Sorafenib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Alberto Plate [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sorafenib is a kinase inhibitor that is FDA approved for the treatment of unresectable hepatocellular carcinoma, advanced renal cell carcinoma, locally recurrent or metastatic, progressive, differentiated thyroid carcinoma refractory to radioactive iodine treatment. Common adverse reactions include hypertension, acral erythema, alopecia, Peeling of skin, rash, hypoalbuminemia, hypocalcemia, hypophosphatemia, raised TSH level, weight decreased, abdominal pain, decrease in appetite, diarrhea, Increased serum lipase level, Loss of appetite, nausea, serum amylase raised, lymphocytopenia, thrombocytopenia, ALT/SGPT level raised, Infectious diseases, fatigue and pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose for Hepatocellular Carcinoma, Renal Cell Carcinoma, and Differentiated Thyroid Carcinoma
- Dosage: 200 mg q12h, taken 1 hour before or 2 hours after a meal. Treatment should continue until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. Temporary interruption of Sorafenib is recommended in patients undergoing major surgical procedures.
- Temporary interruption or permanent discontinuation of Sorafenib may be required for the following:
- Cardiac ischemia or infarction
- Hemorrhage requiring medical intervention
- Severe or persistent hypertension despite adequate anti-hypertensive therapy
- Gastrointestinal perforation
- QTc prolongation
- Severe drug-induced liver injury
- Temporary interruption or permanent discontinuation of Sorafenib may be required for the following:
Dose modifications for Hepatocellular Carcinoma and Renal Cell Carcinoma
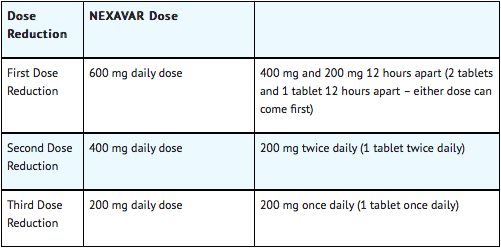
When dose reduction is necessary, the Sorafenib dose may be reduced to 400 mg once daily. If additional dose reduction is required, Sorafenib may be reduced to a single 400 mg dose every other day. If dermatological toxicity presented, the following scheme should be followed:
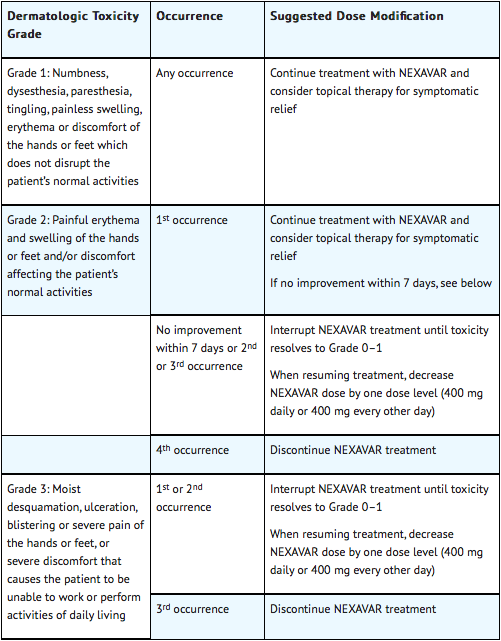
Dose Modifications for Differentiated Thyroid Carcinoma
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sorafenib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sorafenib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sorafenib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sorafenib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sorafenib in pediatric patients.
Contraindications
There is limited information regarding Sorafenib Contraindications in the drug label.
Warnings
There is limited information regarding Sorafenib Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Sorafenib Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Sorafenib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sorafenib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Sorafenib in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sorafenib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sorafenib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sorafenib in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Sorafenib in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Sorafenib in geriatric settings.
Gender
There is no FDA guidance on the use of Sorafenib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sorafenib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sorafenib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sorafenib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sorafenib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sorafenib in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sorafenib Administration in the drug label.
Monitoring
There is limited information regarding Sorafenib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sorafenib and IV administrations.
Overdosage
There is limited information regarding Sorafenib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sorafenib Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sorafenib Mechanism of Action in the drug label.
Structure
There is limited information regarding Sorafenib Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sorafenib Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sorafenib Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sorafenib Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sorafenib Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sorafenib How Supplied in the drug label.
