Sandbox:Prince
Overview
Fungal meningitis results from the infection of the meninges by fungi, most commonly cryptococcus. While cryptococcal meningitis occurs worldwide, other fungal meningitis are endemic to specific regions of the world.[1] Fungal meningitis usually affects immunocompromised patients like HIV patients and transplant recipients on chronic immunosuppression medications. The course of the disease is progressive and may lead to complications if a high dose long term treatment with antifungals are not initiated.[2]
Historical perspective
The first report of human cryptococcosis was published by Busse and Busckhe more than 100 years ago; 10 years later, it was identified as the cause of human meningitis. Cryptococcus neoformans, an encapsulated basidiomycetous yeast, represents one of the most common CNS pathogens encountered in clinical practice today. In 1661, Thomas Willis first described the inflammation of meninges and an epidemic of meningitis. In 1891, Heinrich Quincke provided an early analysis of CSF by introducing a new technique of lumbar puncture. Some specific historical feature usually surround the outbreak of rare causes of fungal meningitis. For example, outbreaks of fungal meningitis in 2002 with Exophiala dermatitidis and more recently in 2012 with Exserohilum rostratum were associated with contamination of compounded corticosteroids with resultant severe complications.[3][4][5] The 1980s witnessed an increase in the number of cases of cryptococcus meningitis in the United States and certain African countries largely due to HIV infection
Classification
Fungal meningitis is usually classified according to the causative organism if identified. It may also be additionally classified according to the severity and duration of the disease as mild, moderate, severe, acute, subacute, chronic and recurrent fungal meningitis.[6][7][8][9][10][11][12][13][14]
Pathophysiology
The pathophysiology of fungal meningitis is not very well studied however, it is known to have a lot of similarities with bacterial meningitis. Fungal meningitis usually occurs in immunocompromised patients. The initial step in fungal meningitis is the pulmonary exposure to the fungi by the inhalation of airborne fungal spores. The pulmonary infection is usually self limited and maybe asymptomatic. Fungal infections are not contagious so they do not spread from one person to another.With an associated impaired immune response the fungus may disseminate. For instance in cryptococcal infection, the fungus may remain dormant in the lungs until the immune system weakens and then can reactivate and disseminate to the CNS. Cryptococcus has predilection for CNS dessimination. Although this remains unclear, the presence of a receptor on glial cells for a ligand on the organism has been suggested to enhance its invasion.[15] Cryptococcal meningitis is most common due to the virulence factors of the organism that enhancing invasion of the blood brain barrier. These factors include: polysaccharide capsule which makes the organism withstand phagocytosis and host immune system, melanin production, ability to thrive at mammalian body temperatures, urease production and phospholipase production.[16][17][18][19][20][21][22][23][24] Once the fungi cross the blood brain barrier they cause an inflammation of the meninges and arachnoid space. The inflammation promotes cytokine release mainly tumor necrosis factor (TNF), interleukin 1, interleukin 2 , interleukin 6, interleukin 12, colony-stimulating factors, and interferon-λ.[25][26][27] The cytokines lead to modulation of host system resuting in fever, increase in the permeability of the blood brain barrier and subsequent cerebral edema and increase in the intracranial pressure. The increase in the permeability of the blood brain barrier is the cause of the observed elevation of the protein level in the cerebral spinal fluid.[28]
Causes
Fungal meningitis is initially caused by the inhalation of airborne fungal spores. The pulmonary infection is usually self limiting and can be asymptomatic. The most common cause of fungal infection is cryptococcus neoformans which is usually found in soil and bird excreta.[29] Other common causes of fungal meningitis include; Aspergillus spp., Blastomyces dermatitidis, Coccidioides immitis, Candida spp., Histoplasma capsulatum and Sporothrix schenckii.[30][31][32][2][33] Rarely, fungal meningitis may be caused by Xylohypha (formerly Cladosporium) trichoides, Curvularia, Mucor, Arthrographis kalrae, Pneumocystis jirovecii,[34][35] Cryptococcus albidus,[36], Alternaria spp,[37], Rhodotorula spp, [38] Acremonium spp,[39] Dreschlera spp,[40] Malassezia spp,[41] Scedosporium spp,[42] Arthrographis spp,[43] Blastoschizomyces,[44][45] Paecilomyces,[46][47] Aureobasidium,[48] Clavispora,[49] Ustilago,[50] Exophiala (Wangiella)[51] and Exserohilum[5]
Differentiating fungak meningitis from other Conditions
The differential diagnosis of fungal meningitis includes a range of medical conditions that can be broadly classified into infectious and non infectious. The cerebrospinal fluid analysis and radiological findings help distinguishing fungal meningitis from other causes of meningitis example bacterial meningeal infection, protozoal meningeal infection, viral meningeal infection, and non infectious causes.
