ciprofloxacin. In rabbits, oral ciprofloxacin dose levels of 30 and 100 mg/kg (approximately 0.4- and 1.3-times the highest recommended therapeutic dose based upon mg/m2) produced gastrointestinal toxicity resulting in maternal weight loss and an increased incidence of abortion, but no teratogenicity was observed at either dose level. After intravenous administration of doses up to 20 mg/kg (approximately 0.3-times the highest recommended therapeutic dose based upon mg/m2) no maternal toxicity was produced and no embryotoxicity or teratogenicity was observed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ciprofloxacin (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ciprofloxacin (injection) during labor and delivery.
Nursing Mothers
- Ciprofloxacin is excreted in human milk. The amount of ciprofloxacin absorbed by the nursing infant is unknown. Because of the potential for serious adverse reactions (including articular damage) in infants nursing from mothers taking ciprofloxacin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Ciprofloxacin, like other quinolones, causes arthropathy and histological changes in weight-bearing joints of juvenile animals resulting in lameness.
Inhalational Anthrax (Post-Exposure)
- Ciprofloxacin is indicated in pediatric patients for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of ciprofloxacin to pediatric patients is appropriate. For information regarding pediatric dosing in inhalational anthrax (post-exposure).
Complicated Urinary Tract Infection and Pyelonephritis
- Ciprofloxacin is indicated for the treatment of complicated urinary tract infections and pyelonephritis due to Escherichia coli. Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse events compared to the controls, including those related to joints and/or surrounding tissues. The rates of these events in pediatric patients with complicated urinary tract infection and pyelonephritis within six weeks of follow-up were 9.3% (31/335) versus 6.0% (21/349) for control agents. The rates of these events occurring at any time up to the one year follow-up were 13.7% (46/335) and 9.5% (33/349), respectively. The rate of all adverse events regardless of drug relationship at six weeks was 41% (138/335) in the ciprofloxacin arm compared to 31% (109/349) in the control arm.
Cystic Fibrosis
- Short-term safety data from a single trial in pediatric cystic fibrosis patients are available. In a randomized, double-blind clinical trial for the treatment of acute pulmonary exacerbations in cystic fibrosis patients (ages 5-17 years), 67 patients received ciprofloxacin I.V. 10 mg/kg/dose q8h for one week followed by ciprofloxacin tablets 20 mg/kg/dose ql2h to complete 10-21 days treatment and 62 patients received the combination of ceftazidime I.V. 50 mg/kg/dose q8h and tobramycin I.V. 3 mg/kg/dose q8h for a total of 10-21 days. Patients less than 5 years of age were not studied. Safety monitoring in the study included periodic range of motion examinations and gait assessments by treatment-blinded examiners. Patients were followed for an average of 23 days after completing treatment (range 0-93 days). This study was not designed to determine long term effects and the safety of repeated exposure to ciprofloxacin. Musculoskeletal adverse events in patients with cystic fibrosis were reported in 22% of the patients in the ciprofloxacin group and 21% in the comparison group. Decreased range of motion was reported in 12% of the subjects in the ciprofloxacin group and 16% in the comparison group. Arthralgia was reported in 10% of the patients in the ciprofloxacin group and 11% in the comparison group. Other adverse events were similar in nature and frequency between treatment arms. One of sixty-seven patients developed arthritis of the knee nine days after a ten day course of treatment with ciprofloxacin. Clinical symptoms resolved, but an MRI showed knee effusion without other abnormalities eight months after treatment. However, the relationship of this event to the patient’s course of ciprofloxacin cannot be definitively determined, particularly since patients with cystic fibrosis may develop arthralgiasis/arthritis as part of their underlying disease process.
Geriatic Use
- Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as Ciprofloxacin Injection, USP. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involves the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing Ciprofloxacin Injection, USP to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue Ciprofloxacin Injection, USP and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur.
