Pemetrexed
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pemetrexed is a antineoplastic agent that is FDA approved for the treatment of non-squamous non-small cell lung cancer, mesothelioma. Common adverse reactions include itching, peeling of skin, constipation, diarrhea, loss of appetite, nausea, pharyngitis, stomatitis, vomiting, anemia, leukopenia, neutropenia, thrombocytopenia, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Combination Use with Cisplatin for Nonsquamous Non-Small Cell Lung Cancer or Malignant Pleural Mesothelioma
The recommended dose of pemetrexed is 500 mg/m2 administered as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle. The recommended dose of cisplatin is 75 mg/m2 infused over 2 hours beginning approximately 30 minutes after the end of pemetrexed administration. See cisplatin package insert for more information.
Single-Agent Use as Maintenance Following First-Line Therapy, or as a Second-Line Therapy
The recommended dose of pemetrexed is 500 mg/m2 administered as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle.
Premedication Regimen and Concurrent Medications
Vitamin Supplementation
- Instruct patients to initiate folic acid 400 mcg to 1000 mcg orally once daily beginning 7 days before the first dose of pemetrexed. Continue folic acid during the full course of therapy and for 21 days after the last dose of pemetrexed.
- Administer vitamin B12 1 mg intramuscularly 1 week prior to the first dose of pemetrexed and every 3 cycles thereafter. Subsequent vitamin B12 injections may be given the same day as treatment with pemetrexed.
Corticosteroids
Administer dexamethasone 4 mg by mouth twice daily the day before, the day of, and the day after pemetrexed administration.
Laboratory Monitoring and Dose Reduction/Discontinuation Recommendations
Monitoring
Complete blood cell counts, including platelet counts, should be performed on all patients receiving pemetrexed. Patients should be monitored for nadir and recovery, which were tested in the clinical study before each dose and on days 8 and 15 of each cycle. Patients should not begin a new cycle of treatment unless the ANC is ≥1500 cells/mm3, the platelet count is ≥100,000 cells/mm3, and creatinine clearance is ≥45 mL/min. Periodic chemistry tests should be performed to evaluate renal and hepatic function.
Dose Reduction Recommendations
Dose adjustments at the start of a subsequent cycle should be based on nadir hematologic counts or maximum nonhematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery, patients should be retreated using the guidelines in Tables 1-3, which are suitable for using pemetrexed as a single-agent or in combination with cisplatin.

If patients develop nonhematologic toxicities (excluding neurotoxicity) ≥Grade 3, treatment should be withheld until resolution to less than or equal to the patient's pre-therapy value. Treatment should be resumed according to guidelines in Table 2.

In the event of neurotoxicity, the recommended dose adjustments for pemetrexed and cisplatin are described in Table 3. Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is experienced.

Discontinuation Recommendation
ALIMTA therapy should be discontinued if a patient experiences any hematologic or nonhematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed.
Renally Impaired Patients
In clinical studies, patients with creatinine clearance ≥45 mL/min required no dose adjustments other than those recommended for all patients. Insufficient numbers of patients with creatinine clearance below 45 mL/min have been treated to make dosage recommendations for this group of patients. Therefore, pemetrexed should not be administered to patients whose creatinine clearance is <45 mL/min using the standard Cockcroft and Gault formula (below) or GFR measured by Tc99m-DTPA serum clearance method:

