Nilotinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
QT PROLONGATION AND SUDDEN DEATHS:
|
Overview
Nilotinib is a kinase inhibitor that is FDA approved for the treatment of newly diagnosed adult patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in chronic phase and chronic phase (CP) and accelerated phase (AP) Ph+ CML in adult patients resistant to or intolerant to prior therapy that included imatinib. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, rash, headache, fatigue, pruritus, vomiting, diarrhea, cough, constipation, arthralgia, nasopharyngitis, pyrexia, and night sweats.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Newly Diagnosed Ph+ CML-CP
- The recommended dose of Tasigna is 300 mg orally twice daily.
Resistant or Intolerant Ph+ CML-CP and CML-AP
- The recommended dose of Tasigna (nilotinib) is 400 mg orally twice daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nilotinib in adult patients.
Non–Guideline-Supported Use
Blastic phase chronic myeloid leukemia, Resistant or intolerant to imatinib
- Nilotinib dose, ranging from 50 to 1200 mg orally once daily and 400 to 600 mg twice daily.[1]
Gastrointestinal stromal tumor, Advanced, resistant to or intolerant of imatinib and/or sunitinib
- Nilotinib 400 mg twice daily.[2]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Nilotinib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nilotinib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nilotinib in pediatric patients.
Contraindications
- Do not use in patients with hypokalemia, hypomagnesemia, or long QT syndrome.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
QT PROLONGATION AND SUDDEN DEATHS:
|
Precautions
- Myelosuppression
- Treatment with Tasigna can cause Grade 3/4 thrombocytopenia, neutropenia and anemia. Perform complete blood counts every 2 weeks for the first 2 months and then monthly thereafter, or as clinically indicated. Myelosuppression was generally reversible and usually managed by withholding Tasigna temporarily or dose reduction.
- QT Prolongation
- Tasigna has been shown to prolong cardiac ventricular repolarization as measured by the QT interval on the surface ECG in a concentration-dependent manner. Prolongation of the QT interval can result in a type of ventricular tachycardia called torsade de pointes, which may result in syncope, seizure, and/or death. ECGs should be performed at baseline, 7 days after initiation of Tasigna, and periodically as clinically indicated and following dose adjustments.
- Tasigna should not be used in patients who have hypokalemia, hypomagnesemia or long QT syndrome. Before initiating Tasigna and periodically, test electrolyte, calcium and magnesium blood levels. Hypokalemia or hypomagnesemia must be corrected prior to initiating Tasigna and these electrolytes should be monitored periodically during therapy.
- Significant prolongation of the QT interval may occur when Tasigna is inappropriately taken with food and/or strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT. Therefore, coadministration with food must be avoided and concomitant use with strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT should be avoided. The presence of hypokalemia and hypomagnesemia may further prolong the QT interval.
- Sudden Deaths
- Sudden deaths have been reported in 0.3% of patients with CML treated with nilotinib in clinical studies of 5,661 patients. The relative early occurrence of some of these deaths relative to the initiation of nilotinib suggests the possibility that ventricular repolarization abnormalities may have contributed to their occurrence.
- Cardiac and Vascular Events
- Cardiovascular events, including arterial vascular occlusive events, were reported in a randomized, clinical trial in newly diagnosed CML patients and observed in the post-marketing reports of patients receiving nilotinib therapy. With a median time on therapy of 48 months in the clinical trial, cases of cardiovascular events included ischemic heart disease-related events (5.0% and 5.8% in the nilotinib 300 mg and 400 mg bid arms respectively, and 1.8% in the imatinib arm), peripheral arterial occlusive disease (1.8% and 2.2% in the nilotinib 300 mg and 400 mg bid arms respectively, and 0% in the imatinib arm), and ischemic cerebrovascular events (1.1% and 1.8% in the nilotinib 300 mg and 400 mg bid arms respectively, and 0.7% in the imatinib arm). If acute signs or symptoms of cardiovascular events occur, advise patients to seek immediate medical attention. The cardiovascular status of patients should be evaluated and cardiovascular risk factors should be monitored and actively managed during Tasigna therapy according to standard guidelines.
- Pancreatitis and Elevated Serum Lipase
- can cause increases in serum lipase. Patients with a previous history of pancreatitis may be at greater risk of elevated serum lipase. If lipase elevations are accompanied by abdominal symptoms, interrupt dosing and consider appropriate diagnostics to exclude pancreatitis. Test serum lipase levels monthly or as clinically indicated.
- Hepatotoxicity
- Tasigna may result in hepatotoxicity as measured by elevations in bilirubin, AST/ALT, and alkaline phosphatase. Monitor hepatic function tests monthly or as clinically indicated.
- Electrolyte Abnormalities
- The use of Tasigna can cause hypophosphatemia, hypokalemia, hyperkalemia, hypocalcemia, and hyponatremia. Electrolyte abnormalities must be corrected prior to initiating Tasigna and these electrolytes should be monitored periodically during therapy.
- Drug Interactions
- Avoid administration of Tasigna with agents that may increase nilotinib exposure (e.g., strong CYP3A4 inhibitors) or anti-arrhythmic drugs (including, but not limited to amiodarone, disopyramide, procainamide, quinidine and sotalol) and other drugs that may prolong QT interval (including, but not limited to chloroquine, clarithromycin, haloperidol, methadone, moxifloxacin and pimozide). Should treatment with any of these agents be required, interrupt therapy with Tasigna. If interruption of treatment with Tasigna is not possible, patients who require treatment with a drug that prolongs QT or strongly inhibits CYP3A4 should be closely monitored for prolongation of the QT interval.
