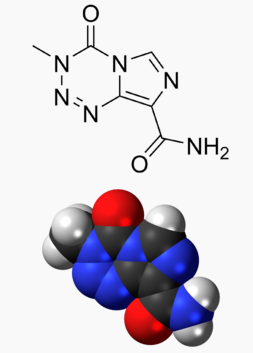
Temozolomide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2];Aparna Vuppala, M.B.B.S. [3]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Temozolomide is an antineoplastic agent that is FDA approved for the treatment of newly diagnosed glioblastoma multiforme (GBM) and refractory anaplastic astrocytoma. Common adverse reactions include alopecia, fatigue, nausea, vomiting, headache, constipation, anorexia, convulsions, rash, hemiparesis, diarrhea, asthenia, fever, dizziness, incoordination, viral infection, amnesia, and insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Newly Diagnosed Glioblastoma Multiforme
- Temozolomide is indicated for the treatment of adult patients with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and then as maintenance treatment.
- Dosage:
- 75 mg/m2 for 42 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1–5 of a 28-day cycle of temozolomide for 6 cycles.
Refractory Anaplastic Astrocytoma
- Temozolomide is indicated for the treatment of adult patients with refractory anaplastic astrocytoma.
- Dosage:
- Initial dose 150 mg/m2 once daily for 5 consecutive days per 28-day treatment cycle.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Temozolomide in adult patients.
Non–Guideline-Supported Use
- Relapsed, refractory, or progressive malignant glioma as monotherapy
- As monotherapy of metastatic malignant melanoma
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Temozolomide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Temozolomide in pediatric patients.
Non–Guideline-Supported Use
- Relapsed, refractory, or progressive malignant glioma as monotherapy
Contraindications
Temozolomide is contraindicated in patients who have a history of hypersensitivity reaction (such as urticaria, allergic reaction including anaphylaxis, toxic epidermal necrolysis, and Stevens-Johnson syndrome) to any of its components. temozolomide is also contraindicated in patients who have a history of hypersensitivity to dacarbazine (DTIC), since both drugs are metabolized to 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC).
Warnings
Myelosuppression
- Patients treated with temozolomide may experience myelosuppression, including prolonged pancytopenia, which may result in aplastic anemia, which in some cases has resulted in a fatal outcome.
- In some cases, exposure to concomitant medications associated with aplastic anemia, including carbamazepine, phenytoin, and sulfamethoxazole/trimethoprim, complicates assessment.
- Prior to dosing, patients must have an absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L and a platelet count greater than or equal to 100 × 109/L.
- A complete blood count should be obtained on Day 22 (21 days after the first dose) or within 48 hours of that day, and weekly until the ANC is above 1.5 × 109/L and platelet count exceeds 100 × 109/L
- Geriatric patients and women have been shown in clinical trials to have a higher risk of developing myelosuppression.
Myelodysplastic Syndrome
- Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukemia, have been observed.
Pneumocystis Pneumonia
- For treatment of newly diagnosed glioblastoma multiforme: Prophylaxis against Pneumocystis pneumonia (PCP) is required for all patients receiving concomitant temozolomide and radiotherapy for the 42-day regimen.
- There may be a higher occurrence of PCP when temozolomide is administered during a longer dosing regimen. However, all patients receiving temozolomide, particularly patients receiving steroids, should be observed closely for the development of PCP regardless of the regimen.
Laboratory Tests
- For the concomitant treatment phase with RT, a complete blood count should be obtained prior to initiation of treatment and weekly during treatment.
- For the 28-day treatment cycles, a complete blood count should be obtained prior to treatment on Day 1 and on Day 22 (21 days after the first dose) of each cycle. Blood counts should be performed weekly until recovery if the ANC falls below 1.5 × 109/L and the platelet count falls below 100 × 109/L.
Hepatotoxicity
- Fatal and severe hepatotoxicity have been reported in patients receiving temozolomide. Perform liver function tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately two to four weeks after the last dose of temozolomide.
Use in Pregnancy
- Temozolomide can cause fetal harm when administered to a pregnant woman. Administration of temozolomide to rats and rabbits during organogenesis at 0.38 and 0.75 times the maximum recommended human dose (75 and 150 mg/m2), respectively, caused numerous fetal malformations of the external organs, soft tissues, and skeleton in both species.
Infusion Time
- As bioequivalence has been established only when temozolomide for Injection was given over 90 minutes, infusion over a shorter or longer period of time may result in suboptimal dosing. Additionally, the possibility of an increase in infusion-related adverse reactions cannot be ruled out.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed Glioblastoma Multiforme
During the concomitant phase (temozolomide+radiotherapy), adverse reactions including thrombocytopenia, nausea, vomiting, anorexia, and constipation were more frequent in the temozolomide+RT arm. The incidence of other adverse reactions was comparable in the two arms. The most common adverse reactions across the cumulative temozolomide experience were alopecia, nausea, vomiting, anorexia, headache, and constipation. Forty-nine percent (49%) of patients treated with temozolomide reported one or more severe or life-threatening reactions, most commonly fatigue (13%), convulsions (6%), headache (5%), and thrombocytopenia (5%). Overall, the pattern of reactions during the maintenance phase was consistent with the known safety profile of temozolomide.

Myelosuppression (neutropenia and thrombocytopenia), which is a known dose-limiting toxicity for most cytotoxic agents, including temozolomide, was observed. When laboratory abnormalities and adverse reactions were combined, Grade 3 or Grade 4 neutrophil abnormalities including neutropenic reactions were observed in 8% of the patients, and Grade 3 or Grade 4 platelet abnormalities, including thrombocytopenic reactions, were observed in 14% of the patients treated with temozolomide.