Caution should be exercised when administering pemetrexed concurrently with NSAIDs to patients whose creatinine clearance is <80 mL/min [see Drug Interactions (7.1)].
Preparation and Administration Precautions
As with other potentially toxic anticancer agents, care should be exercised in the handling and preparation of infusion solutions of pemetrexed. The use of gloves is recommended. If a solution of pemetrexed contacts the skin, wash the skin immediately and thoroughly with soap and water. If pemetrexed contacts the mucous membranes, flush thoroughly with water. Several published guidelines for handling and disposal of anticancer agents are available.
pemetrexed is not a vesicant. There is no specific antidote for extravasation of pemetrexed. To date, there have been few reported cases of pemetrexed extravasation, which were not assessed as serious by the investigator. Pemetrexed extravasation should be managed with local standard practice for extravasation as with other non-vesicants.
Preparation for Intravenous Infusion Administration
- Use aseptic technique during the reconstitution and further dilution of pemetrexed for intravenous infusion administration.
- Calculate the dose of pemetrexed and determine the number of vials needed. Vials contain either 100 mg or 500 mg of pemetrexed. The vials contain an excess of pemetrexed to facilitate delivery of label amount.
- Reconstitute each 100-mg vial with 4.2 ml of 0.9% Sodium Chloride Injection (preservative free). Reconstitute each 500-mg vial with 20 mL of 0.9% Sodium Chloride Injection (preservative free). Reconstitution of either size vial gives a solution containing 25 mg/mL pemetrexed. Gently swirl each vial until the powder is completely dissolved. The resulting solution is clear and ranges in color from colorless to yellow or green-yellow without adversely affecting product quality. The pH of the reconstituted pemetrexed solution is between 6.6 and 7.8. FURTHER DILUTION IS REQUIRED.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter is observed, do not administer.
- An appropriate quantity of the reconstituted pemetrexed solution must be further diluted into a solution of 0.9% Sodium Chloride Injection (preservative free), so that the total volume of solution is 100 ml. pemetrexed is administered as an intravenous infusion over 10 minutes.
- Chemical and physical stability of reconstituted and infusion solutions of pemetrexed were demonstrated for up to 24 hours following initial reconstitution, when stored refrigerated. When prepared as directed, reconstitution and infusion solutions of pemetrexed contain no antimicrobial preservatives. Discard any unused portion.
Reconstitution and further dilution prior to intravenous infusion is only recommended with 0.9% Sodium Chloride Injection (preservative free). pemetrexed is physically incompatible with diluents containing calcium, including Lactated Ringer's Injection, USP and Ringer's Injection, USP and therefore these should not be used. Coadministration of pemetrexed with other drugs and diluents has not been studied, and therefore is not recommended. Pemetrexed is compatible with standard polyvinyl chloride (PVC) administration sets and intravenous solution bags.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pemetrexed in adult patients.
Non–Guideline-Supported Use
Pleural Mesothelioma
- Dosing Information
Recurrent Ovarian Cancer
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pemetrexed FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pemetrexed in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pemetrexed in pediatric patients.
Contraindications
Pemetrexed is contraindicated in patients who have a history of severe hypersensitivity reaction to pemetrexed.
Warnings
Requirement for Premedication and Concomitant Medication to Reduce Toxicity
Vitamin Supplementation
Prior to treatment with pemetrxed, initiate supplementation with oral folic acid and intramuscular vitamin B12 to reduce the severity of hematologic and gastrointestinal toxicity of pemetrxed. Do not substitute oral vitamin B12 for intramuscular vitamin B12. In clinical studies, the incidence of the following Grade 3-4 toxicities were higher in patients with mesothelioma who were never supplemented as compared to patients who were fully supplemented with folic acid and vitamin B12 prior to and throughout pemetrxed treatment: neutropenia [38% versus 23%], thrombocytopenia [9% versus 5%], febrile neutropenia [9% versus 0.6%], and infection with neutropenia [6% versus. 0].
Corticosteroids
Administer dexamethasone the day before, the day of, and the day after pemetrxed administration.
Bone Marrow Suppression
Pemetrxed can suppress bone marrow function, as manifested by neutropenia, thrombocytopenia, and anemia (or pancytopenia); myelosuppression is usually the dose-limiting toxicity. Dose reductions for subsequent cycles are based on nadir ANC, platelet count, and maximum nonhematologic toxicity seen in the previous cycle.
Decreased Renal Function
Pemetrxed is primarily eliminated unchanged by renal excretion. No dosage adjustment is needed in patients with creatinine clearance ≥45 mL/min. Insufficient numbers of patients have been studied with creatinine clearance <45 mL/min to give a dose recommendation. Therefore, pemetrxed should not be administered to patients whose creatinine clearance is < 45 mL/min.
One patient with severe renal impairment (creatinine clearance 19 mL/min) who did not receive folic acid and vitamin B12 died of drug-related toxicity following administration of pemetrxed alone.
Use with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) with Mild to Moderate Renal Insufficiency
Caution should be used when administering NSAIDs concurrently with pemetrxed to patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 mL/min).
Required Laboratory Monitoring
Obtain a complete blood count and renal function tests at the beginning of each cycle and as needed. Do not initiate a cycle of treatment unless the ANC is ≥1500 cells/mm3, the platelet count is ≥100,000 cells/mm3, and creatinine clearance is ≥45 mL/min.
Pregnancy Category D
Based on its mechanism of action, pemetrxed can cause fetal harm when administered to a pregnant woman. Pemetrexed administered intraperitoneally to mice during organogenesis was embryotoxic, fetotoxic and teratogenic in mice at greater than 1/833rd the recommended human dose. If pemetrxed is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant. Women should be advised to use effective contraceptive measures to prevent pregnancy during treatment with pemetrxed.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
In clinical trials, the most common adverse reactions (incidence ≥20%) during therapy with pemetrexed as a single-agent were fatigue, nausea, and anorexia. Additional common adverse reactions (incidence ≥20%) during therapy with pemetrexed when used in combination with cisplatin included vomiting, neutropenia, leukopenia, anemia, stomatitis/pharyngitis, thrombocytopenia, and constipation.
Non-Small Cell Lung Cancer (NSCLC) – Pemetrexed in Combination with Cisplatin
- Table 4 provides the frequency and severity of adverse reactions that have been reported in > 5% of 839 patients with NSCLC who were randomized to study and received pemetrexed plus cisplatin and 830 patients with NSCLC who were randomized to study and received gemcitabine plus cisplatin. All patients received study therapy as initial treatment for locally advanced or metastatic NSCLC and patients in both treatment groups were fully supplemented with folic acid and vitamin B12.

- No clinically relevant differences in adverse reactions were seen in patients based on histology.
- In addition to the lower incidence of hematologic toxicity on the pemetrexed and cisplatin arm, use of transfusions (RBC and platelet) and hematopoietic growth factors was lower in the pemetrexed and cisplatin arm compared to the gemcitabine and cisplatin arm.
The following additional adverse reactions were observed in patients with non-small cell lung cancer randomly assigned to receive pemetrexed plus cisplatin
Incidence 1% to 5%
- Body as a Whole: febrile neutropenia, infection, pyrexia
- General Disorders: dehydration
- Metabolism and Nutrition: increased AST, increased ALT
- Renal: creatinine clearance decrease, renal failure
- Special Senses: conjunctivitis
Incidence Less than 1%
- Cardiovascular: arrhythmia
- General Disorders: chest pain
- Metabolism and Nutrition: increased GGT
- Neurology: motor neuropathy
Non-Small Cell Lung Cancer (NSCLC) – Maintenance
Pemetrexed Maintenance Following Non-Pemetrexed Containing, Platinum-Based Induction Therapy
- Table 5 provides the frequency and severity of adverse reactions reported in >5% of the 438 patients with NSCLC who received pemetrexed maintenance and the 218 patients with NSCLC who received placebo following a platinum-based induction therapy.
- All patients received study therapy immediately following 4 cycles of platinum-based treatment for locally advanced or metastatic NSCLC. Patients in both study arms were fully supplemented with folic acid and vitamin B12.

- No clinically relevant differences in Grade 3/4 adverse reactions were seen in patients based on age, gender, ethnic origin, or histology except a higher incidence of Grade 3/4 fatigue for Caucasian patients compared to non-Caucasian patients (6.5% versus 0.6%).
- Safety was assessed by exposure for patients who received at least one dose of pemetrexed (N=438). The incidence of adverse reactions was evaluated for patients who received ≤6 cycles of pemetrexed, and compared to patients who received >6 cycles of pemetrexed. Increases in adverse reactions (all grades) were observed with longer exposure; however no clinically relevant differences in Grade 3/4 adverse reactions were seen.
- Consistent with the higher incidence of anemia (all grades) on the pemetrexed arm, use of transfusions (mainly RBC) and erythropoiesis stimulating agents (ESAs; erythropoietin and darbepoetin) were higher in the pemetrexed arm compared to the placebo arm (transfusions 9.5% versus 3.2%, ESAs 5.9% versus 1.8%).
The following additional adverse reactions were observed in patients with non-small cell lung cancer who received pemetrexed
Incidence 1% to 5%
- Dermatology/Skin: alopecia, pruritis/itching
- Gastrointestinal: constipation
- General Disorders: edema, fever (in the absence of neutropenia)
- Hematologic: thrombocytopenia
- Renal: decreased creatinine clearance, increased creatinine, decreased glomerular filtration rate
- Special Senses: ocular surface disease (including conjunctivitis), increased lacrimation
Incidence Less than 1%
- Cardiovascular: supraventricular arrhythmia
- Dermatology/Skin: erythema multiforme
- General Disorders: febrile neutropenia, allergic reaction/hypersensitivity
- Neurology: motor neuropathy
- Renal: renal failure
Continuation of Pemetrexed as Maintenance Following Pemetrexed Plus Platinum Induction Therapy
- Table 6 provides the frequency and severity of adverse reactions reported in >5% of the 500 patients with non-squamous NSCLC who received at least one cycle of pemetrexed maintenance (n=333) or placebo (n=167) on the continuation maintenance trial.
- The median of maintenance cycles administered to patients receiving one or more doses of maintenance therapy was 4 on both the pemetrexed and placebo arms. Dose reductions for adverse events occurred in 3.3% of patients in the pemetrexed arm and 0.6% in the placebo arm. Dose delays for adverse events occurred in 22% of patients in the pemetrexed arm and 16% in the placebo arm. Patients in both study arms were supplemented with folic acid and vitamin B12.