- Food Effects
- The bioavailability of nilotinib is increased with food, thus Tasigna must not be taken with food. No food should be consumed for at least 2 hours before and for at least 1 hour after the dose is taken. Also avoid grapefruit products and other foods that are known to inhibit CYP3A4.
- Hepatic Impairment
- Nilotinib exposure is increased in patients with impaired hepatic function. Use a lower starting dose for patients with mild to severe hepatic impairment (at baseline) and monitor the QT interval frequently.
- Tumor Lysis Syndrome
- Tumor lysis syndrome cases have been reported in Tasigna treated patients with resistant or intolerant CML. Malignant disease progression, high WBC counts and/or dehydration were present in the majority of these cases. Due to potential for tumor lysis syndrome, maintain adequate hydration and correct uric acid levels prior to initiating therapy with Tasigna.
- Total Gastrectomy
- Since the exposure of nilotinib is reduced in patients with total gastrectomy, perform more frequent monitoring of these patients. Consider dose increase or alternative therapy in patients with total gastrectomy.
- Lactose
- Since the capsules contain lactose, Tasigna is not recommended for patients with rare hereditary problems of galactose intolerance, severe lactase deficiency with a severe degree of intolerance to lactose-containing products, or of glucose-galactose malabsorption.
- Monitoring Laboratory Tests
- Complete blood counts should be performed every 2 weeks for the first 2 months and then monthly thereafter. Chemistry panels, including electrolytes, calcium, magnesium, lipid profile, and glucose should be checked prior to therapy and periodically. ECGs should be obtained at baseline, 7 days after initiation and periodically thereafter, as well as following dose adjustments. Laboratory monitoring for patients receiving Tasigna may need to be performed more or less frequently at the physician’s discretion.
- Embryo-Fetal Toxicity
- There are no adequate and well controlled studies of Tasigna in pregnant women. However, Tasigna may cause fetal harm when administered to a pregnant woman. Nilotinib caused embryo-fetal toxicities in animals at maternal exposures that were lower than the expected human exposure at the recommended doses of nilotinib. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of child-bearing potential should avoid becoming pregnant while taking Tasigna.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Patients with Newly Diagnosed Ph+ CML-CP
- The data below reflect exposure to Tasigna from a randomized trial in patients with newly diagnosed Ph+ CML in chronic phase treated at the recommended dose of 300 mg twice daily (n=279). The median time on treatment in the nilotinib 300 mg twice daily group was 48 months (range 0.1 to 59 months). The median actual dose intensity was 594 mg/day in the nilotinib 300 mg twice daily group.
- The most common (>10%) non-hematologic adverse drug reactions were rash, pruritus, headache, nausea, fatigue, alopecia and myalgia. Upper abdominal pain, constipation, diarrhea, dry skin, muscle spasms, arthralgia, abdominal pain, peripheral edema, vomiting, and asthenia were observed less commonly (≤10% and >5%) and have been of mild to moderate severity, manageable and generally did not require dose reduction. Pleural and pericardial effusions, occurred in 1% and <;<1% of patients, respectively. Gastrointestinal hemorrhage was reported in 3% of patients.
- Increase in QTcF >60 msec from baseline was observed in 1 patient (0.4%) in the 300 mg twice daily treatment group. No patient had an absolute QTcF of >500 msec while on study drug.
- The most common hematologic adverse drug reactions (all grades) were myelosuppression including: thrombocytopenia (18%), neutropenia (15%) and anemia (7%). See Table 7 for Grade 3/4 laboratory abnormalities.
- Discontinuation due to adverse reactions, regardless of relationship to study drug, was observed in 10% of patients.
In Patients with Resistant or Intolerant Ph+ CML-CP and CML-AP
- In the single open-label multicenter clinical trial, a total of 458 patients with Ph+ CML-CP and CML-AP resistant to or intolerant to at least one prior therapy including imatinib were treated (CML-CP=321; CML-AP=137) at the recommended dose of 400 mg twice daily.
- The median duration of exposure in days for CML-CP and CML-AP patients is 561 (range 1 to 1096) and 264 (range 2 to 1160), respectively. The median dose intensity for patients with CML-CP and CML-AP is 789 mg/day (range 151 to 1110) and 780 mg/day (range 150 to 1149), respectively and corresponded to the planned 400 mg twice daily dosing.
- The median cumulative duration in days of dose interruptions for the CML-CP patients was 20 (range 1 to 345), and the median duration in days of dose interruptions for the CML-AP patients was 23 (range 1 to 234).
- In patients with CML-CP, the most commonly reported non-hematologic adverse drug reactions (≥10%) were rash, pruritus, nausea, fatigue, headache, constipation, diarrhea, vomiting and myalgia. The common serious drug-related adverse reactions (≥1% and <10%) were thrombocytopenia, neutropenia and anemia.
- In patients with CML-AP, the most commonly reported non-hematologic adverse drug reactions (≥10%) were rash, pruritus and fatigue. The common serious adverse drug reactions (≥1% and <;<10%) were thrombocytopenia, neutropenia, febrile neutropenia, pneumonia, leukopenia, intracranial hemorrhage, elevated lipase and pyrexia.
- Sudden deaths and QT prolongation were reported. The maximum mean QTcF change from baseline at steady-state was 10 msec. Increase in QTcF >60 msec from baseline was observed in 4.1% of the patients and QTcF of >500 msec was observed in 4 patients (<1%).
- Discontinuation due to adverse drug reactions was observed in 16% of CML-CP and 10% of CML-AP patients.