- In a retrospective analysis of 23 multiple-dose controlled clinical trials of ciprofloxacin encompassing over 3500 ciprofloxacin treated patients, 25% of patients were greater than or equal to 65 years of age and 10% were greater than or equal to 75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals on any drug therapy cannot be ruled out. Ciprofloxacin is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. No alteration of dosage is necessary for patients greater than 65 years of age with normal renal function. However, since some older individuals experience reduced renal function by virtue of their advanced age, care should be taken in dose selection for elderly patients, and renal function monitoring may be useful in these patients.
- In general, elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using ciprofloxacin with concomitant drugs that can result in prolongation of the QT interval (for example, class IA or class III antiarrhythmics) or in patients with risk factors for torsade de pointes (for example, known QT prolongation, uncorrected hypokalemia).
Gender
There is no FDA guidance on the use of Ciprofloxacin (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ciprofloxacin (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ciprofloxacin (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ciprofloxacin (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ciprofloxacin (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ciprofloxacin (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Preparation of Ciprofloxacin Injection, USP for Administration Vials (Injection Concentrate)
THIS PREPARATION MUST BE DILUTED BEFORE USE
- The intravenous dose should be prepared by aseptically withdrawing the concentrate from the vial of Ciprofloxacin Injection, USP. This should be diluted with a suitable intravenous solution to a final concentration of l-2mg/mL.The resulting solution should be infused over a period of 60 minutes by direct infusion or through a Y-type intravenous infusion set which may already be in place.
- If the Y-type or "piggyback" method of administration is used, it is advisable to discontinue temporarily the administration of any other solutions during the infusion of Ciprofloxacin Injection, USP. If the concomitant use of Ciprofloxacin Injection, USP and another drug is necessary each drug should be given separately in accordance with the recommended dosage and route of administration for each drug.
Flexible Containers
- Ciprofloxacin Injection, USP is also available as a 0.2% premixed solution in 5% dextrose in flexible containers of 100 mL or 200 mL. The solutions in flexible containers do not need to be diluted and may be infused as described above.
Compatibility and Stability
- Ciprofloxacin injection 1% (10 mg/mL), when diluted with the following intravenous solutions to concentrations of 0.5 to 2.0 mg/mL, is stable for up to 14 days at refrigerated or room temperature storage.
- 0.9% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- Sterile Water for Injection
- 10% Dextrose for Injection
- 5% Dextrose and 0.225% Sodium Chloride for Injection
- 5% Dextrose and 0.45% Sodium Chloride for Injection
- Lactated Ringer’s for Injection
Monitoring
There is limited information regarding Monitoring of Ciprofloxacin (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ciprofloxacin (injection) in the drug label.
Overdosage
There is limited information regarding Ciprofloxacin (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- The bactericidal action of ciprofloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV (both Type II topoisomerases), which are required for bacterial DNA replication, transcription, repair, and recombination.
Structure
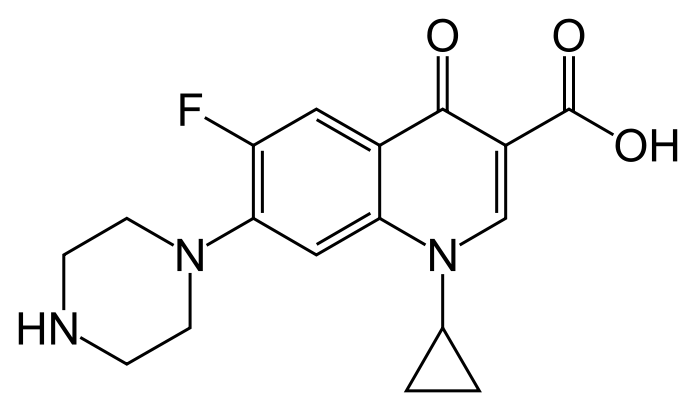
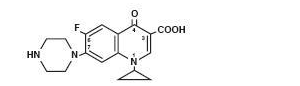
Ciprofloxacin Injection, USP (ciprofloxacin) is a synthetic broad-s pectrum antimicrobial agent for intravenous (I.V.) administration. Ciprofloxacin, a fluoroquinolone, is l-cyclopropyl-6-fluoro-l,4-dihydro-4-oxo-7-(l-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its chemical structure is:
- Ciprofloxacin is a faint to light yellow crystalline powder with a molecular weight of 331.4. It is soluble in dilute (0.1N) hydrochloric acid and is practically insoluble in water and ethanol. Ciprofloxacin Injection, USP solutions are available as sterile 1.0% aqueous concentrates, which are intended for dilution prior to administration, and as 0.2% ready-for-use infusion solutions in 5% Dextrose Injection. All formulas contain lactic acid as a solubilizing agent and hydrochloric acid for pH adjustment. The pH range for the 1.0% aqueous concentrates in vials is 3.3 to 3.9. The pH range for the 0.2% ready-for-use infusion solutions is 3.5 to 4.6.