Epidemiology and Demographics
While cryptococcus and candida infections occur worldwide, other fungal infections tend to cluster in specific geographical regions.[52][2] There is an increasing trend of fungal meningitis. This has been attributed to enlarging population of high-risk immunosuppressed patients, more successful pharmacological immunosuppression and chemotherapies, increase in numbers of patients living with human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS), migration of susceptible persons into hyperendemic areas and aging of the population.[2][53] Cryptococcal meningitis occurs worldwide but it is highly prevalent in southeast Asia and southern and east Africa where the prevalence of HIV is high.[28] The incidence of cryptococcal meningitis is almost the same as in meningococcal meningitis[54] with an incidence of one case per 100,000 persons.[54] Prior to the introduction of highly active antiretroviral therapy (HAART) in the United States, yearly incidence rate of cryptococcal meningitis was on ascendancy with incidence of 6600 cases per 100,000 persons with AIDS[55] The incidence has decreased with the advent of HAART [56] although cases are still reported.[57] The worldwide incidence of cryptococcal meningitis is pegged at 1,000,000 annually according to an estimate by Centers for Disease Control and Prevention CDC in 2009[58] with approximately half of these resulting in death.[58] The prevalence of fungal meningitis does not vary with gender.[59] Non-Caucasian race have a higher prediclection to developing fungal meningitis especially coccidioidal meningitis[60] The prevalence of fungal meningitis does not vary with age.[59] The major factor accounting for age predilection has to do with the clinical state of the patient and the immune response. For example candida meningitis may occur in older children[61][62] and in adults with neutropenia, often presenting with brain abscesses rather than meningitis.[63] Children with certain conditions have higher incidence of fungal meningitis example myeloperoxidase deficiency,[64][65] chronic granulomatous disease of childhood[66][67] and chronic mucocutaneous candidiasis.[68]
Screening
It is recommended that patients with CD4 counts ≤ 100 cells/μl, should have routine cryptococcal antigen screening. Patients with positive result are offered preemptive anti-fungal therapy.[69][70]
Risk Factors
Fungal meningitis rarely occurs in otherwise healthy individuals. Co-existing medical conditions, immunosuppression and travel history to areas where specific fungi are endemic are risk factors for fungal meningitis. [30][31][32][2][53][71][72][73]
Natural History, Complications and Prognosis
If left untreated, neurological complications might occur. Fungal meningitis may be complicated by abscesses, bone invasion, fluid collections, neurological deficits, ocular invasion, papilledema, neurodevelopmental delays in children, and seizures[74][75][76][77][78] The mortality associated with fungal meningitis is high. Better prognosis is associated with early diagnosis, early initiation of the treatment and compliance of patients with medications.
Diagnosis
History and Symptoms
Fungal meningitis can occur in two main clinical pictures: subacute meningitis and chronic meningitis. Chronic meningitis is characterized by the presence of symptoms for more than four weeks. Commonly, patients with fungal meningitis have a history of one or more of the following chronic sinusitis, Granulocytopenia, chronic renal failure, Diabetes, Pregnancy, persons with diabetes, IV drug abuse, prolonged intravenous therapy, exposure history example travel to or residence inendemic regions of the pathogen, immune suppression examples systemic neoplasia, organ transplantation, Human immunodeficiency virus (HIV) / acquired immunodeficiency syndrome (AIDS), water aspiration as in a case of Pseudoallescheria boydii and traumatic inoculation as in a case of Sporothrix schenckii.[13][52][30][31][32][2][53][71][72][73][79]On presentation patients may have the following symptoms, Headache, Neck stiffness, fever, Fatigue, Night sweats, cranial nerves involvement,[28] Hydrocephalus, cranial neuropathy, Radiculopathy and cognitive and personality changes.[28]
Physical Examination
As in the case of any disease, a complete physical exam must be done on the patient looking for positive and negative symptoms. The clinical presentation of fungal meningitis is usually obscure as are the findings on physical exam. The pertinent findings are low grade fever and possible neurological signs like focal weakness, loss of sensation and cranial nerves involvement. Physical exam findings, including presence of rashes, lymphadenopathy, hepatomegaly, pulmonary disease, ocular pathology (eg, endophthalmitis, vitritis, chorioretinitis, uveitis, optic nerve involvement), and cranial nerve (CN) palsies, may narrow the differential. Papilledema and abducens nerve palsy suggest the presence of increased intracranial pressure (ICP). Kernig's sign and brudzinski's sign are not typically present in fungal meningitis.