Most Frequently Reported Adverse Reactions
- Tables 5 and 6 show the percentage of patients experiencing non-hematologic adverse reactions (excluding laboratory abnormalities) regardless of relationship to study drug. Adverse reactions reported in greater than 10% of patients who received at least 1 dose of Tasigna are listed.
Additional Data from Clinical Trials
- The following adverse drug reactions were reported in patients in the Tasigna clinical studies at the recommended doses. These adverse drug reactions are ranked under a heading of frequency, the most frequent first using the following convention: common (≥1% and <10%), uncommon (≥0.1% and <1%), and unknown frequency (single events). For laboratory abnormalities, very common events (≥10%), which were not included in Tables 5 and 6, are also reported. These adverse reactions are included based on clinical relevance and ranked in order of decreasing seriousness within each category, obtained from 2 clinical studies:
- Newly diagnosed Ph+ CML-CP 48 month analysis and,
- Resistant or intolerant Ph+ CML-CP and CMP-AP 24 months’ analysis.
Infections and Infestations
Common: folliculitis, upper respiratory tract infection (including pharyngitis, nasopharyngitis, rhinitis). Uncommon: pneumonia, bronchitis, urinary tract infection, candidiasis (including oral candidiasis), gastroenteritis. Unknown frequency: sepsis, subcutaneous abscess, anal abscess, furuncle, tinea pedis.
Neoplasms Benign, Malignant, and Unspecified
Common: skin papilloma. Unknown frequency: oral papilloma, paraproteinemia.
Blood and Lymphatic System Disorders
Common: eosinophilia, febrile neutropenia, pancytopenia, lymphopenia. Unknown frequency: thrombocythemia, leukocytosis.
Immune System Disorders
Unknown frequency: hypersensitivity.
Endocrine Disorders
Uncommon: hyperthyroidism, hypothyroidism. Unknown frequency: hyperparathyroidism secondary, thyroiditis.
Metabolism and Nutrition Disorders
Very Common: hypophosphatemia. Common: electrolyte imbalance (including hypomagnesemia, hyperkalemia, hypokalemia, hyponatremia, hypocalcemia, hypercalcemia, hyperphosphatemia), diabetes mellitus, hyperglycemia, hypercholesterolemia, hyperlipidemia, hypertriglyceridemia. Uncommon: gout, dehydration, increased appetite. Unknown frequency: hyperuricemia, hypoglycemia.
Psychiatric Disorders
Common: depression, insomnia, anxiety. Unknown frequency: disorientation, confusional state, amnesia, dysphoria.
Nervous System Disorders
Common: dizziness, peripheral neuropathy, hypoesthesia, paresthesia. Uncommon: intracranial hemorrhage, migraine, loss of consciousness (including syncope), tremor, disturbance in attention, hyperesthesia. Unknown frequency: transient ischemic attack, brain edema, optic neuritis, lethargy, dysesthesia, restless legs syndrome.
Eye Disorders
Common: eye hemorrhage, periorbital edema, eye pruritus, conjunctivitis, dry eye (including xerophthalmia). Uncommon: vision impairment, vision blurred, visual acuity reduced, photopsia, hyperemia (scleral, conjunctival, ocular), eye irritation, conjunctival hemorrhage. Unknown frequency: papilloedema, diplopia, photophobia, eye swelling, blepharitis, eye pain, chorioretinopathy, conjunctivitis allergic, ocular surface disease.
Ear and Labyrinth Disorders
Common: vertigo. Unknown frequency: hearing impaired, ear pain, tinnitus.
Cardiac Disorders
Common: angina pectoris, arrhythmia (including atrioventricular block, cardiac flutter, extrasystoles, atrial fibrillation, tachycardia, bradycardia), palpitations, electrocardiogram QT prolonged. Uncommon: cardiac failure, pericardial effusion, coronary artery disease, cyanosis, cardiac murmur. Unknown frequency: myocardial infarction, ventricular dysfunction, pericarditis, ejection fraction decrease.
Vascular Disorders
Common: hypertension, flushing. Uncommon: hypertensive crisis, peripheral arterial occlusive disease, intermittent claudication, arterial stenosis limb, hematoma, arteriosclerosis. Unknown frequency: shock hemorrhagic, hypotension, thrombosis.
Respiratory, Thoracic and Mediastinal Disorders
Common: dyspnea, dyspnea exertional, epistaxis, cough, dysphonia. Uncommon: pulmonary edema, pleural effusion, interstitial lung disease, pleuritic pain, pleurisy, pharyngolaryngeal pain, throat irritation. Unknown frequency: pulmonary hypertension, wheezing, oropharyngeal pain.
Gastrointestinal Disorders
Common: pancreatitis, abdominal discomfort, abdominal distension, dyspepsia, dysgeusia, flatulence. Uncommon: gastrointestinal hemorrhage, melena, mouth ulceration, gastroesophageal reflux, stomatitis, esophageal pain, dry mouth, gastritis, sensitivity of teeth. Unknown frequency: gastrointestinal ulcer perforation, retroperitoneal hemorrhage, hematemesis, gastric ulcer, esophagitis ulcerative, subileus, enterocolitis, hemorrhoids, hiatus hernia, rectal hemorrhage, gingivitis.
Hepatobiliary Disorders
Very Common: hyperbilirubinemia. Common: hepatic function abnormal. Uncommon: hepatotoxicity, toxic hepatitis, jaundice. Unknown frequency: cholestasis, hepatomegaly.