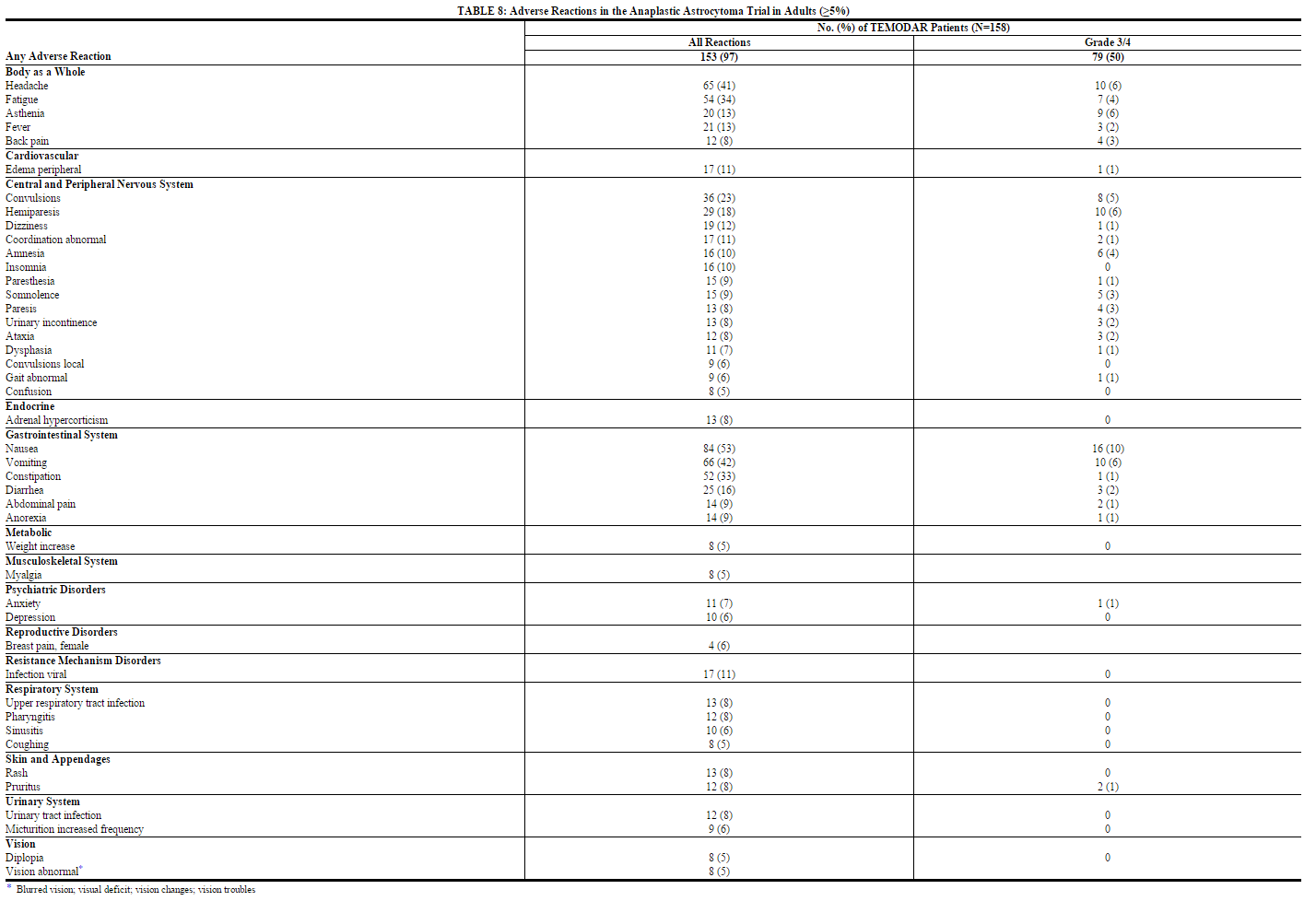
Refractory Anaplastic Astrocytoma
TABLES 8 and 9 show the incidence of adverse reactions in the 158 patients in the anaplastic astrocytoma study for whom data are available. In the absence of a control group, it is not clear in many cases whether these reactions should be attributed to temozolomide or the patients' underlying conditions, but nausea, vomiting, fatigue, and hematologic effects appear to be clearly drug-related. The most frequently occurring adverse reactions were nausea, vomiting, headache, and fatigue. The adverse reactions were usually NCI Common Toxicity Criteria (CTC) Grade 1 or 2 (mild to moderate in severity) and were self-limiting, with nausea and vomiting readily controlled with antiemetics. The incidence of severe nausea and vomiting (CTC Grade 3 or 4) was 10% and 6%, respectively. Myelosuppression (thrombocytopenia and neutropenia) was the dose-limiting adverse reaction. It usually occurred within the first few cycles of therapy and was not cumulative.
Myelosuppression occurred late in the treatment cycle and returned to normal, on average, within 14 days of nadir counts. The median nadirs occurred at 26 days for platelets (range: 21–40 days) and 28 days for neutrophils (range: 1–44 days). Only 14% (22/158) of patients had a neutrophil nadir and 20% (32/158) of patients had a platelet nadir, which may have delayed the start of the next cycle. Less than 10% of patients required hospitalization, blood transfusion, or discontinuation of therapy due to myelosuppression.
In clinical trial experience with 110 to 111 women and 169 to 174 men (depending on measurements), there were higher rates of Grade 4 neutropenia (ANC less than 500 cells/µL) and thrombocytopenia (less than 20,000 cells/µL) in women than men in the first cycle of therapy (12% vs. 5% and 9% vs. 3%, respectively).
In the entire safety database for which hematologic data exist (N=932), 7% (4/61) and 9.5% (6/63) of patients over age 70 experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. For patients less than or equal to age 70, 7% (62/871) and 5.5% (48/879) experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. Pancytopenia, leukopenia, and anemia have also been reported.