Laboratory Findings
A lumbar puncture is essential for the diagnosis of fungal meningitis and initiation of the appropriate treatment. The cerebrospinal fluid (CSF) of a patient having fungal meningitis is distinguished by the presence of lymphocytosis, low glucose level and high proteins level. Specific CSF stains and cultures as well as serologies help in determining the specific nature of the causative fungi. Biopsy of skin lesions, chest radiography, ophthalmologic examination, computed tomography or MRI of the brain, in addition to cultures of CSF, blood, and sputum, may provide essential diagnostic clues.
CT
The diagnosis of fungal meningitis mainly relies on the results of the cerebrospinal fluid (CSF) analysis, stain and culture. The role of imaging is to rule out other differential diagnosis of the initial presentation. In addition, brain imaging must be done when the patient has signs of increased intracranial pressure to prevent brain herniation.
MRI
The diagnosis of fungal meningitis mainly relies on the results of the cerebrospinal fluid (CSF) analysis, stain and culture. The role of imaging is to rule out other differential diagnosis of the initial presentation. In addition, brain imaging must be done when the patient has signs of increased intracranial pressure to prevent brain herniation. Also, MRI can detect meningeal enhancement, tumors and para-meningeal infections (brain abscess).[28]
Treatment
Fungal meningitis, such as cryptococcal meningitis, is treated with long courses of high dose antifungals. In addition, frequent lumbar punctures are recommended in order to relieve the increased intracranial pressure[80].
References
- ↑ Bovers M, Hagen F, Kuramae EE, Diaz MR, Spanjaard L, Dromer F; et al. (2006). "Unique hybrids between the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii". FEMS Yeast Res. 6 (4): 599–607. doi:10.1111/j.1567-1364.2006.00082.x. PMID 16696655.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Gottfredsson M, Perfect JR (2000). "Fungal meningitis". Semin Neurol. 20 (3): 307–22. doi:10.1055/s-2000-9394. PMID 11051295.
- ↑ Invalid
<ref>tag; no text was provided for refs namedpmid12532960 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid23252499 - ↑ 5.0 5.1 Pettit AC, Pugh ME (2013). "Index case for the fungal meningitis outbreak, United States". N Engl J Med. 368 (10): 970. doi:10.1056/NEJMc1300630. PMID 23465119.
- ↑ Zheng H, Chen Q, Xie Z, Wang D, Li M, Zhang X; et al. (2016). "A retrospective research of HIV-negative cryptococcal meningoencephalitis patients with acute/subacute onset". Eur J Clin Microbiol Infect Dis. 35 (2): 299–303. doi:10.1007/s10096-015-2545-0. PMID 26792138.
- ↑ Zunt JR, Baldwin KJ (2012). "Chronic and subacute meningitis". Continuum (Minneap Minn). 18 (6 Infectious Disease): 1290–318. doi:10.1212/01.CON.0000423848.17276.21. PMID 23221842.
- ↑ Chimalizeni Y, Tickell D, Connell T (2010). "Evidence behind the WHO guidelines: hospital care for children: what is the most appropriate anti-fungal treatment for acute cryptococcal meningitis in children with HIV?". J Trop Pediatr. 56 (1): 4–12. doi:10.1093/tropej/fmp123. PMID 20097705.
- ↑ Malessa R, Krams M, Hengge U, Weiller C, Reinhardt V, Volbracht L; et al. (1994). "Elevation of intracranial pressure in acute AIDS-related cryptococcal meningitis". Clin Investig. 72 (12): 1020–6. PMID 7711408.
- ↑ Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK; et al. (1992). "Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group". N Engl J Med. 326 (2): 83–9. doi:10.1056/NEJM199201093260202. PMID 1727236.
- ↑ Sloan D, Dlamini S, Paul N, Dedicoat M (2008). "Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings". Cochrane Database Syst Rev (4): CD005647. doi:10.1002/14651858.CD005647.pub2. PMID 18843697.
- ↑ Witt MD, Lewis RJ, Larsen RA, Milefchik EN, Leal MA, Haubrich RH; et al. (1996). "Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing". Clin Infect Dis. 22 (2): 322–8. PMID 8838190.