Skin and Subcutaneous Tissue Disorders
Common: night sweats, eczema, urticaria, erythema, hyperhidrosis, contusion, acne, dermatitis (including allergic, exfoliative and acneiform), dry skin. Uncommon: exfoliative rash, drug eruption, pain of skin, ecchymosis, swelling of face. Unknown frequency: psoriasis, erythema multiforme, erythema nodosum, skin ulcer, palmar-plantar erythrodysesthesia syndrome, petechiae, photosensitivity, blister, dermal cyst, sebaceous hyperplasia, skin atrophy, skin discoloration, skin exfoliation, skin hyperpigmentation, skin hypertrophy, hyperkeratosis.
Musculoskeletal and Connective Tissue Disorders
Common: bone pain, musculoskeletal chest pain, musculoskeletal pain, back pain, neck pain, flank pain. Uncommon: musculoskeletal stiffness, muscular weakness, joint swelling. Unknown frequency: arthritis.
Renal and Urinary Disorders
Common: pollakiuria. Uncommon: dysuria, micturition urgency, nocturia. Unknown frequency: renal failure, hematuria, urinary incontinence, chromaturia.
Reproductive System and Breast Disorders
Uncommon: breast pain, gynecomastia, erectile dysfunction. Unknown frequency: breast induration, menorrhagia, nipple swelling.
General Disorders and Administration Site Conditions
Common: pyrexia, chest pain (including non-cardiac chest pain), pain, chest discomfort, malaise. Uncommon: face edema, gravitational edema, influenza-like illness, chills, feeling body temperature change (including feeling hot, feeling cold). Unknown frequency: localized edema.
Investigations
Very Common: alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased. Common: hemoglobin decreased, blood amylase increased, gamma-glutamyltransferase increased, blood creatinine phosphokinase increased, blood alkaline phosphatase increased, weight decreased, weight increased, lipoprotein increased (including very low density and high density). Uncommon: blood lactate dehydrogenase increased, blood urea increased, globulins decreased. Unknown frequency: troponin increased, blood bilirubin unconjugated increased, insulin C-peptide decreased, blood parathyroid hormone increased.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nilotinib in the drug label.
Drug Interactions
- Effects of Nilotinib on Drug Metabolizing Enzymes and Drug Transport Systems
- Nilotinib is a competitive inhibitor of CYP3A4, CYP2C8, CYP2C9, CYP2D6 and UGT1A1 in vitro, potentially increasing the concentrations of drugs eliminated by these enzymes. In vitro studies also suggest that nilotinib may induce CYP2B6, CYP2C8 and CYP2C9, and decrease the concentrations of drugs which are eliminated by these enzymes.
- In patients with CML, multiple doses of Tasigna increased the systemic exposure of oral midazolam (a substrate of CYP3A4) 2.6-fold. Tasigna is a moderate CYP3A4 inhibitor. As a result, the systemic exposure of drugs metabolized by CYP3A4 (e.g., certain HMG-CoA reductase inhibitors) may be increased when co-administered with Tasigna. Dose adjustment may be necessary for drugs that are CYP3A4 substrates, especially those that have narrow therapeutic indices (e.g., alfentanil, cyclosporine, dihydroergotamine, ergotamine, fentanyl, sirolimus and tacrolimus) when co-administered with Tasigna.
- Single-dose administration of Tasigna to healthy subjects did not change the pharmacokinetics and pharmacodynamics of warfarin (a CYP2C9 substrate). The ability of multiple doses of Tasigna to induce metabolism of drugs other than midazolam has not been determined in vivo. Monitor patients closely when co-administering Tasigna with drugs that have a narrow therapeutic index and are substrates for CYP2B6, CYP2C8, or CYP2C9 enzymes.
- Nilotinib inhibits human P-glycoprotein (P-gp). If Tasigna is administered with drugs that are substrates of P-gp, increased concentrations of the substrate drug are likely, and caution should be exercised.
- Drugs that Inhibit or Induce Cytochrome P450 3A4 Enzymes
- Nilotinib undergoes metabolism by CYP3A4, and concomitant administration of strong inhibitors or inducers of CYP3A4 can increase or decrease nilotinib concentrations significantly. The administration of Tasigna with agents that are strong CYP3A4 inhibitors should be avoided. Concomitant use of Tasigna with medicinal products and herbal preparations that are potent inducers of CYP3A4 is likely to reduce exposure to nilotinib to a clinically relevant extent. Therefore, in patients receiving Tasigna, concomitant use of alternative therapeutic agents with less potential for CYP3A4 induction should be selected.
- Ketoconazole: In healthy subjects receiving ketoconazole, a CYP3A4 inhibitor, at 400 mg once daily for 6 days, systemic exposure (AUC) to nilotinib was increased approximately 3-fold.
- Rifampicin: In healthy subjects receiving the CYP3A4 inducer, rifampicin, at 600 mg daily for 12 days, systemic exposure (AUC) to nilotinib was decreased approximately 80%.
- Drugs that Affect Gastric pH
- Nilotinib has pH-dependent solubility, with decreased solubility at higher pH. Drugs such as proton pump inhibitors that inhibit gastric acid secretion to elevate the gastric pH may decrease the solubility of nilotinib and reduce its bioavailability. In healthy subjects, coadministration of a single 400 mg dose of Tasigna with multiple doses of esomeprazole (a proton pump inhibitor) at 40 mg daily decreased the nilotinib AUC by 34%. Increasing the dose of Tasigna when coadministered with such agents is not likely to compensate for the loss of exposure. Since proton pump inhibitors affect pH of the upper GI tract for an extended period, separation of doses may not eliminate the interaction. The concomitant use of proton pump inhibitors with Tasigna is not recommended.