Temozolomide for injection delivers equivalent temozolomide dose and exposure to both temozolomide and 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC) as the corresponding temozolomide capsules. Adverse reactions probably related to treatment that were reported from the 2 studies with the intravenous formulation (n=35) that were not reported in studies using the temozolomide capsules were: pain, irritation, pruritus, warmth, swelling, and erythema at infusion site as well as the following adverse reactions: petechiae and hematoma.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of temozolomide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
- Dermatologic disorders: Toxic epidermal necrolysis and Stevens-Johnson syndrome
- Immune system disorders: Allergic reactions, including anaphylaxis. Erythema multiforme, which resolved after discontinuation of temozolomide and, in some cases, recurred upon rechallenge.
- Hematopoietic disorders: Prolonged pancytopenia, which may result in aplastic anemia and fatal outcomes.
- Hepatobiliary disorders: Fatal and severe hepatotoxicity, elevation of liver enzymes, hyperbilirubinemia, cholestasis, and hepatitis.
- Infections and infestations: Opportunistic infections including Pneumocystis pneumonia (PCP), reactivation of infections such as cytomegalovirus and hepatitis B.
- Pulmonary disorders: Interstitial pneumonitis, pneumonitis, alveolitis, and pulmonary fibrosis.
- Endocrine disorders: Diabetes insipidus
Drug Interactions
Administration of valproic acid decreases oral clearance of temozolomide by about 5%. The clinical implication of this effect is not known
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
temozolomide can cause fetal harm when administered to a pregnant woman. Five consecutive days of oral temozolomide administration of 0.38 and 0.75 times the highest recommended human dose (75 and 150 mg/m2) in rats and rabbits, respectively, during the period of organogenesis caused numerous malformations of the external and internal soft tissues and skeleton in both species. Doses equivalent to 0.75 times the highest recommended human dose (150 mg/m2) caused embryolethality in rats and rabbits as indicated by increased resorptions. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Women of childbearing potential should be advised to avoid becoming pregnant during therapy with temozolomide.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Temozolomide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Temozolomide during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants and tumorigenicity shown for temozolomide in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of temozolomide to the mother.
Pediatric Use
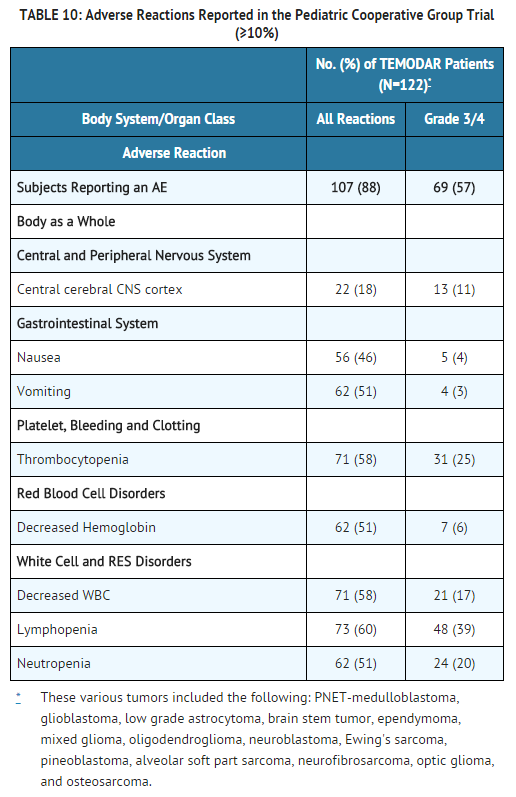
Safety and effectiveness in pediatric patients have not been established. temozolomide Capsules have been studied in 2 open-label studies in pediatric patients (aged 3–18 years) at a dose of 160 to 200 mg/m2 daily for 5 days every 28 days. In one trial, 29 patients with recurrent brain stem glioma and 34 patients with recurrent high grade astrocytoma were enrolled. All patients had recurrence following surgery and radiation therapy, while 31% also had disease progression following chemotherapy. In a second study conducted by the Children's Oncology Group (COG), 122 patients were enrolled, including patients with medulloblastoma/PNET (29), high grade astrocytoma (23), low grade astrocytoma (22), brain stem glioma (16), ependymoma (14), other CNS tumors (9), and non-CNS tumors (9). The temozolomide toxicity profile in pediatric patients is similar to adults.
Geriatic Use
Clinical studies of temozolomide did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
In the anaplastic astrocytoma study population, patients 70 years of age or older had a higher incidence of Grade 4 neutropenia and Grade 4 thrombocytopenia (2/8; 25%, P=0.31 and 2/10; 20%, P=0.09, respectively) in the first cycle of therapy than patients under 70 years of age.
In newly diagnosed patients with glioblastoma multiforme, the adverse reaction profile was similar in younger patients (<65 years) vs. older (≥65 years).
Gender
A population pharmacokinetic analysis indicated that women have an approximately 5% lower clearance (adjusted for body surface area) for temozolomide than men.
Race
The effect of race on the pharmacokinetics of temozolomide has not been studied.
Renal Impairment
- Caution should be exercised when temozolomide is administered to patients with severe renal impairment.
- A population pharmacokinetic analysis indicated that creatinine clearance over the range of 36 to 130 mL/min/m2 has no effect on the clearance of temozolomide after oral administration. The pharmacokinetics of temozolomide have not been studied in patients with severely impaired renal function (CLcr <36 mL/min/m2). Caution should be exercised when temozolomide is administered to patients with severe renal impairment. Temozolomide has not been studied in patients on dialysis.
Hepatic Impairment
- Caution should be exercised when temozolomide is administered to patients with severe hepatic impairment.