- The Plastic container is latex-free and is fabricated from a specially formulated plastic. Ciprofloxacin Injection, USP is supplied in both PVC and PVC free containers. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di(2-ethylhexyl) phthalate (DEHP), up to 5 parts per million for polyvinyl chloride (PVC) containers. The suitability of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
- The glucose content for the 100 mL bag is 5 g and 10 g for the 200 mL flexible container.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ciprofloxacin (injection) in the drug label.
Pharmacokinetics
Absorption
- Following 60-minute intravenous infusions of 200 mg and 400 mg ciprofloxacin to normal volunteers, the mean maximum serum concentrations achieved were 2.1 and 4.6 mcg/mL, respectively; the concentrations at 12 hours were 0.1 and 0.2 mcg/mL, respectively.
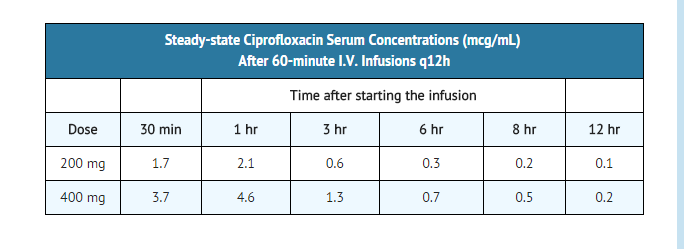
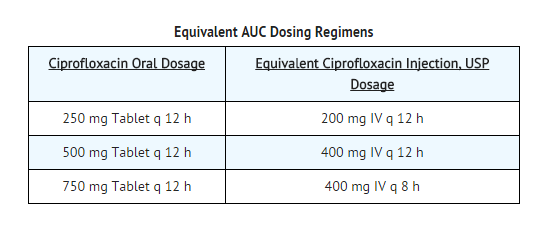
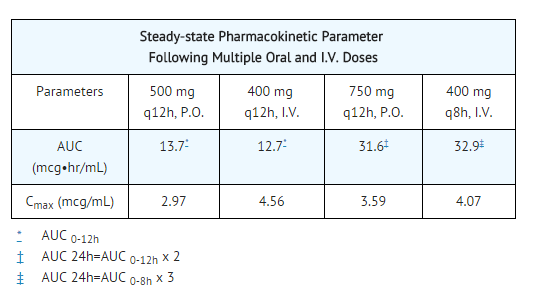
- The pharmacokinetics of ciprofloxacin are linear over the dose range of 200 to 400 mg administered intravenously. Comparison of the pharmacokinetic parameters following the 1st and 5th I.V. dose on a q 12 h regimen indicates no evidence of drug accumulation. The absolute bioavailability of oral ciprofloxacin is within a range of 70-80% with no substantial loss by first pass metabolism. An intravenous infusion of 400-mg ciprofloxacin given over 60 minutes every 12 hours has been shown to produce an area under the serum concentration time curve (AUC) equivalent to that produced by a 500-mg oral dose given every 12 hours. An intravenous infusion of 400 mg ciprofloxacin given over 60 minutes every 8 hours has been shown to produce an AUC at steady-state equivalent to that produced by a 750-mg oral dose given every 12 hours. A 400-mg I.V. dose results in a Cmax similar to that observed with a 750-mg oral dose. An infusion of 200 mg ciprofloxacin given every 12 hours produces an AUC equivalent to that produced by a 250-mg oral dose given every 12 hours.