- In healthy subjects, no significant change in nilotinib pharmacokinetics was observed when a single 400 mg dose of Tasigna was administered 10 hours after and 2 hours before famotidine (an H2 blocker). Therefore, when the concurrent use of a H2 blocker is necessary, it may be administered approximately 10 hours before and approximately 2 hours after the dose of Tasigna.
- Administration of an antacid (aluminum hydroxide/magnesium hydroxide/simethicone) to healthy subjects, 2 hours before or 2 hours after a single 400 mg dose of Tasigna did not alter nilotinib pharmacokinetics. Therefore, if necessary, an antacid may be administered approximately 2 hours before or approximately 2 hours after the dose of Tasigna.
- Drugs that Inhibit Drug Transport Systems
- Nilotinib is a substrate of the efflux transporter P-glycoprotein (P-gp, ABCB1). If Tasigna is administered with drugs that inhibit P-gp, increased concentrations of nilotinib are likely, and caution should be exercised.
- Drugs that May Prolong the QT Interval
- The administration of Tasigna with agents that may prolong the QT interval such as anti-arrhythmic medicines should be avoided.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Risk Summary
- Based on its mechanism of action and findings in animals, Tasigna may cause fetal harm when administered to a pregnant woman. Women should be advised to avoid becoming pregnant while on Tasigna. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Animal Data
- Nilotinib was studied for effects on embryo-fetal development in pregnant rats and rabbits given oral doses of 10, 30, 100 mg/kg/day, and 30, 100, 300 mg/kg/day, respectively, during organogenesis. In rats, nilotinib at doses of 100 mg/kg/day (approximately 5.7 times the AUC in patients at the dose of 400 mg twice daily) was associated with maternal toxicity (decreased gestation weight, gravid uterine weight, net weight gain, and food consumption). Nilotinib at doses ≥30 mg/kg/day (approximately 2 times the AUC in patients at the dose of 400 mg twice daily) resulted in embryo-fetal toxicity as shown by increased resorption and post-implantation loss, and at 100 mg/kg/day, a decrease in viable fetuses. In rabbits, maternal toxicity at 300 mg/kg/day (approximately one-half the human exposure based on AUC) was associated with mortality, abortion, decreased gestation weights and decreased food consumption. Embryonic toxicity (increased resorption) and minor skeletal anomalies were observed at a dose of 300 mg/kg/day. Nilotinib is not considered teratogenic.
- When pregnant rats were dosed with nilotinib during organogenesis and through lactation, the adverse effects included a longer gestational period, lower pup body weights until weaning and decreased fertility indices in the pups when they reached maturity, all at a maternal dose of 360 mg/m2 (approximately 0.7 times the clinical dose of 400 mg twice daily based on body surface area). At doses up to 120 mg/m2 (approximately 0.25 times the clinical dose of 400 mg twice daily based on body surface area) no adverse effects were seen in the maternal animals or the pups.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nilotinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nilotinib during labor and delivery.
Nursing Mothers
- It is not known whether nilotinib is excreted in human milk. One study in lactating rats demonstrates that nilotinib is excreted into milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Tasigna, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Tasigna in pediatric patients have not been established.
Geriatic Use
- In the clinical trials of Tasigna (patients with newly diagnosed Ph+ CML-CP and resistant or intolerant Ph+ CML-CP and CML-AP), approximately 12% and 30% of patients were 65 years or over respectively.
- Patients with newly diagnosed Ph+ CML-CP: There was no difference in major molecular response between patients aged <65 years and those ≥65 years.
- Patients with resistant or intolerant CML-CP: There was no difference in major cytogenetic response rate between patients aged <65 years and those ≥65 years.
- Patients with resistant or intolerant CML-AP: The hematologic response rate was 44% in patients <65 years of age and 29% in patients ≥65 years.
- No major differences for safety were observed in patients ≥65 years of age as compared to patients <;<65 years.
Gender
There is no FDA guidance on the use of Nilotinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nilotinib with respect to specific racial populations.
Renal Impairment
- Clinical studies have not been performed in patients with impaired renal function. Clinical studies have excluded patients with serum creatinine concentration >1.5 times the upper limit of the normal range.
- Since nilotinib and its metabolites are not renally excreted, a decrease in total body clearance is not anticipated in patients with renal impairment.
Hepatic Impairment
- Nilotinib exposure is increased in patients with impaired hepatic function. In a study of subjects with mild to severe hepatic impairment following a single dose administration of 200 mg of Tasigna, the mean AUC values were increased on average of 35%, 35%, and 56% in subjects with mild (Child-Pugh class A, score 5 to 6), moderate (Child-Pugh class B, score 7 to 9) and severe hepatic impairment (Child-Pugh class C, score 10 to 15), respectively, compared to a control group of subjects with normal hepatic function. Table 8 summarizes the Child-Pugh Liver Function Classification applied in this study. A lower starting dose is recommended in patients with hepatic impairment and the QT interval should be monitored closely in these patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nilotinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nilotinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Nilotinib in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Nilotinib in the drug label.
Overdosage
Acute Overdose
- Overdose with nilotinib has been reported, where an unspecified number of Tasigna capsules were ingested in combination with alcohol and other drugs. Events included neutropenia, vomiting, and drowsiness. In the event of overdose, the patient should be observed and appropriate supportive treatment given.
Chronic Overdose
There is limited information regarding Chronic Overdose of Nilotinib in the drug label.