- A study showed that the pharmacokinetics of temozolomide in patients with mild-to-moderate hepatic impairment (Child-Pugh Class I – II) were similar to those observed in patients with normal hepatic function. Caution should be exercised when temozolomide is administered to patients with severe hepatic impairment
Females of Reproductive Potential and Males
Temozolomide impairs male fertility. Temozolomide caused syncytial cells/immature sperm formation at 0.25 and 0.63 times the maximum recommended human dose (50 and 125 mg/m2) in rats and dogs, respectively, and testicular atrophy in dogs at 0.63 times the maximum recommended human dose (125 mg/m2).
Immunocompromised Patients
There is no FDA guidance one the use of Temozolomide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Temozolomide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Temozolomide and IV administrations.
Overdosage
Doses of 500, 750, 1000, and 1250 mg/m2 (total dose per cycle over 5 days) have been evaluated clinically in patients. Dose-limiting toxicity was hematologic and was reported with any dose but is expected to be more severe at higher doses. An overdose of 2000 mg per day for 5 days was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia, multi-organ failure, and death. There are reports of patients who have taken more than 5 days of treatment (up to 64 days), with adverse reactions reported including bone marrow suppression, which in some cases was severe and prolonged, and infections and resulted in death. In the event of an overdose, hematologic evaluation is needed. Supportive measures should be provided as necessary.
Pharmacology
Mechanism of Action
Temozolomide is not directly active but undergoes rapid nonenzymatic conversion at physiologic pH to the reactive compound 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC). The cytotoxicity of MTIC is thought to be primarily due to alkylation of DNA. Alkylation (methylation) occurs mainly at the O6 and N7 positions of guanine.
Structure
The chemical name of temozolomide is 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide. The structural formula is:

Pharmacodynamics
There is limited information regarding Temozolomide Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
Temozolomide is rapidly and completely absorbed after oral administration with a peak plasma concentration (Cmax) achieved in a median Tmax of 1 hour. Food reduces the rate and extent of temozolomide absorption. Mean peak plasma concentration and AUC decreased by 32% and 9%, respectively, and median Tmax increased by 2-fold (from 1–2.25 hours) when temozolomide was administered after a modified high-fat breakfast.
A pharmacokinetic study comparing oral and intravenous temozolomide in 19 patients with primary CNS malignancies showed that 150 mg/m2 temozolomide for injection administered over 90 minutes is bioequivalent to 150 mg/m2 temozolomide oral capsules with respect to both Cmax and AUC of temozolomide and MTIC. Following a single 90-minute intravenous infusion of 150 mg/m2, the geometric mean Cmax values for temozolomide and MTIC were 7.3 mcg/mL and 276 ng/mL, respectively. Following a single oral dose of 150 mg/m2, the geometric mean Cmax values for temozolomide and MTIC were 7.5 mcg/mL and 282 ng/mL, respectively. Following a single 90-minute intravenous infusion of 150 mg/m2, the geometric mean AUC values for temozolomide and MTIC were 24.6 mcg∙hr/mL and 891 ng∙hr/mL, respectively. Following a single oral dose of 150 mg/m2, the geometric mean AUC values for temozolomide and MTIC were 23.4 mcg∙hr/mL and 864 ng∙hr/mL, respectively.
Distribution
Temozolomide has a mean apparent volume of distribution of 0.4 L/kg (%CV=13%). It is weakly bound to human plasma proteins; the mean percent bound of drug-related total radioactivity is 15%.
Metabolism and Elimination
Temozolomide is spontaneously hydrolyzed at physiologic pH to the active species, MTIC and to temozolomide acid metabolite. MTIC is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC), which is known to be an intermediate in purine and nucleic acid biosynthesis, and to methylhydrazine, which is believed to be the active alkylating species. Cytochrome P450 enzymes play only a minor role in the metabolism of temozolomide and MTIC. Relative to the AUC of temozolomide, the exposure to MTIC and AIC is 2.4% and 23%, respectively.
Excretion
About 38% of the administered temozolomide total radioactive dose is recovered over 7 days: 37.7% in urine and 0.8% in feces. The majority of the recovery of radioactivity in urine is unchanged temozolomide (5.6%), AIC (12%), temozolomide acid metabolite (2.3%), and unidentified polar metabolite(s) (17%). Overall clearance of temozolomide is about 5.5 L/hr/m2. Temozolomide is rapidly eliminated, with a mean elimination half-life of 1.8 hours, and exhibits linear kinetics over the therapeutic dosing range of 75 to 250 mg/m2/day.
Tobacco Use
A population pharmacokinetic analysis indicated that the oral clearance of temozolomide is similar in smokers and nonsmokers.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
Temozolomide is carcinogenic in rats at doses less than the maximum recommended human dose. Temozolomide induced mammary carcinomas in both males and females at doses 0.13 to 0.63 times the maximum human dose (25–125 mg/m2) when administered orally on 5 consecutive days every 28 days for 6 cycles. Temozolomide also induced fibrosarcomas of the heart, eye, seminal vesicles, salivary glands, abdominal cavity, uterus, and prostate, carcinomas of the seminal vesicles, schwannomas of the heart, optic nerve, and harderian gland, and adenomas of the skin, lung, pituitary, and thyroid at doses 0.5 times the maximum daily dose. Mammary tumors were also induced following 3 cycles of temozolomide at the maximum recommended daily dose.
Temozolomide is a mutagen and a clastogen. In a reverse bacterial mutagenesis assay (Ames assay), temozolomide increased revertant frequency in the absence and presence of metabolic activation. Temozolomide was clastogenic in human lymphocytes in the presence and absence of metabolic activation.