- Administration of RBC (13% versus 4.8%) and platelet (1.5% versus 0.6%) transfusions, erythropoiesis stimulating agents (12% versus 7%), and granulocyte colony stimulating factors (6% versus 0) were higher in the pemetrexed arm compared to the placebo arm.
The following additional Grade 3 or 4 adverse reactions were observed more frequently in the pemetrexed arm
Incidence 1% to 5%
- Blood/Bone Marrow: thrombocytopenia
- General Disorders: febrile neutropenia
Incidence Less than 1%
- Cardiovascular: ventricular tachycardia, syncope
- General Disorders: pain
- Gastrointestinal: gastrointestinal obstruction
- Neurologic: depression
- Renal: renal failure
- Vascular: pulmonary embolism
Non-Small Cell Lung Cancer (NSCLC) – After Prior Chemotherapy
- Table 7 provides the frequency and severity of adverse reactions that have been reported in >5% of 265 patients randomly assigned to receive single-agent pemetrexed with folic acid and vitamin B12 supplementation and 276 patients randomly assigned to receive single-agent docetaxel. All patients were diagnosed with locally advanced or metastatic NSCLC and received prior chemotherapy.

- No clinically relevant differences in adverse reactions were seen in patients based on histology.
- Clinically relevant adverse reactions occurring in <5% of patients that received pemetrexed treatment but >5% of patients that received docetaxel include CTC Grade 3/4 febrile neutropenia (1.9% pemetrexed, 12.7% docetaxel).
The following additional adverse reactions were observed in patients with non-small cell lung cancer randomly assigned to receive pemetrexed
Incidence 1% to 5%
- Body as a Whole: abdominal pain, allergic reaction/hypersensitivity, febrile neutropenia, infection
- Dermatology/Skin: erythema multiforme
- Neurology: motor neuropathy, sensory neuropathy
- Renal: increased creatinine
Incidence Less than 1%
- Cardiovascular: supraventricular arrhythmias
Malignant Pleural Mesothelioma (MPM)
- Table 8 provides the frequency and severity of adverse reactions that have been reported in >5% of 168 patients with mesothelioma who were randomly assigned to receive cisplatin and pemetrexed and 163 patients with mesothelioma randomly assigned to receive single-agent cisplatin. In both treatment arms, these chemonaive patients were fully supplemented with folic acid and vitamin B12.
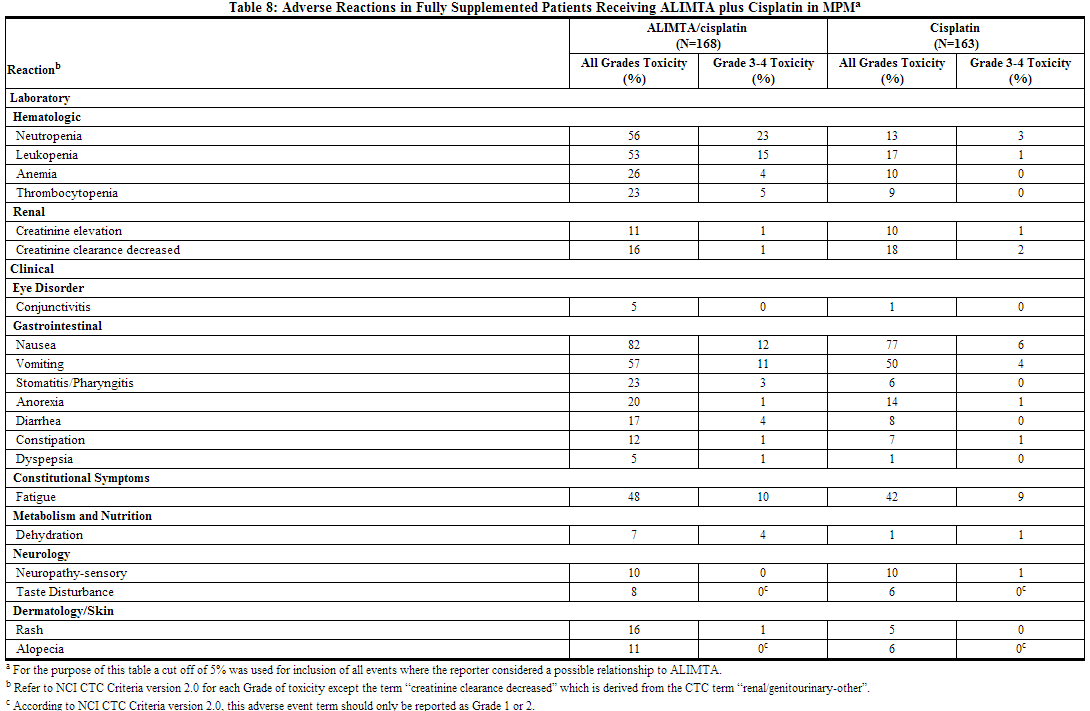
The following additional adverse reactions were observed in patients with malignant pleural mesothelioma randomly assigned to receive pemetrexed plus cisplatin. Incidence 1% to 5% Body as a Whole: febrile neutropenia, infection, pyrexia Dermatology/Skin: urticaria General Disorders: chest pain Metabolism and Nutrition: increased AST, increased ALT, increased GGT Renal: renal failure
Incidence Less than 1% Cardiovascular: arrhythmia Neurology: motor neuropathy
Effects of Vitamin Supplementations on Toxicity
- Table 9 compares the incidence (percentage of patients) of CTC Grade 3/4 toxicities in patients who received vitamin supplementation with daily folic acid and vitamin B12 from the time of enrollment in the study (fully supplemented) with the incidence in patients who never received vitamin supplementation (never supplemented) during the study in the pemetrexed plus cisplatin arm.