Distribution
- After intravenous administration, ciprofloxacin is present in saliva, nasal and bronchial secretions, sputum, skin blister fluid, lymph, peritoneal fluid, bile, and prostatic secretions. It has also been detected in the lung, skin, fat, muscle, cartilage, and bone. Although the drug diffuses into cerebrospinal fluid (CSF), CSF concentrations are generally less than 10% of peak serum concentrations. Levels of the drug in the aqueous and vitreous chambers of the eye are lower than in serum.
Metabolism
- After I.V. administration, three metabolites of ciprofloxacin have been identified in human urine which together account for approximately 10% of the intravenous dose. The binding of ciprofloxacin to serum proteins is 20 to 40%. Ciprofloxacin is an inhibitor of human cytochrome P450 1A2 (CYP1A2) mediated metabolism. Coadministration of ciprofloxacin with other drugs primarily metabolized by CYP1A2 results in increased plasma concentrations of these drugs and could lead to clinically significant adverse events of the coadministered drug.
Excretion
- The serum elimination half-life is approximately 5-6 hours and the total clearance is around 35 L/hr. After intravenous administration, approximately 50% to 70% of the dose is excreted in the urine as unchanged drug. Following a 200-mg I.V. dose, concentrations in the urine usually exceed 200 mcg/mL 0-2 hours after dosing and are generally greater than 15 mcg/mL 8-12 hours after dosing. Following a 400-mg I.V. dose, urine concentrations generally exceed 400 mcg/mL 0-2 hours after dosing and are usually greater than 30 mcg/mL 8-12 hours after dosing. The renal clearance is approximately 22 L/hr. The urinary excretion of ciprofloxacin is virtually complete by 24 hours after dosing.
- Although bile concentrations of ciprofloxacin are several fold higher than serum concentrations after intravenous dosing, only a small amount of the administered dose (< 1%) is recovered from the bile as unchanged drug. Approximately 15% of an I.V. dose is recovered from the feces within 5 days after dosing.
Special Populations
- Pharmacokinetic studies of the oral (single dose) and intravenous (single and multiple dose) forms of ciprofloxacin indicate that plasma concentrations of ciprofloxacin are higher in elderly subjects (> 65 years) as compared to young adults. Although the Cmax is increased 16-40%, the increase in mean AUC is approximately 30%, and can be at least partially attributed to decreased renal clearance in the elderly. Elimination half-life is only slightly (~20%) prolonged in the elderly. These differences are not considered clinically significant.
Patient with Renal Impairment
- In patients with reduced renal function, the half-life of ciprofloxacin is slightly prolonged and dosage adjustments may be required.
Patient with Hepatic Impairment
- In preliminary studies in patients with stable chronic liver cirrhosis, no significant changes in ciprofloxacin pharmacokinetics have been observed. However, the kinetics of ciprofloxacin in patients with acute hepatic insufficiency have not been fully elucidated.
Pediatrics
- Following a single oral dose of 10 mg/kg ciprofloxacin suspension to 16 children ranging in age from 4 months to 7 years, the mean Cmax was 2.4 mcg/mL (range: 1.5 – 3.4 mcg /mL) and the mean AUC was 9.2 mcg *h/mL (range: 5.8 – 14.9 mcg *h/mL). There was no apparent age-dependence, and no notable increase in Cmax or AUC upon multiple dosing (10 mg/kg TID). In children with severe sepsis who were given intravenous ciprofloxacin (10 mg/kg as a 1-hour infusion), the mean Cmax was 6.1 mcg /mL (range: 4.6 – 8.3 mcg/mL) in 10 children less than 1 year of age; and 7.2 mcg /mL (range: 4.7 – 11.8 mcg/mL) in 10 children between 1 and 5 years of age. The AUC values were 17.4 mcg *h/mL (range: 11.8 – 32.0 mcg *h/mL) and 16.5 mcg *h/mL (range: 11.0 – 23.8 mcg *h/mL) in the respective age groups. These values are within the range reported for adults at therapeutic doses. Based on population pharmacokinetic analysis of pediatric patients with various infections, the predicted mean half-life in children is approximately 4-5 hours, and the bioavailability of the oral suspension is approximately 60%.