Animal Toxicology and/or Pharmacology
Toxicology studies in rats and dogs identified a low incidence of hemorrhage, degeneration, and necrosis of the retina at temozolomide doses equal to or greater than 0.63 times the maximum recommended human dose (125 mg/m2). These changes were most commonly seen at doses where mortality was observed.
Clinical Studies
Newly Diagnosed Glioblastoma Multiforme
Five hundred and seventy-three patients were randomized to receive either temozolomide (TMZ)+Radiotherapy (RT) (n=287) or RT alone (n=286). Patients in the temozolomide+RT arm received concomitant temozolomide (75 mg/m2) once daily, starting the first day of RT until the last day of RT, for 42 days (with a maximum of 49 days). This was followed by 6 cycles of temozolomide alone (150 or 200 mg/m2) on Days 1 to 5 of every 28-day cycle, starting 4 weeks after the end of RT. Patients in the control arm received RT only. In both arms, focal radiation therapy was delivered as 60 Gy/30 fractions. Focal RT includes the tumor bed or resection site with a 2- to 3-cm margin. Pneumocystis pneumonia (PCP) prophylaxis was required during the TMZ+RT, regardless of lymphocyte count, and was to continue until recovery of lymphocyte count to less than or equal to Grade 1.
At the time of disease progression, temozolomide was administered as salvage therapy in 161 patients of the 282 (57%) in the RT alone arm, and 62 patients of the 277 (22%) in the temozolomide+RT arm.