- The following adverse events were greater in the fully supplemented group compared to the never supplemented group: hypertension (11%, 3%), chest pain (8%, 6%), and thrombosis/embolism (6%, 3%).
- No relevant effect for pemetrexed safety due to gender or race was identified, except an increased incidence of rash in men (24%) compared to women (16%).
Additional Experience Across Clinical Trials
- Sepsis, which in some cases was fatal, occurred in approximately 1% of patients.
- Esophagitis occurred in less than 1% of patients.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pemetrexed. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions occurred with pemetrexed when used as a single-agent and in combination therapies:
- Blood and Lymphatic System: immune-mediated hemolytic anemia
- Gastrointestinal: colitis, pancreatitis
- General Disorders and Administration Site Conditions: edema
- Injury, poisoning, and procedural complications: Radiation recall has been reported in patients who have previously received radiotherapy.
- Respiratory: interstitial pneumonitis
- Skin: Bullous conditions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Some cases were fatal.
Drug Interactions
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Although ibuprofen (400 mg four times a day) can decrease the clearance of pemetrexed, it can be administered with pemetrexed in patients with normal renal function (creatinine clearance ≥80 mL/min). No dose adjustment of pemetrexed is needed with concomitant NSAIDs in patients with normal renal function.
- Caution should be used when administering NSAIDs concurrently with pemetrexed to patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 mL/min). NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of 2 days before, the day of, and 2 days following administration of pemetrexed.
- In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least 5 days before, the day of, and 2 days following pemetrexed administration. If concomitant administration of NSAIDs is necessary, patients should be monitored closely for toxicity, especially myelosuppression, renal, and gastrointestinal toxicity.
Nephrotoxic Drugs
- Pemetrexed is primarily eliminated unchanged renally as a result of glomerular filtration and tubular secretion. Concomitant administration of nephrotoxic drugs could result in delayed clearance of pemetrexed. Concomitant administration of substances that are also tubularly secreted (e.g., probenecid) could potentially result in delayed clearance of pemetrexed.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Based on its mechanism of action, pemetrexed can cause fetal harm when administered to a pregnant woman. There are no adequate and well controlled studies of pemetrexed in pregnant women. Pemetrexed was embryotoxic, fetotoxic, and teratogenic in mice. In mice, repeated intraperitoneal doses of pemetrexed when given during organogenesis caused fetal malformations (incomplete ossification of talus and skull bone; about 1/833rd the recommended intravenous human dose on a mg/m2 basis), and cleft palate (1/33rd the recommended intravenous human dose on a mg/m2 basis). Embryotoxicity was characterized by increased embryo-fetal deaths and reduced litter sizes. If pemetrexed is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to use effective contraceptive measures to prevent pregnancy during the treatment with pemetrexed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pemetrexed in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pemetrexed during labor and delivery.
Nursing Mothers
It is not known whether pemetrexed or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from pemetrexed, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother.
Pediatric Use
Efficacy of pemetrexed in pediatric patients has not been demonstrated. Pemetrexed was administered as an intravenous infusion over 10 minutes on Day 1 of a 21 day cycle to pediatric patients with recurrent solid tumors in a Phase 1 study (32 patients) and a Phase 2 study (72 patients). All patients received pretreatment with vitamin B12 and folic acid supplementation and dexamethasone. The dose escalation in the Phase 1 study determined the maximum tolerated dose was 1910 mg/m2 and this dose (or 60 mg/kg for patients <12 months old) was evaluated in the Phase 2 study of patients with relapsed or refractory osteosarcoma, Ewing sarcoma/peripheral PNET, rhabdomyosarcoma, neuroblastoma, ependymoma, medulloblastoma/supratentorial PNET, or non-brainstem high grade glioma. No responses were observed among the 72 patients in this Phase 2 trial. The most common toxicities reported were hematological (leukopenia, neutropenia/granulocytopenia, anemia, thrombocytopenia, and lymphopenia), liver function abnormalities (increased ALT/AST), fatigue, and nausea.
The single dose pharmacokinetics of pemetrexed administered in doses ranging from 400 to 2480 mg/m2 were evaluated in the Phase 1 trial in 22 patients (13 males and 9 females) aged 4 to 18 years (average age 12 years). Pemetrexed exposure (AUC and Cmax) appeared to increase proportionally with dose. The average pemetrexed clearance (2.30 L/h/m2) and half-life (2.3 hours) in pediatric patients were comparable to values reported in adults.
Geriatic Use
Pemetrexed is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Renal function monitoring is recommended with administration of pemetrexed. No dose reductions other than those recommended for all patients are necessary for patients 65 years of age or older . Of 3,946 patients (34.0% ≥65) studied across the five clinical trials, the effect of pemetrexed on survival was similar in patients <65 compared to ≥65 years of age. There were no differences in safety with the exception of the following Grade 3-4 adverse reactions, which were noted in at least one of the five trials to be greater in patients 65 years of age and older as compared to younger patients: anemia, fatigue, thrombocytopenia, hypertension, and neutropenia.
Gender
Of 3,946 patients (Male 70.5%) studied across the five registration studies for pemetrexed indications, the effect of pemetrexed on survival was similar in female and male patients.
Race
Of 3,946 patients (Caucasian 78.6%) studied across the five registration studies for pemetrexed indications, the effect of pemetrexed on survival was similar in the Caucasian and non-Caucasian patients.
Renal Impairment
Pemetrexed is known to be primarily excreted by the kidneys. Decreased renal function will result in reduced clearance and greater exposure (AUC) to pemetrexed compared with patients with normal renal function. Cisplatin coadministration with pemetrexed has not been studied in patients with moderate renal impairment.
Hepatic Impairment
There was no effect of elevated AST, ALT, or total bilirubin on the pharmacokinetics of pemetrexed. However, no formal studies have been conducted to examine the pharmacokinetics of pemetrexed in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pemetrexed in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pemetrexed in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
Complete blood cell counts, including platelet counts, should be performed on all patients receiving pemetrexed. Patients should be monitored for nadir and recovery, which were tested in the clinical study before each dose and on days 8 and 15 of each cycle. Patients should not begin a new cycle of treatment unless the ANC is ≥1500 cells/mm3, the platelet count is ≥100,000 cells/mm3, and creatinine clearance is ≥45 mL/min. Periodic chemistry tests should be performed to evaluate renal and hepatic function.
IV Compatibility
Reconstitution and further dilution prior to intravenous infusion is only recommended with 0.9% Sodium Chloride Injection (preservative free). Pemetrexed is physically incompatible with diluents containing calcium, including Lactated Ringer's Injection, USP and Ringer's Injection, USP and therefore these should not be used. Coadministration of pemetrexed with other drugs and diluents has not been studied, and therefore is not recommended. Pemetrexed is compatible with standard polyvinyl chloride (PVC) administration sets and intravenous solution bags.
Overdosage
- There have been few cases of pemetrexed overdose. Reported toxicities included neutropenia, anemia, thrombocytopenia, mucositis, and rash. Anticipated complications of overdose include bone marrow suppression as manifested by neutropenia, thrombocytopenia, and anemia. In addition, infection with or without fever, diarrhea, and mucositis may be seen. If an overdose occurs, general supportive measures should be instituted as deemed necessary by the treating physician.
- In clinical trials, leucovorin was permitted for CTC Grade 4 leukopenia lasting ≥3 days, CTC Grade 4 neutropenia lasting ≥3 days, and immediately for CTC Grade 4 thrombocytopenia, bleeding associated with Grade 3 thrombocytopenia, or Grade 3 or 4 mucositis. The following intravenous doses and schedules of leucovorin were recommended for intravenous use: 100 mg/m2, intravenously once, followed by leucovorin, 50 mg/m2, intravenously every 6 hours for 8 days.
- The ability of pemetrexed to be dialyzed is unknown.
Pharmacology
| |
Pemetrexed
| |
| Systematic (IUPAC) name | |
| 2-[4-[2-(4-amino-2-oxo-3,5,7-triazabicyclo[4.3.0] nona-3,8,10-trien-9-yl)ethyl] benzoyl] aminopentanedioic acid | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 427.411 g/mol |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 81% |
| Metabolism | Negligible |
| Half life | 3.5 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
D(US) |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV |
Mechanism of Action
Pemetrexed for injection, is a folate analog metabolic inhibitor that exerts its action by disrupting folate-dependent metabolic processes essential for cell replication. In vitro studies have shown that pemetrexed inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), which are folate-dependent enzymes involved in the de novo biosynthesis of thymidine and purine nucleotides. Pemetrexed is taken into cells by membrane carriers such as the reduced folate carrier and membrane folate binding protein transport systems. Once in the cell, pemetrexed is converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase. The polyglutamate forms are retained in cells and are inhibitors of TS and GARFT. Polyglutamation is a time- and concentration-dependent process that occurs in tumor cells and, is thought to occur to a lesser extent, in normal tissues. Polyglutamated metabolites are thought to have an increased intracellular half-life resulting in prolonged drug action in malignant cells.
Structure
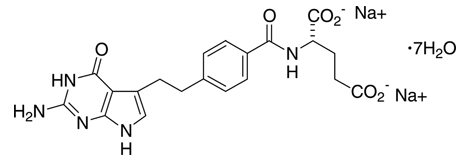
Pemetrexed disodium heptahydrate has the chemical name L-Glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-, disodium salt, heptahydrate. It is a white to almost-white solid with a molecular formula of C20H19N5Na2O6•7H2O and a molecular weight of 597.49. The structural formula is as follows:

Pemetrexed is supplied as a sterile lyophilized powder for intravenous infusion available in single-dose vials. The product is a white to either light yellow or green-yellow lyophilized solid. Each 100-mg or 500-mg vial of pemetrexed contains pemetrexed disodium equivalent to 100 mg pemetrexed and 106 mg mannitol or 500 mg pemetrexed and 500 mg mannitol, respectively. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH.
Pharmacodynamics
- Preclinical studies have shown that pemetrexed inhibits the in vitro growth of mesothelioma cell lines (MSTO-211H, NCI-H2052). Studies with the MSTO-211H mesothelioma cell line showed synergistic effects when pemetrexed was combined concurrently with cisplatin.
- Absolute neutrophil counts (ANC) following single-agent administration of pemetrexed to patients not receiving folic acid and vitamin B12 supplementation were characterized using population pharmacodynamic analyses. Severity of hematologic toxicity, as measured by the depth of the ANC nadir, correlates with the systemic exposure, or area under the curve (AUC) of pemetrexed. It was also observed that lower ANC nadirs occurred in patients with elevated baseline cystathionine or homocysteine concentrations. The levels of these substances can be reduced by folic acid and vitamin B12 supplementation. There is no cumulative effect of pemetrexed exposure on ANC nadir over multiple treatment cycles.
- Time to ANC nadir with pemetrexed systemic exposure (AUC), varied between 8 to 9.6 days over a range of exposures from 38.3 to 316.8 mcg•hr/mL. Return to baseline ANC occurred 4.2 to 7.5 days after the nadir over the same range of exposures.
Pharmacokinetics
Absorption
- The pharmacokinetics of pemetrexed administered as a single-agent in doses ranging from 0.2 to 838 mg/m2 infused over a 10-minute period have been evaluated in 426 cancer patients with a variety of solid tumors. Pemetrexed total systemic exposure (AUC) and maximum plasma concentration (Cmax) increase proportionally with dose. The pharmacokinetics of pemetrexed do not change over multiple treatment cycles.
Distribution
- Pemetrexed has a steady-state volume of distribution of 16.1 liters. In vitro studies indicate that pemetrexed is approximately 81% bound to plasma proteins. Binding is not affected by degree of renal impairment.
Metabolism and Excretion
- Pemetrexed is not metabolized to an appreciable extent and is primarily eliminated in the urine, with 70% to 90% of the dose recovered unchanged within the first 24 hours following administration. The clearance decreases, and exposure (AUC) increases, as renal function decreases. The total systemic clearance of pemetrexed is 91.8 mL/min and the elimination half-life of pemetrexed is 3.5 hours in patients with normal renal function (creatinine clearance of 90 mL/min).
- The pharmacokinetics of pemetrexed in special populations were examined in about 400 patients in controlled and single arm studies.
- In vitro studies indicate that pemetrexed is a substrate of OAT3 (organic anion transporter 3), a transporter that may play a role in active secretion of pemetrexed.
Effect of Age, Gender or Race
- No effect of age on the pharmacokinetics of pemetrexed was observed over a range of 26 to 80 years.
- The pharmacokinetics of pemetrexed were not different in male and female patients.
- The pharmacokinetics of pemetrexed were similar in Caucasians and patients of African descent. Insufficient data are available to compare pharmacokinetics for other ethnic groups.
Effect of Hepatic Insufficiency
- There was no effect of elevated AST, ALT, or total bilirubin on the pharmacokinetics of pemetrexed. However, studies of hepatically impaired patients have not been conducted.
Effect of Renal Insufficiency
- Pharmacokinetic analyses of pemetrexed included 127 patients with reduced renal function. Plasma clearance of pemetrexed decreases as renal function decreases, with a resultant increase in systemic exposure. Patients with creatinine clearances of 45, 50, and 80 mL/min had 65%, 54%, and 13% increases, respectively in pemetrexed total systemic exposure (AUC) compared to patients with creatinine clearance of 100 mL/min.
Effect of Third Space Fluid
- The effect of third space fluid, such as pleural effusion and ascites, on pemetrexed is not fully defined. A study of pemetrexed 500 mg/m2 was performed in 31 solid tumor patients with stable third space fluid (All but 2 of the 31 patients included in study had mild or moderate amounts of third space fluid). Moderate pleural effusion was defined in the study as less than 1/3 the way up on one side with obscuring of the entire hemidiaphragm. Moderate ascites was defined as that detectable on physical exam. The pemetrexed plasma concentrations in these patients were comparable to those observed in previous clinical trials in patients without third space fluid collections. Thus, drainage of mild or moderate third space fluid collection prior to pemetrexed treatment should be considered, but is probably not necessary. The effect of severe third space fluid on pharmacokinetics is not known.
Effect of Ibuprofen
- Ibuprofen doses of 400 mg four times a day reduce pemetrexed's clearance by about 20% (and increase AUC by 20%) in patients with normal renal function. The effect of greater doses of ibuprofen on pemetrexed pharmacokinetics is unknown.
Effect of Aspirin
- Aspirin, administered in low to moderate doses (325 mg every 6 hours), does not affect the pharmacokinetics of pemetrexed. The effect of greater doses of aspirin on pemetrexed pharmacokinetics is unknown.
Effect of Cisplatin
- Cisplatin does not affect the pharmacokinetics of pemetrexed and the pharmacokinetics of total platinum are unaltered by pemetrexed.
Effect of Vitamins
- Coadministration of oral folic acid or intramuscular vitamin B12 does not affect the pharmacokinetics of pemetrexed.
Drugs Metabolized by Cytochrome P450 Enzymes
- Results from in vitro studies with human liver microsomes predict that pemetrexed would not cause clinically significant inhibition of metabolic clearance of drugs metabolized by CYP3A, CYP2D6, CYP2C9, and CYP1A2.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with pemetrexed. Pemetrexed was clastogenic in the in vivo micronucleus assay in mouse bone marrow but was not mutagenic in multiple in vitro tests (Ames assay, CHO cell assay). Pemetrexed administered at i.v. doses of 0.1 mg/kg/day or greater to male mice (about 1/1666 the recommended human dose on a mg/m2 basis) resulted in reduced fertility, hypospermia, and testicular atrophy.
Clinical Studies
Non-Small Cell Lung Cancer (NSCLC) – Combination with Cisplatin
A multi-center, randomized, open-label study in 1725 chemonaive patients with Stage IIIb/IV NSCLC was conducted to compare the overall survival following treatment with pemetrexed in combination with cisplatin (AC) versus gemcitabine in combination with cisplatin (GC). Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 with cisplatin administered intravenously at a dose of 75 mg/m2 after pemetrexed administration, on Day 1 of each 21-day cycle. Gemcitabine was administered at a dose of 1250 mg/m2 on Day 1 and Day 8, and cisplatin was administered intravenously at a dose of 75 mg/m2 after administration of gemcitabine, on Day 1 of each 21-day cycle. Treatment was administered up to a total of 6 cycles, and patients in both treatment arms received folic acid, vitamin B12, and dexamethasone.
Patient demographics of the intent to treat (ITT) population are shown in Table 10. The demographics and disease characteristics were well balanced.