Drug-Drug Interactions
- Concomitant administration with tizanidine is contraindicated. The potential for pharmacokinetic drug interactions between ciprofloxacin and theophylline, caffeine, cyclosporins, phenytoin, sulfonylurea glyburide, metronidazole, warfarin, probenecid, and piperacillin sodium has been evaluated.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ciprofloxacin (injection) in the drug label.
Clinical Studies
Empirical Therapy In Adult Febrile Neutropenic Patients
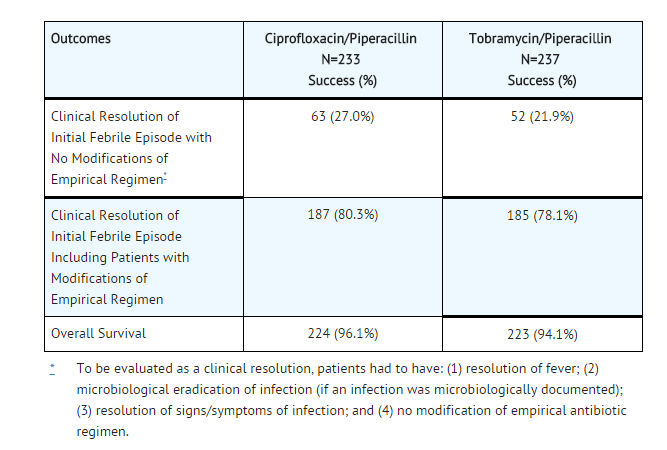
- The safety and efficacy of ciprofloxacin, 400 mg IV q 8h, in combination with piperacillin sodium, 50 mg/kg IV q 4h, for the empirical therapy of febrile neutropenic patients were studied in one large pivotal multicenter, randomized trial and were compared to those of tobramycin, 2 mg/kg IV q 8h, in combination with piperacillin sodium, 50 mg/kg IV q 4h. Clinical response rates observed in this study were as follows:
Complicated Urinary Tract Infection and Pyelonephritis - Efficacy in Pediatric Patients:
- NOTE: Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse events compared to controls, including events related to joints and/or surrounding tissues.
- Ciprofloxacin, administered I.V. and/or orally, was compared to a cephalosporin for treatment of complicated urinary tract infections (cUTI) and pyelonephritis in pediatric patients 1 to 17 years of age (mean age of 6 ± 4 years). The trial was conducted in the US, Canada, Argentina, Peru, Costa Rica, Mexico, South Africa, and Germany. The duration of therapy was 10 to 21 days (mean duration of treatment was 11 days with a range of 1 to 88 days). The primary objective of the study was to assess musculoskeletal and neurological safety.
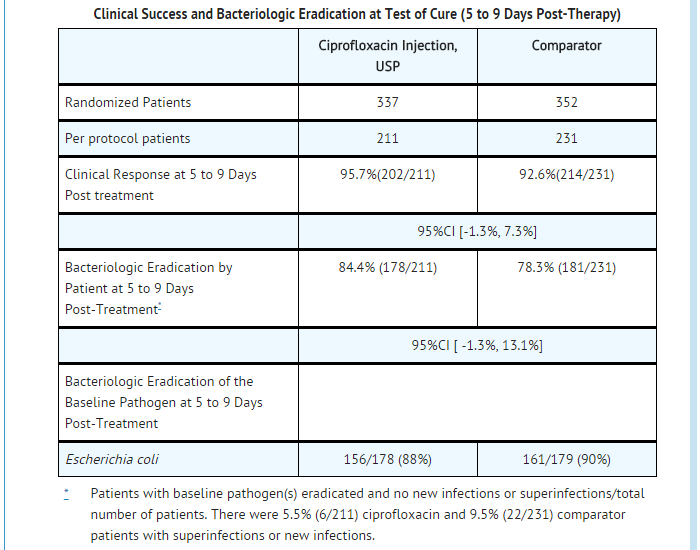
- Patients were evaluated for clinical success and bacteriological eradication of the baseline organism(s) with no new infection or superinfection at 5 to 9 days post-therapy (Test of Cure or TOC). The Per Protocol population had a causative organism(s) with protocol specified colony count(s) at baseline, no protocol violation, and no premature discontinuation or loss to follow-up (among other criteria).