The addition of concomitant and maintenance temozolomide to radiotherapy in the treatment of patients with newly diagnosed GBM showed a statistically significant improvement in overall survival compared to radiotherapy alone. The hazard ratio (HR) for overall survival was 0.63 (95% CI for HR=0.52-0.75) with a log-rank P<0.0001 in favor of the temozolomide arm. The median survival was increased by 2.5 months in the temozolomide arm.

Refractory Anaplastic Astrocytoma
A single-arm, multicenter study was conducted in 162 patients who had anaplastic astrocytoma at first relapse and who had a baseline Karnofsky performance status of 70 or greater. Patients had previously received radiation therapy and may also have previously received a nitrosourea with or without other chemotherapy. Fifty-four patients had disease progression on prior therapy with both a nitrosourea and procarbazine, and their malignancy was considered refractory to chemotherapy (refractory anaplastic astrocytoma population). Median age of this subgroup of 54 patients was 42 years (19–76). Sixty-five percent were male. Seventy-two percent of patients had a KPS of >80. Sixty-three percent of patients had surgery other than a biopsy at the time of initial diagnosis. Of those patients undergoing resection, 73% underwent a subtotal resection and 27% underwent a gross total resection. Eighteen percent of patients had surgery at the time of first relapse. The median time from initial diagnosis to first relapse was 13.8 months (4.2–75.4).
temozolomide Capsules were given for the first 5 consecutive days of a 28-day cycle at a starting dose of 150 mg/m2/day. If the nadir and day of dosing (Day 29, Day 1 of next cycle) absolute neutrophil count was greater than or equal to 1.5 × 109/L (1500/µL) and the nadir and Day 29, Day 1 of next cycle platelet count was greater than or equal to 100 × 109/L (100,000/µL), the temozolomide dose was increased to 200 mg/m2/day for the first 5 consecutive days of a 28-day cycle.