Pharmacology
Mechanism of Action
- Nilotinib is an inhibitor of the BCR-ABL kinase. Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. In vitro, nilotinib inhibited BCR-ABL mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ CML. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from BCR-ABL kinase mutations, in 32 out of 33 mutations tested. In vivo, nilotinib reduced the tumor size in a murine BCR-ABL xenograft model. Nilotinib inhibited the autophosphorylation of the following kinases at IC50 values as indicated: BCR-ABL (20 to 60 nM), PDGFR (69 nM), c-KIT (210 nM), CSF-1R (125 to 250 nM), and DDR1 (3.7 nM).
Structure
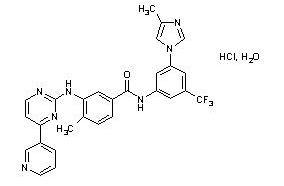
- Tasigna (nilotinib) belongs to a pharmacologic class of drugs known as kinase inhibitors.
- Nilotinib drug substance, a monohydrate monohydrochloride, is a white to slightly yellowish to slightly greenish yellow powder with the anhydrous molecular formula and weight, respectively, of C28H22F3N7O•HCl • H2O and 584. The solubility of nilotinib in aqueous solutions decreases with increasing pH. Nilotinib is not optically active. The pKa1 was determined to be 2.1; pKa2 was estimated to be 5.4.
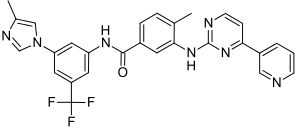
- The chemical name of nilotinib is 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[4-(3-pyridinyl)-2-pyrimidinyl]amino]-benzamide, monohydrochloride, monohydrate. Its structure is shown below:
- Tasigna (nilotinib) capsules, for oral use, contain 150 mg or 200 mg nilotinib base, anhydrous (as hydrochloride, monohydrate) with the following inactive ingredients: colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate and poloxamer 188. The capsules contain gelatin, iron oxide (red), iron oxide (yellow), iron oxide (black), and titanium dioxide.
Pharmacodynamics
- asigna can increase bilirubin levels. A pharmacogenetic analysis of 97 patients evaluated the polymorphisms of UGT1A1 and its potential association with hyperbilirubinemia during Tasigna treatment. In this study, the (TA)7/(TA)7 genotype was associated with a statistically significant increase in the risk of hyperbilirubinemia relative to the (TA)6/(TA)6 and (TA)6/(TA)7 genotypes. However, the largest increases in bilirubin were observed in the (TA)7/(TA)7 genotype (UGT1A1*28) patients.
- QT/QTc Prolongation
- In a placebo-controlled study in healthy volunteers designed to assess the effects of Tasigna on the QT interval, administration of Tasigna was associated with concentration-dependent QT prolongation; the maximum mean placebo-adjusted QTcF change from baseline was 18 msec (1-sided 95% Upper CI: 26 msec). A positive control was not included in the QT study of healthy volunteers. Peak plasma concentrations in the QT study were 26% lower than those observed in patients enrolled in the single-arm study.
Pharmacokinetics
- Absorption and Distribution
- The absolute bioavailability of nilotinib has not been determined. As compared to an oral drink solution (pH of 1.2 to 1.3), relative bioavailability of nilotinib capsule is approximately 50%. Peak concentrations of nilotinib are reached 3 hours after oral administration.
- Steady-state nilotinib exposure was dose-dependent with less than dose-proportional increases in systemic exposure at dose levels higher than 400 mg given as once daily dosing. Daily serum exposure to nilotinib following 400 mg twice daily dosing at steady state was 35% higher than with 800 mg once daily dosing. Steady state exposure (AUC) of nilotinib with 400 mg twice daily dosing was 13% higher than with 300 mg twice daily dosing. The average steady state nilotinib trough and peak concentrations did not change over 12 months. There was no relevant increase in exposure to nilotinib when the dose was increased from 400 mg twice daily to 600 mg twice daily.
- The bioavailability of nilotinib was increased when given with a meal. Compared to the fasted state, the systemic exposure (AUC) increased by 82% when the dose was given 30 minutes after a high fat meal.
- Single dose administration of two 200 mg nilotinib capsules each dispersed in 1 teaspoon of applesauce and administered within 15 minutes was shown to be bioequivalent to a single dose administration of two 200 mg intact capsules. The blood-to-serum ratio of nilotinib is 0.68. Serum protein binding is approximately 98% on the basis of in vitro experiments.
- Median steady-state trough concentration of nilotinib was decreased by 53% in patients with total gastrectomy compared to patients who had not undergone surgeries [see Warnings and Precautions (5.12)].
- Pharmacokinetics, Metabolism and Excretion
- The apparent elimination half-life estimated from the multiple dose pharmacokinetic studies with daily dosing was approximately 17 hours. Inter-patient variability in nilotinib AUC was 32% to 64%. Steady state conditions were achieved by Day 8. An increase in serum exposure to nilotinib between the first dose and steady state was approximately 2-fold for daily dosing and 3.8-fold for twice-daily dosing.
- Main metabolic pathways identified in healthy subjects are oxidation and hydroxylation. Nilotinib is the main circulating component in the serum. None of the metabolites contribute significantly to the pharmacological activity of nilotinib.
- After a single dose of radiolabeled nilotinib in healthy subjects, more than 90% of the administered dose was eliminated within 7 days: mainly in feces (93% of the dose). Parent drug accounted for 69% of the dose.
- Age, body weight, gender, or ethnic origin did not significantly affect the pharmacokinetics of nilotinib.
- Drug-Drug Interactions
- In a Phase 1 trial of nilotinib 400 mg twice daily in combination with imatinib 400 mg daily or 400 mg twice daily, the AUC increased 30% to 50% for nilotinib and approximately 20% for imatinib.