- The clinical success and bacteriologic eradication rates in the Per Protocol population were similar between ciprofloxacin and the comparator group as shown below.
How Supplied
- Ciprofloxacin Injection, USP (ciprofloxacin) is available as a clear, colorless to slightly yellowish solution. Ciprofloxacin Injection, USP and Ciprofloxacin in Dextrose (5%) Injection,USP are available in 200 mg and 400 mg strengths. The concentrate is supplied in vials while the premixed solution is supplied in flexible containers as follows:
VIAL: manufactured for Claris Lifesciences Inc. by Claris Lifesciences Ltd., India.
SIZE STRENGTH NDC NUMBER
20 mL 200 mg, 1% 36000-010-01
40 mL 400 mg, 1% 36000-011-01
FLEXIBLE CONTAINER: manufactured for Claris Lifesciences Inc. by Claris Lifesciences Ltd., India.
SIZE STRENGTH NDC NUMBER
100 mL 5% Dextrose 200 mg, 0.2% 36000-008-24 – PVC Containers
100 mL 5% Dextrose 200 mg, 0.2% 36000-029-24 – PVC Free Containers
200 mL 5% Dextrose 400 mg, 0.2% 36000-009-24 – PVC Containers
200 mL 5% Dextrose 400 mg, 0.2% 36000-030-24 – PVC Free Containers
Storage
There is limited information regarding Ciprofloxacin (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ciprofloxacin (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

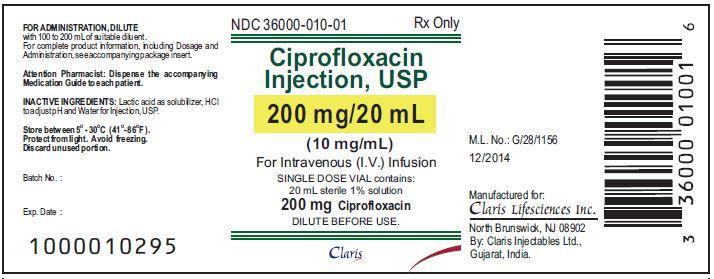




{{#ask: Label Page::Ciprofloxacin (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information For Patients:
Patients should be advised:
- To contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue Ciprofloxacin Injection, USP treatment. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- That fluoroquinolones like Ciprofloxacin Injection, USP may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Patients should call their healthcare provider right away if they have any worsening muscle weakness or breathing problems.
- That antibacterial drugs including Ciprofloxacin Injection, USP should only be used to treat bacterial infections. They do not treat viral infections (for example, the common cold). When Ciprofloxacin Injection, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Ciprofloxacin Injection, USP or other antibacterial drugs in the future.
- That ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
- That photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
- That ciprofloxacin may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
That ciprofloxacin increases the effects of tizanidine (Zanaflex®). Patients should not use ciprofloxacin if they are already taking tizanidine.
- That ciprofloxacin may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking ciprofloxacin.
- That peripheral neuropathies have been associated with ciprofloxacin use. If symptoms may occur soon after intimation of therapy and may be irreversible if symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, they should discontinue treatment and contact their physicians.
That ciprofloxacin has been associated with an increased rate of adverse events involving joints and surrounding tissue structures (like tendons) in pediatric patients (less than 18 years of age). Parents should inform their child’s physician if the child has a history of joint-related problems before taking this drug. Parents of pediatric patients should also notify their child’s physician of any joint-related problems that occur during or following ciprofloxacin therapy.
- That diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
- Alcohol-Ciprofloxacin (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CIPROFLOXACIN®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J, GL (September 1986). "Absolute oral bioavailability of ciprofloxacin". Antimicrob Agents Chemother. 30 (3): 444–6. doi:10.1128/aac.30.3.444. ISSN 0066-4804. PMC 180577. PMID 3777908.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help) - ↑ "ciprofloxacin injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)