Patients received a median of 5 cycles of treatment in both study arms. Patients treated with pemetrexed plus cisplatin received a relative dose intensity of 94.8% of the protocol-specified pemetrexed dose intensity and 95.0% of the protocol-specified cisplatin dose intensity. Patients treated with gemcitabine plus cisplatin received a relative dose intensity of 85.8% of the protocol-specified gemcitabine dose intensity and 93.5% of the protocol-specified cisplatin dose intensity.
The primary endpoint in this study was overall survival. The median survival time was 10.3 months in the pemetrexed plus cisplatin treatment arm and 10.3 months in the gemcitabine plus cisplatin arm, with an adjusted hazard ratio of 0.94.


A pre-specified analysis of the impact of NSCLC histology on overall survival was examined. Clinically relevant differences in survival according to histology were observed and are shown in Table 12. This difference in treatment effect for pemetrexed based on histology demonstrating a lack of efficacy in squamous cell histology was also observed in the single-agent, second-line study and the maintenance study.


Non-Small Cell Lung Cancer – Maintenance
Pemetrexed Maintenance Following Non-Pemetrexed Containing Platinum-Based, Induction Therapy
A multi-center, randomized, double-blind, placebo-controlled study was conducted in 663 patients with Stage IIIb/IV NSCLC who did not progress after four cycles of platinum-based chemotherapy. Patients who did not progress were randomized 2:1 to receive pemetrexed or placebo immediately following platinum-based chemotherapy. Of the randomized patients, 47.2% versus 52.7% achieved a complete or partial response to induction therapy and 51.9% versus 47.3% had stable disease after induction therapy in the pemetrexed and placebo arms, respectively. Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 on Day 1 of each 21-day cycle, until disease progression. Patients in both study arms received folic acid, vitamin B12, and dexamethasone.
The study was designed to demonstrate superior progression-free survival and overall survival of pemetrexed over placebo. Progression-free survival (PFS) was assessed by independent review. Patient characteristics of the intent to treat (ITT) population are shown in Table 13. The demographics and baseline disease characteristics were well balanced between study arms.