Storage
There is limited information regarding Sorafenib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sorafenib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sorafenib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sorafenib Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sorafenib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sorafenib Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sorafenib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Clinical data | |
|---|---|
| Synonyms | Nexavar Sorafenib tosylate |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 29-49% |
| Protein binding | 99.5% |
| Metabolism | Hepatic oxidation and glucuronidation (CYP3A4-mediated) |
| Elimination half-life | 25–48 hours |
| Excretion | Fecal (77%) and renal (19%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H16ClF3N4O3 |
| Molar mass | 464.825 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Sorafenib |
|
Articles |
|---|
|
Most recent articles on Sorafenib |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sorafenib at Clinical Trials.gov Clinical Trials on Sorafenib at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sorafenib
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sorafenib Discussion groups on Sorafenib Directions to Hospitals Treating Sorafenib Risk calculators and risk factors for Sorafenib
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sorafenib |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Sorafenib (rINN), marketed as Nexavar by Bayer, is a drug approved for the treatment of advanced renal cell carcinoma (primary kidney cancer). It has also received "Fast Track" designation by the FDA[1] for the treatment of advanced hepatocellular carcinoma (primary liver cancer), and has since performed well in Phase III trials[2].
Pharmacology
It is a small molecular inhibitor of Raf kinase, PDGF (platelet-derived growth factor), VEGF receptor 2 & 3 kinases and c Kit the receptor for Stem cell factor. A growing number of drugs target most of these pathways. The originality of Sorafenib lays in its simultaneous targeting of the Raf/Mek/Erk pathway.[3]
Approval
Sorafenib was approved by the U.S. Food and Drug Administration (FDA) on December 20, 2005 and received a E.U. marketing authorisation on July 19, 2006[4].
The European Commission has granted marketing authorization to Nexavar® (sorafenib) tablets for the treatment of patients with hepatocellular carcinoma (HCC), the most common form of liver cancer, on October 30, 2007. [5].
Studies
A New England Journal of Medicine article published in January 2007 showed compared with placebo, treatment with sorafenib prolongs progression-free survival in patients with advanced clear-cell renal-cell carcinoma in whom previous therapy has failed; the median progression-free survival was 5.5 months in the sorafenib group and 2.8 months in the placebo group (hazard ratio for disease progression in the sorafenib group, 0.44; 95% confidence interval [CI], 0.35 to 0.55; P<0.01). The first interim analysis of overall survival in May 2005 showed that sorafenib reduced the risk of death, as compared with placebo (hazard ratio, 0.72; 95% CI, 0.54 to 0.94; P=0.02), although this benefit was not statistically significant according to the O'Brien–Fleming threshold. Partial responses were reported as the best response in 10% of patients receiving sorafenib and in 2% of those receiving placebo (P<0.001).
At ASCO 2007, results from the SHARP trial were presented, which showed efficacy of sorafenib in hepatocellular carcinoma. The primary endpoint was overall survival, which showed a 44% improvement in patients who received sorafenib compared to placebo (hazard ratio 0.69; 95% CI, 0.55 to 0.87; p=0.0001). Both median survival and time to progression showed 3-month improvements.
Regulatory filing is planned.
Side effects
Side effects of sorafenib included skin rash, hand-foot skin reactions, diarrhea, and hypertension.
Footnotes
- ↑ https://lnn605.aus.us.siteprotect.com/medicalnews.php?newsid=45119
- ↑ http://www.healthtech.com/news/top_news/2007/June/Nexavar_Extends_Survival.asp
- ↑ "SorafenibSunitinibdifferences". Retrieved 2007-08-15.
- ↑ European Commission - Enterprise and industry. Nexavar. Retrieved April 24,2007.
- ↑ DGnews [1]. Retrieved November 02, 2007.
External links
- Nexavar.com – Manufacturer's website
- Prescribing Information – includes data from the key studies justifying the use of sorafenib for the treatment of kidney cancer (particularly clear cell renal cell carcinoma, which is associated with the von Hippel-Lindau gene)
- Patient Information from FDA
- Sorafenib in Treating Patients With Soft Tissue Sarcomas
- Clinical trial number NCT00217399 at ClinicalTrials.gov - Sorafenib and Anastrozole in Treating Postmenopausal Women With Metastatic Breast Cancer
- [http:/mpablog.typepad.com/david_foster] Two year experience blog on nexavar, sutent, side effects, etc.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Cancer treatments
- Orphan drugs