Nonclinical Toxicology
- A 2-year carcinogenicity study was conducted orally in rats at nilotinib doses of 5, 15, and 40 mg/kg/day. Exposures in animals at the highest dose tested were approximately 2 to 3 fold the human exposure (based on AUC) at the nilotinib dose of 400 mg twice daily. The study was negative for carcinogenic findings.
- Nilotinib was not mutagenic in a bacterial mutagenesis (Ames) assay, was not clastogenic in a chromosome aberration assay in human lymphocytes, did not induce DNA damage (comet assay) in L5178Y mouse lymphoma cells, nor was it clastogenic in an in vivo rat bone marrow micronucleus assay with two oral treatments at doses up to 2000 mg/kg/dose.
- There were no effects on male or female rat and female rabbit mating or fertility at doses up to 180 mg/kg in rats (approximately 4 to 7 fold for males and females, respectively, the AUC in patients at the dose of 400 mg twice daily) or 300 mg/kg in rabbits (approximately one-half the AUC in patients at the dose of 400 mg twice daily). The effect of Tasigna on human fertility is unknown. In a study where male and female rats were treated with nilotinib at oral doses of 20 to 180 mg/kg/day (approximately 1 to 6.6 fold the AUC in patients at the dose of 400 mg twice daily) during the pre-mating and mating periods and then mated, and dosing of pregnant rats continued through gestation Day 6, nilotinib increased post-implantation loss and early resorption, and decreased the number of viable fetuses and litter size at all doses tested.
Clinical Studies
Newly Diagnosed Ph+ CML-CP
- An open-label, multicenter, randomized trial was conducted to determine the efficacy of Tasigna versus imatinib tablets in adult patients with cytogenetically confirmed newly diagnosed Ph+ CML-CP. Patients were within 6 months of diagnosis and were previously untreated for CML-CP, except for hydroxyurea and/or anagrelide. Efficacy was based on a total of 846 patients: 283 patients in the imatinib 400 mg once daily group, 282 patients in the nilotinib 300 mg twice daily group, 281 patients in the nilotinib 400 mg twice daily group.
- Median age was 46 years in the imatinib group and 47 years in both nilotinib groups, with 12%, 13%, and 10% of patients ≥65 years of age in imatinib 400 mg once daily, nilotinib 300 mg twice daily and nilotinib 400 mg twice daily treatment groups, respectively. There were slightly more male than female patients in all groups (56%, 56%, and 62% in imatinib 400 mg once daily, nilotinib 300 mg twice daily and nilotinib 400 mg twice daily treatment groups, respectively). More than 60% of all patients were Caucasian, and 25% were Asian.
- The primary data analysis was performed when all 846 patients completed 12 months of treatment (or discontinued earlier). Subsequent analyses were done when patients completed 24, 36, and 48 months of treatment (or discontinued earlier). The median time on treatment was approximately 48 months in all three treatment groups. This study is on-going and further data will be required to determine long-term outcome.
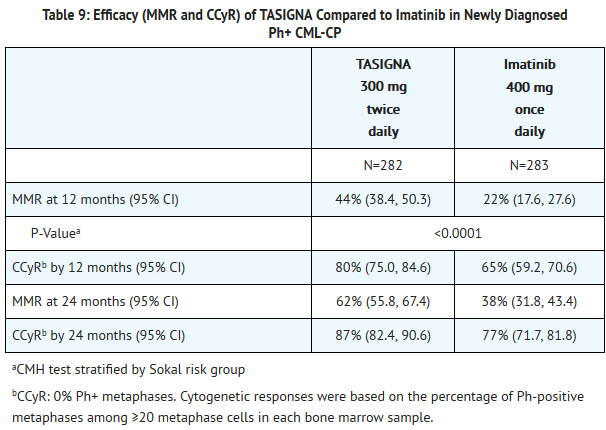
- The primary efficacy endpoint was major molecular response (MMR) at 12 months after the start of study medication. MMR was defined as ≤0.1% BCR-ABL/ABL % by international scale measured by RQ-PCR, which corresponds to a ≥3 log reduction of BCR-ABL transcript from standardized baseline. Efficacy endpoints are summarized in Table 9 below.
- Three patients in the nilotinib arm progressed to either accelerated phase (including clonal evolution) or blast crisis (2 within the first 6 months of treatment and 1 within 36 to 48 months while 17 patients on the imatinib arm progressed to either accelerated phase (including clonal evolution) or blast crisis (8 patients within first 6 months, 4 within 6 to 12 months, 4 within 12 to 18 months and 1 within 18 to 24 months).
Patients with Resistant or Intolerant Ph+ CML-CP and CML-AP
- A single-arm, open-label, multicenter study was conducted to evaluate the efficacy and safety of Tasigna (400 mg twice daily) in patients with imatinib-resistant or -intolerant CML with separate cohorts for chronic and accelerated phase disease. The definition of imatinib resistance included failure to achieve a complete hematologic response (by 3 months), cytogenetic response (by 6 months) or major cytogenetic response (by 12 months) or progression of disease after a previous cytogenetic or hematologic response. Imatinib intolerance was defined as discontinuation of treatment due to toxicity and lack of a major cytogenetic response at time of study entry. At the time of data cut-off, 321 patients with CML-CP and 137 patients with CML-AP with a minimum follow-up of 24 months were enrolled. In this study, about 50% of CML-CP and CML-AP patients were males, over 90% (CML-CP) and 80% (CML-AP) were Caucasian, and approximately 30% were age 65 years or older.