In the refractory anaplastic astrocytoma population, the overall tumor response rate (CR+PR) was 22% (12/54 patients) and the complete response rate was 9% (5/54 patients). The median duration of all responses was 50 weeks (range: 16–114 weeks) and the median duration of complete responses was 64 weeks (range: 52–114 weeks). In this population, progression-free survival at 6 months was 45% (95% CI: 31%–58%) and progression-free survival at 12 months was 29% (95% CI: 16%–42%). Median progression-free survival was 4.4 months. Overall survival at 6 months was 74% (95% CI: 62%–86%) and 12-month overall survival was 65% (95% CI: 52%–78%). Median overall survival was 15.9 months.
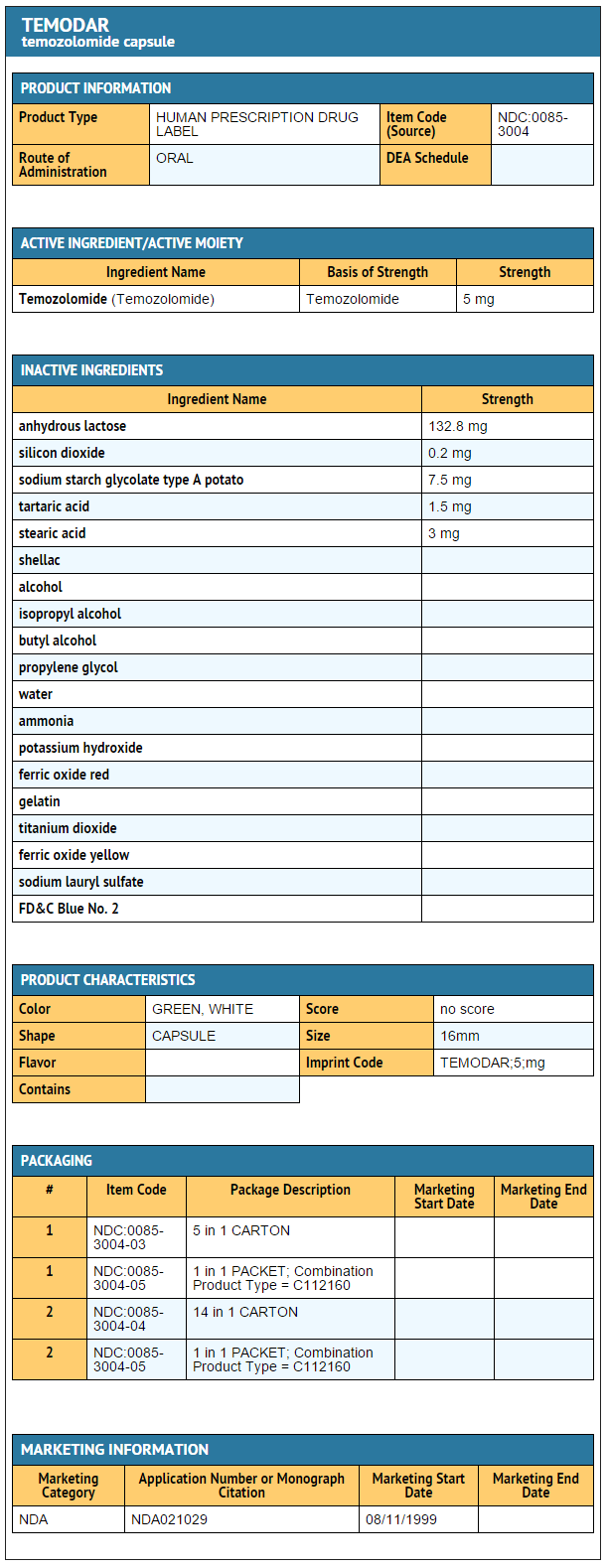
How Supplied
Temozolomide Capsules
- Temozolomide Capsules 5 mg
- 5-count – NDC 0085-3004-03
- 14-count – NDC 0085-3004-04
- Temozolomide Capsules 20 mg
- 5-count – NDC 0085-1519-03
- 14-count – NDC 0085-1519-04
- Temozolomide Capsules 100 mg:
- 5-count – NDC 0085-1366-03
- 14-count – NDC 0085-1366-04
- Temozolomide Capsules 140 mg
- 5-count – NDC 0085-1425-03
- 14-count – NDC 0085-1425-04
- Temozolomide Capsules 180 mg
- 5-count – NDC 0085-1430-03
- 14-count – NDC 0085-1430-04
- Temozolomide Capsules 250 mg
- 5-count – NDC 0085-1417-02
Temozolomide for Injection
- Temozolomide for Injection 100 mg
- NDC 0085-1381-01
Storage
- Store temozolomidecapsules at 25°C (77°F)
- Store temozolomide for Injection refrigerated at 2–8°C (36–46°F).
Images
Drug Images
{{#ask: Page Name::Temozolomide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