Patients received a median of 5 cycles of pemetrexed and 3.5 cycles of placebo. Patients randomized to ALIMTA received a relative dose intensity of 95.7%. A total of 213 patients (48.3%) completed ≥6 cycles and a total of 98 patients (22.6%) completed ≥10 cycles of treatment with ALIMTA.
In the overall study population, pemetrexed was statistically superior to placebo in terms of overall survival (OS) (median 13.4 months versus 10.6 months, HR=0.79 (95% CI: 0.65-0.95), p-value=0.012) and PFS (median 4.0 months versus 2.0 months, HR=0.60 (95% CI: 0.49-0.73), p-value<0.00001). A difference in treatment outcomes was observed according to histologic classification. For the population of patients with nonsquamous NSCLC, pemetrexed was superior to placebo for OS (median 15.5 months versus 10.3 months, HR=0.70 (95% CI: 0.56-0.88)) and PFS (median 4.4 months versus 1.8 months, HR=0.47 (95% CI: 0.37-0.60)). For the population of patients with squamous NSCLC, pemetrexed did not improve OS compared to placebo (median 9.9 months versus 10.8 months, HR=1.07 (95% CI: 0.77-1.50)) or PFS (median 2.4 months versus 2.5 months, HR=1.03 (95% CI: 0.71-1.49)). This difference in treatment effect for pemetrexed based on histology demonstrating lack of benefit in squamous cell histology was also observed in the first-line and second-line studies.
Efficacy results for the overall patient population are presented in Table 14 and Figure 3, and efficacy results by pre-specified histologic subgroups are presented in Table 15 and Figure 4, below.




Continuation of Pemetrexed as Maintenance Following Pemetrexed Plus Platinum Induction Therapy
A multi-center, randomized, double-blind, placebo-controlled study was conducted to evaluate continuation of pemetrexed in patients with Stage IIIb/IV nonsquamous NSCLC. Patients completing induction treatment of four cycles of pemetrexed plus cisplatin with stable disease or better and PS 0/1 were randomized (2:1) to maintenance treatment with pemetrexed or placebo. Randomization was stratified by response to induction (complete response (CR)/partial response (PR) versus stable disease (SD)), disease stage (IIIb versus IV), and ECOG performance status (0 versus 1). Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 on Day 1 of each 21-day cycle and continued until disease progression. Patients in both study arms received folic acid, vitamin B12, and dexamethasone. The main efficacy outcome was investigator-assessed progression-free survival.
A total of 539 patients were randomized; all completed four cycles of pemetrexed and cisplatin induction prior to randomization. Of the randomized patients, 44% versus 42% achieved a complete or partial response to induction therapy and 53% versus 53% had stable disease after induction treatment in the pemetrexed or the placebo arms respectively.
Patient demographics of the intent to treat (ITT) population are shown in Table 16.

Patients received a median of four cycles of pemetrexed maintenance or placebo. The percentages of patients that received post-study treatment were similar (64% in the pemetrexed arm and 72% in the placebo arm).
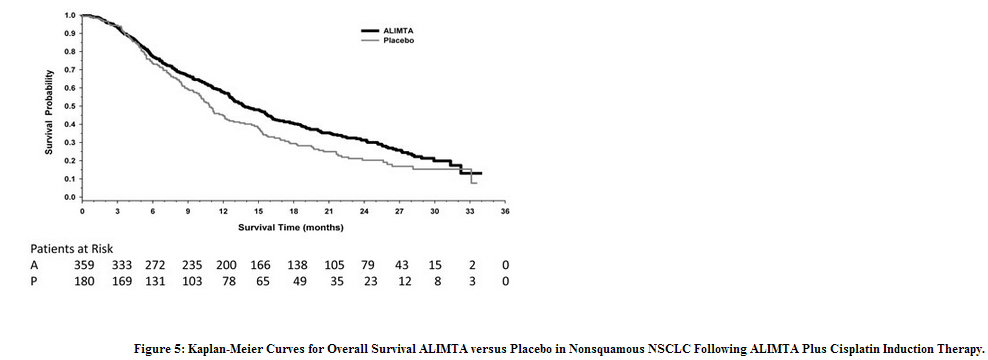
The trial showed a statistically significant improvement in progression-free survival and in overall survival for patients randomized to pemetrexed maintenance. Efficacy results are presented in Table 17 and Figure 5.

Non-Small Cell Lung Cancer – After Prior Chemotherapy
A multi-center, randomized, open label study was conducted in patients with Stage III or IV NSCLC after prior chemotherapy to compare the overall survival following treatment with pemetrexed versus docetaxel. Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 and docetaxel was administered at 75 mg/m2 as a 1-hour intravenous infusion. Both drugs were given on Day 1 of each 21-day cycle. All patients treated with pemetrexed received vitamin supplementation with folic acid and vitamin B12. The study was intended to show either an overall survival superiority or non-inferiority of pemetrexed to docetaxel. Patient demographics of the intent to treat (ITT) population are shown in Table 18.

The primary endpoint in this study was overall survival. The median survival time was 8.3 months in the pemetrexed treatment arm and 7.9 months in the docetaxel arm, with a hazard ratio of 0.99 (see Table 19). The study did not show an overall survival superiority of pemetrexed.

A retrospective analysis of the impact of NSCLC histology on overall survival was examined. Clinically relevant differences in survival according to histology were observed and are shown in Table 20. This difference in treatment effect for pemetrexed based on histology demonstrating a lack of efficacy in squamous cell histology was also observed in the first-line combination study and in the maintenance study.

Malignant Pleural Mesothelioma
A multi-center, randomized, single-blind study in 448 chemonaive patients with malignant pleural mesothelioma (MPM) compared survival in patients treated with pemetrexed in combination with cisplatin to survival in patients receiving cisplatin alone. Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 and cisplatin was administered intravenously over 2 hours at a dose of 75 mg/m2 beginning approximately 30 minutes after the end of administration of pemetrexed. Both drugs were given on Day 1 of each 21-day cycle. After 117 patients were treated, white cell and GI toxicity led to a change in protocol whereby all patients were given folic acid and vitamin B12 supplementation.
The primary analysis of this study was performed on the population of all patients randomly assigned to treatment who received study drug (randomized and treated). An analysis was also performed on patients who received folic acid and vitamin B12 supplementation during the entire course of study therapy (fully supplemented), as supplementation is recommended. Results in all patients and those fully supplemented were similar. Patient demographics are shown in Table 21.