- Overall, 73% of patients were imatinib resistant while 27% were imatinib intolerant. The median time of prior imatinib treatment was approximately 32 (CML-CP) and 28 (CML-AP) months. Prior therapy included hydroxyurea in 85% of patients, interferon in 56% and stem cell or bone marrow transplant in 8%. The median highest prior imatinib dose was 600 mg/day for patients with CML-CP and CML-AP, and the highest prior imatinib dose was ≥600 mg/day in 74% of all patients with 40% of patients receiving imatinib doses ≥800 mg/day.
- Median duration of nilotinib treatment was 18.4 months in patients with CML-CP and 8.7 months in patients with CML-AP.
- The efficacy endpoint in CML-CP was unconfirmed major cytogenetic response (MCyR) which included complete and partial cytogenetic responses.
- The efficacy endpoint in CML-AP was confirmed hematologic response (HR), defined as either a complete hematologic response (CHR) or no evidence of leukemia (NEL). The rates of response for CML-CP and CML-AP patients are reported in Table 10.
- Median durations of response had not been reached at the time of data analysis.
Patients with Chronic Phase
- The MCyR rate in 321 CML-CP patients was 51%. The median time to MCyR among responders was 2.8 months (range 1 to 28 months). The median duration of MCyR cannot be estimated. The median duration of exposure on this single arm-trial was 18.4 months. Among the CML-CP patients who achieved MCyR, 62% of them had MCyR lasting more than 18 months. The CCyR rate was 37%.
Patients with Accelerated Phase
- The overall confirmed hematologic response rate in 137 patients with CML-AP was 39%. The median time to first hematologic response among responders was 1 month (range 1 to 14 months). Among the CML-AP patients who achieved HR, 44% of them had a response lasting for more than 18 months.
- After imatinib failure, 24 different BCR-ABL mutations were noted in 42% of chronic phase and 54% of accelerated phase CML patients who were evaluated for mutations.
How Supplied
- Tasigna (nilotinib) 150 mg capsules are red opaque hard gelatin capsules, size 1 with black axial imprint “NVR/BCR”. Tasigna (nilotinib) 200 mg capsules are light yellow opaque hard gelatin capsules, size 0 with the red axial imprint “NVR/TKI.” Tasigna capsules are supplied in blister packs.
- 150 mg
- Carton of 4 blister packs of (4x28) ………………………….…….NDC 0078-0592-87
- Blisters of 28 capsules……………………………………….…….NDC 0078-0592-51
- 200 mg
- Carton of 4 blister packs of (4x28) ………………………….…….NDC 0078-0526-87
- Blisters of 28 capsules……………………………………….…….NDC 0078-0526-51
- Tasigna (nilotinib) capsules should be stored at 25°C (77°F); excursions permitted between 15° to 30°C (59° to 86°F).
Storage
There is limited information regarding Nilotinib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nilotinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nilotinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Cardiac and Vascular Events
- Advise patients that cardiovascular events (including ischemic heart disease, peripheral arterial occlusive disease, and ischemic cerebrovascular events) have been reported. Advise patients to seek immediate medical attention with any symptoms suggestive of a cardiovascular event. Cardiovascular status of patients should be evaluated and cardiovascular risk factors should be monitored and managed during Tasigna therapy according to standard guidelines.
- Taking Tasigna
- Advise patients to take Tasigna doses twice daily approximately 12 hours apart. The capsules should be swallowed whole with water.
- Advise patients to take Tasigna on an empty stomach. No food should be consumed for at least 2 hours before the dose is taken and for at least 1 hour after the dose is taken. Patients should not consume grapefruit products and other foods that are known to inhibit CYP3A4 at any time during Tasigna treatment.
- If the patient missed a dose of Tasigna, the patient should take the next scheduled dose at its regular time. The patient should not take two doses at the same time.
- Should patients be unable to swallow capsules, the contents of each capsule may be dispersed in one teaspoon of applesauce and the mixture swallowed immediately (within 15 minutes).
- Drug Interactions
- Tasigna and certain other medicines, including over the counter medications or herbal supplements (such as St. John’s Wort), can interact with each other.
- Pregnancy
- Advise patients that the use of Tasigna during pregnancy may cause harm to the fetus and that Tasigna should not be taken during pregnancy unless necessary. Women of childbearing potential should use highly effective contraceptives while taking Tasigna. Sexually active female patients taking Tasigna should use adequate contraception.
- Compliance
- Advise patients of the following:
- Continue taking Tasigna every day for as long as their doctor tells them.
- This is a long-term treatment.
- Do not change dose or stop taking Tasigna without first consulting their doctor.
- If a dose is missed, take the next dose as scheduled. Do not take a double dose to make up for the missed capsules.
- Advise patients of the following:
Precautions with Alcohol
- Alcohol-Nilotinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TASIGNA®[4]
Look-Alike Drug Names
There is limited information regarding Nilotinib Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B; et al. (2006). "Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL". N Engl J Med. 354 (24): 2542–51. doi:10.1056/NEJMoa055104. PMID 16775235.
- ↑ Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B; et al. (2012). "Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib". Ann Oncol. 23 (7): 1680–7. doi:10.1093/annonc/mdr598. PMID 22357255.
- ↑ 3.0 3.1 Invalid
<ref>tag; no text was provided for refs namedMSR - ↑ "TASIGNA nilotinib capsule".
{{#subobject:
|Page Name=Nilotinib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Nilotinib |Label Name=Nilotinib12.png
}}
{{#subobject:
|Label Page=Nilotinib |Label Name=Nilotinib13.png
}}
{{#subobject:
|Label Page=Nilotinib |Label Name=Nilotinib14.png
}}