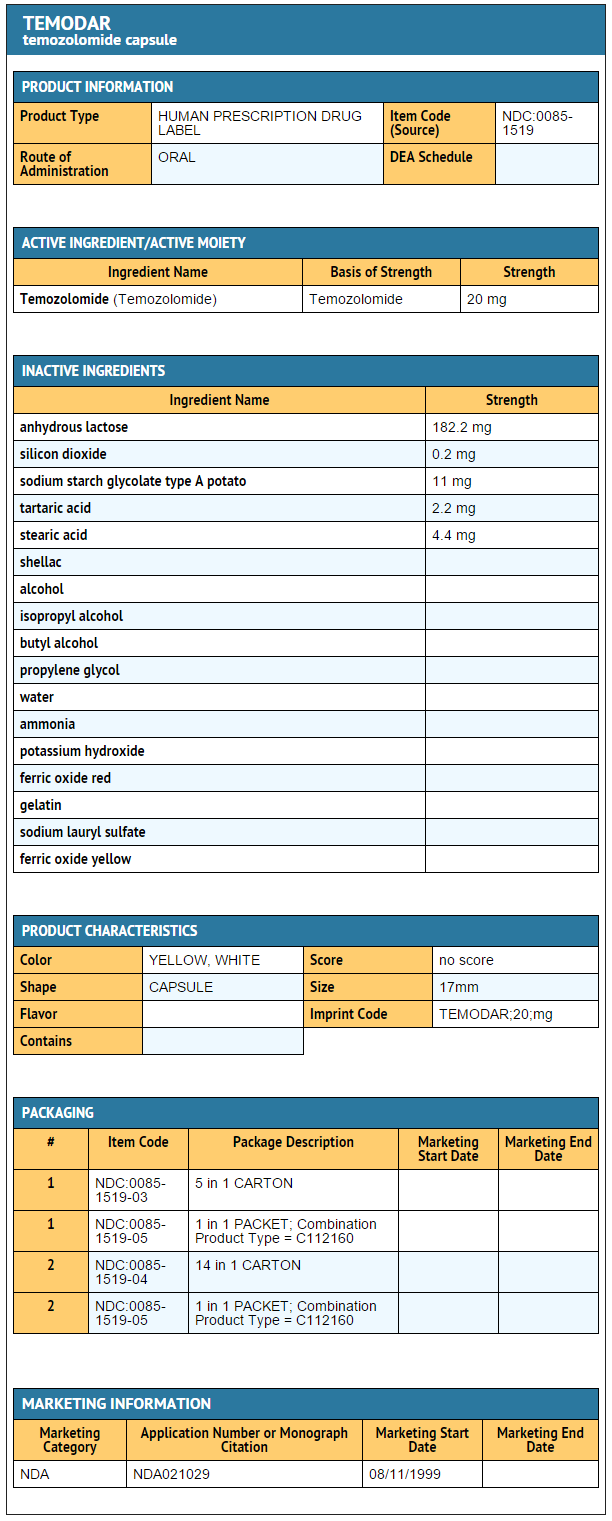

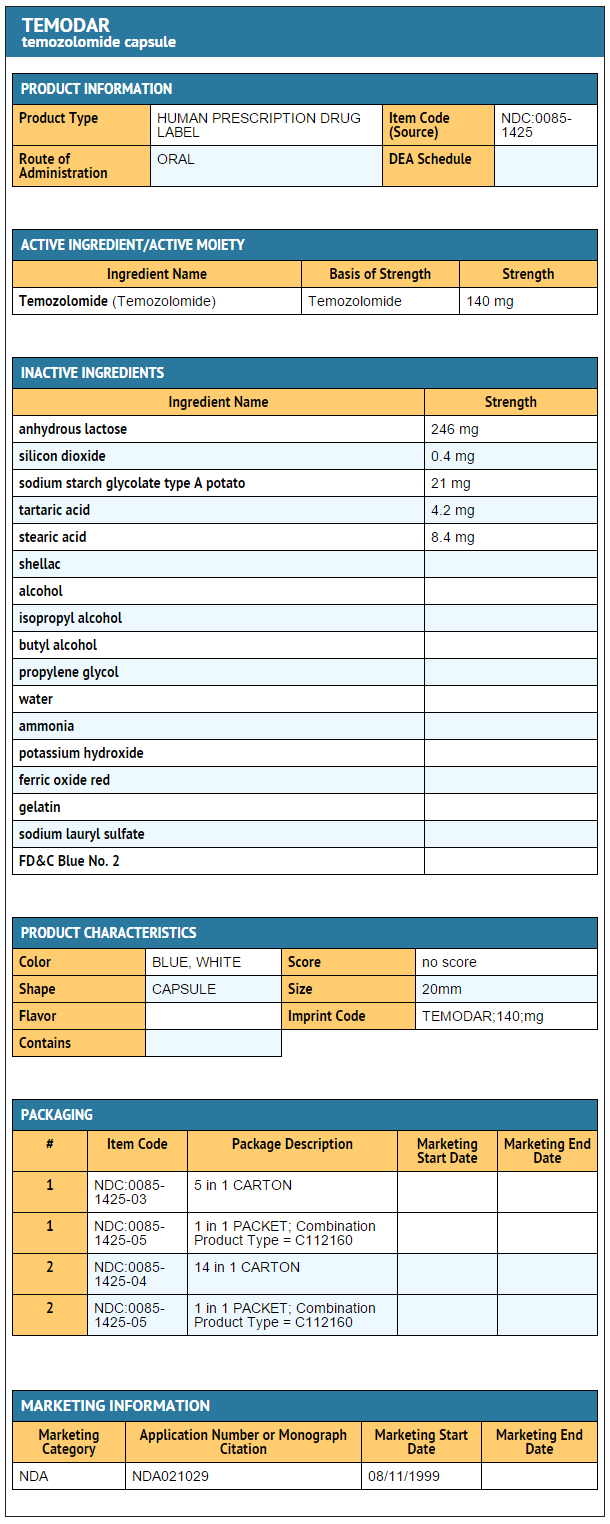
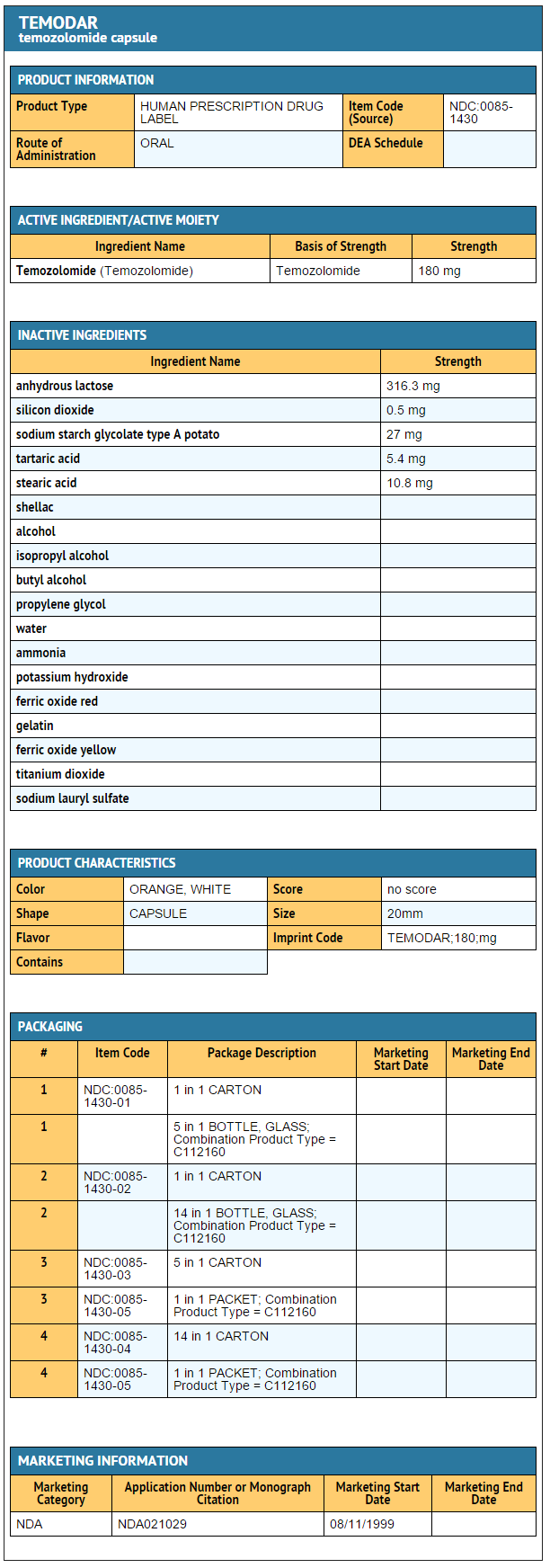
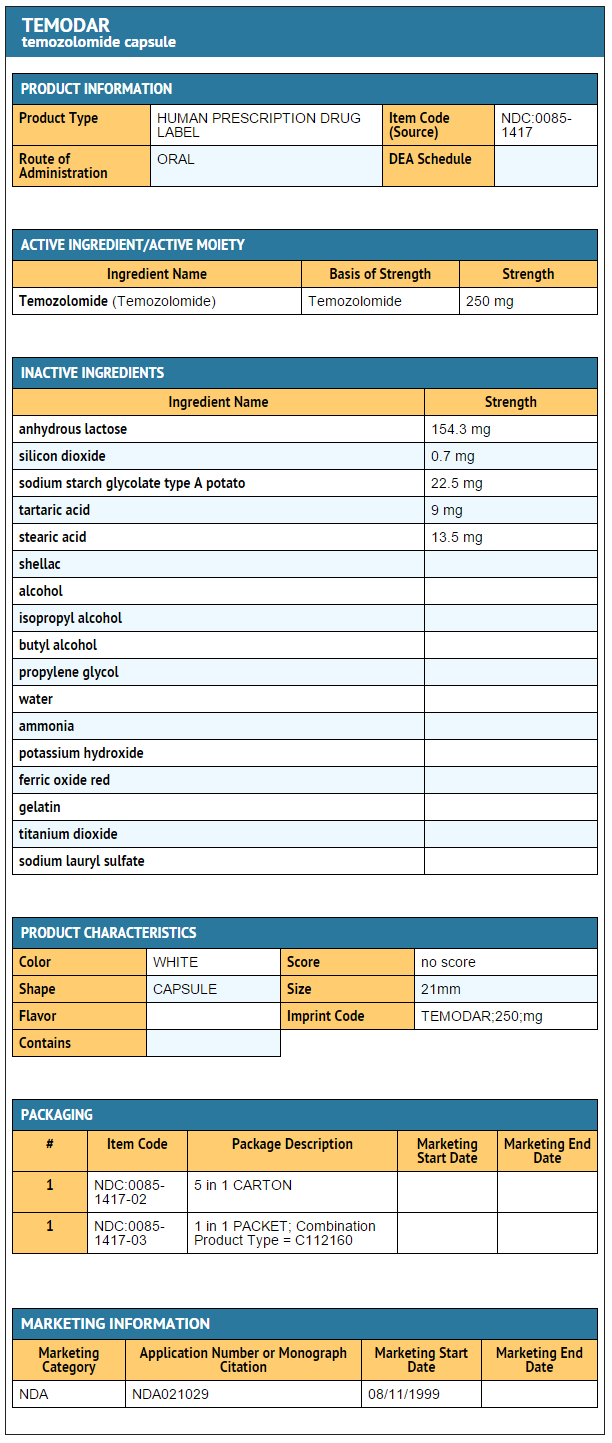
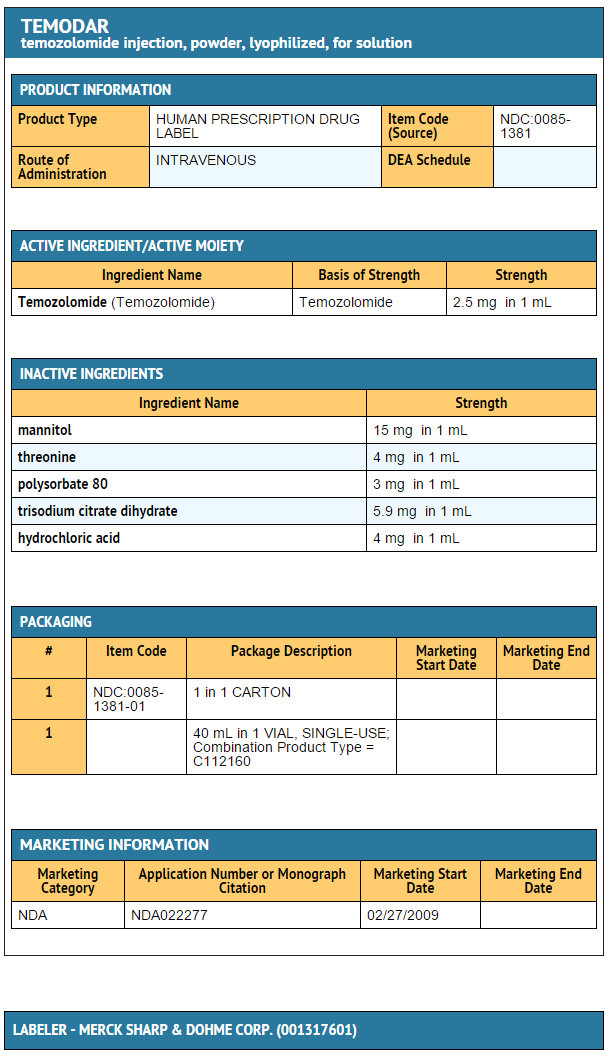
{{#ask: Label Page::Temozolomide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Physicians should discuss the following with their patients:
- Nausea and vomiting are the most frequently occurring adverse reactions. Nausea and vomiting are usually either self-limiting or readily controlled with standard antiemetic therapy.
- Capsules should not be opened. If capsules are accidentally opened or damaged, rigorous precautions should be taken with the capsule contents to avoid inhalation or contact with the skin or mucous membranes.
- The medication should be kept away from children and pets.
Precautions with Alcohol
Alcohol-Temozolomide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Temodar [1]
Look-Alike Drug Names
There is limited information regarding Temozolomide Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "FDA LABEL: TEMODAR- temozolomide capsule TEMODAR- temozolomide injection, powder, lyophilized, for solution". line feed character in
|title=at position 42 (help)
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 5mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 20mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 100mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 140mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 180mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide capsule 250mg.png
}}
{{#subobject:
|Label Page=Temozolomide |Label Name=Temozolomide for injection 100 mg.png
}}