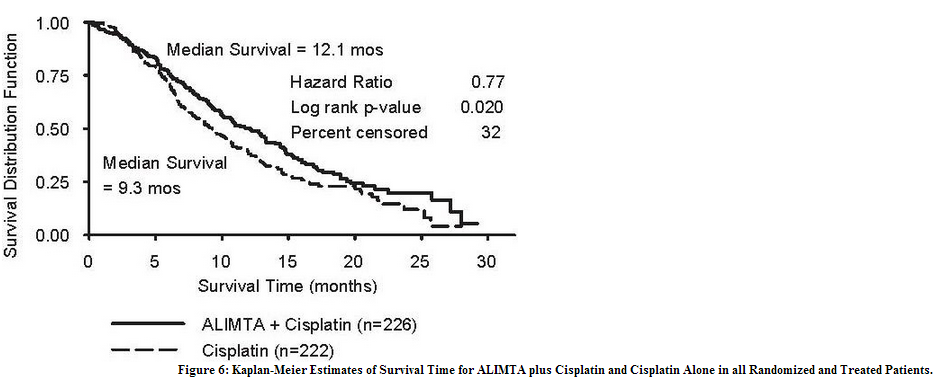
Table 22 and Figure 6 summarize the survival results for all randomized and treated patients regardless of vitamin supplementation status and those patients receiving vitamin supplementation from the time of enrollment in the trial.

Similar results were seen in the analysis of patients (N=303) with confirmed histologic diagnosis of malignant pleural mesothelioma. There were too few non-white patients to assess possible ethnic differences. The effect in women (median survival 15.7 months with the combination versus 7.5 months on cisplatin alone), however, was larger than the effect in males (median survival 11 versus 9.4 respectively). As with any exploratory analysis, it is not clear whether this difference is real or is a chance finding.
Objective tumor response for malignant pleural mesothelioma is difficult to measure and response criteria are not universally agreed upon. However, based upon prospectively defined criteria, the objective tumor response rate for pemetrexed plus cisplatin was greater than the objective tumor response rate for cisplatin alone. There was also improvement in lung function (forced vital capacity) in the pemetrexed plus cisplatin arm compared to the control arm.
Patients who received full supplementation with folic acid and vitamin B12 during study therapy received a median of 6 and 4 cycles in the pemetrexed/cisplatin (N=168) and cisplatin (N=163) arms, respectively. Patients who never received folic acid and vitamin B12 during study therapy received a median of 2 cycles in both treatment arms (N=32 and N=38 for the pemetrexed/cisplatin and cisplatin arm, respectively). Patients receiving pemetrexed in the fully supplemented group received a relative dose intensity of 93% of the protocol specified pemetrexed dose intensity; patients treated with cisplatin in the same group received 94% of the projected dose intensity. Patients treated with cisplatin alone had a dose intensity of 96%.
How Supplied
- ALIMTA, pemetrexed for injection, is available in sterile single-use vials containing 100 mg pemetrexed. NDC 0002-7640-01 (VL7640): single-use vial with ivory flip-off cap individually packaged in a carton.
- ALIMTA, pemetrexed for injection, is available in sterile single-use vials containing 500 mg pemetrexed. NDC 0002-7623-01 (VL7623): single-use vial with ivory flip-off cap individually packaged in a carton.
Storage
- Pemetrexed for injection, should be stored at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
- Chemical and physical stability of reconstituted and infusion solutions of pemetrexed were demonstrated for up to 24 hours following initial reconstitution, when stored refrigerated, 2-8°C (36-46°F).
- When prepared as directed, reconstituted and infusion solutions of pemetrexed contain no antimicrobial preservatives.
- Discard unused portion
Images
Drug Images
{{#ask: Page Name::Pemetrexed |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pemetrexed |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Instruct patients to read the patient package insert before initiating pemetrexed.
- Instruct patients on the need for folic acid and vitamin B12 supplementation to reduce treatment-related hematologic and gastrointestinal toxicity and of the need for corticosteroids to reduce treatment-related dermatologic toxicity.
- Inform patients of the risk of low blood cell counts and instruct them to immediately contact their physician for signs of infection, including fever, bleeding or symptoms of anemia.
- Instruct patients to contact their physician if persistent vomiting, diarrhea, or signs of dehydration appear.
- Instruct patients to inform their physician of all concomitant prescription or over-the-counter medications they are taking, particularly those for pain or inflammation such as non-steroidal anti-inflammatory drugs.
Precautions with Alcohol
Alcohol-Pemetrexed interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Alimta
Look-Alike Drug Names
- Pemetrexed - Pralatrexate
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Katirtzoglou N, Gkiozos I, Makrilia N, Tsaroucha E, Rapti A, Stratakos G; et al. (2010). "Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: a phase II study". Clin Lung Cancer. 11 (1): 30–5. doi:10.3816/CLC.2010.n.005. PMID 20085865.
- ↑ Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C; et al. (2003). "Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma". J Clin Oncol. 21 (8): 1556–61. doi:10.1200/JCO.2003.06.122. PMID 12697881.
- ↑ Vergote I, Calvert H, Kania M, Kaiser C, Zimmermann AH, Sehouli J (2009). "A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian or primary peritoneal cancer". Eur J Cancer. 45 (8): 1415–23. doi:10.1016/j.ejca.2008.12.013. PMID 19168349.
- ↑ Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML; et al. (2009). "Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group". J Clin Oncol. 27 (16): 2686–91. doi:10.1200/JCO.2008.19.2963. PMC 2690393. PMID 19332726.
- ↑ Sehouli J, Alvarez AM, Manouchehrpour S, Ghatage P, Szczylik C, Zimmermann A; et al. (2012). "A phase II trial of pemetrexed in combination with carboplatin in patients with recurrent ovarian or primary peritoneal cancer". Gynecol Oncol. 124 (2): 205–9. doi:10.1016/j.ygyno.2011.09.007. PMID 22044606.
{{#subobject:
|Label Page=Pemetrexed |Label Name=PemetrexedPackage1.png
}}
{{#subobject:
|Label Page=Pemetrexed |Label Name=PemetrexedPackage2.png
}}