- ↑ 13.0 13.1 Morgand M, Rammaert B, Poirée S, Bougnoux ME, Tran H, Kania R; et al. (2015). "Chronic Invasive Aspergillus Sinusitis and Otitis with Meningeal Extension Successfully Treated with Voriconazole". Antimicrob Agents Chemother. 59 (12): 7857–61. doi:10.1128/AAC.01506-15. PMC 4649149. PMID 26392507.
- ↑ Banarer M, Cost K, Rychwalski P, Bryant KA (2005). "Chronic lymphocytic meningitis in an adolescent". J Pediatr. 147 (5): 686–90. doi:10.1016/j.jpeds.2005.07.010. PMID 16291364.
- ↑ Invalid
<ref>tag; no text was provided for refs namedpmid8483058 - ↑ Granger DL, Perfect JR, Durack DT (1985). "Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide". J Clin Invest. 76 (2): 508–16. doi:10.1172/JCI112000. PMC 423853. PMID 3928681.
- ↑ Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK; et al. (2012). "Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection". J Biol Chem. 287 (19): 15298–306. doi:10.1074/jbc.M112.353375. PMC 3346080. PMID 22418440.
- ↑ Kwon-Chung KJ, Rhodes JC (1986). "Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans". Infect Immun. 51 (1): 218–23. PMC 261090. PMID 3079732.
- ↑ Polacheck I, Platt Y, Aronovitch J (1990). "Catecholamines and virulence of Cryptococcus neoformans". Infect Immun. 58 (9): 2919–22. PMC 313587. PMID 2117574.
- ↑ Jacobson ES, Emery HS (1991). "Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans". J Bacteriol. 173 (1): 401–3. PMC 207201. PMID 1898925.
- ↑ Jacobson ES, Tinnell SB (1993). "Antioxidant function of fungal melanin". J Bacteriol. 175 (21): 7102–4. PMC 206840. PMID 8226653.
- ↑ Chang YC, Kwon-Chung KJ (1994). "Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence". Mol Cell Biol. 14 (7): 4912–9. PMC 358863. PMID 8007987.
- ↑ Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR (2000). "Urease as a virulence factor in experimental cryptococcosis". Infect Immun. 68 (2): 443–8. PMC 97161. PMID 10639402.
- ↑ Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC; et al. (2001). "Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans". Mol Microbiol. 39 (1): 166–75. PMID 11123698.
- ↑ Invalid
<ref>tag; no text was provided for refs namedpmid7682573 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid8014494 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid3110308 - ↑ 28.0 28.1 28.2 28.3 28.4 Roos KL, Tyler KL. Chapter 381. Meningitis, Encephalitis, Brain Abscess, and Empyema. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012.
- ↑ Koroshetz WJ. Chapter 382. Chronic and Recurrent Meningitis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012.
- ↑ 30.0 30.1 30.2 Chiller TM, Galgiani JN, Stevens DA (2003). "Coccidioidomycosis". Infect Dis Clin North Am. 17 (1): 41–57, viii. PMID 12751260.
- ↑ 31.0 31.1 31.2 Singh N, Husain S (2000). "Infections of the central nervous system in transplant recipients". Transpl Infect Dis. 2 (3): 101–11. PMID 11429020.
- ↑ 32.0 32.1 32.2 Rosenstein NE, Emery KW, Werner SB, Kao A, Johnson R, Rogers D; et al. (2001). "Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996". Clin Infect Dis. 32 (5): 708–15. doi:10.1086/319203. PMID 11229838.
- ↑ del Brutto OH (2000). "[Central nervous system mycotic infections]". Rev Neurol. 30 (5): 447–59. PMID 10775973.
- ↑ Villanueva JL, Cordero E, Caballero-Granado FJ, Regordan C, Becerril B, Pachón J (1997). "Pneumocystis carinii meningoradiculitis in a patient with AIDS". Eur J Clin Microbiol Infect Dis. 16 (12): 940–2. PMID 9495679.
- ↑ Baena Luna MR, Muñoz García J, Grancha Bertolín L, Sanz García M (1998). "[Presence of Pneumocystis carinii in cerebrospinal fluid]". An Med Interna. 15 (5): 265–6. PMID 9629775.
- ↑ Melo JC, Srinivasan S, Scott ML, Raff MJ (1980). "Cryptococcus albidus meningitis". J Infect. 2 (1): 79–82. PMID 7185917.
- ↑ OHASHI Y (1960). "On a rare disease due to Alternaria tenuis Nees (alternariasis)". Tohoku J Exp Med. 72: 78–82. PMID 13730495.
- ↑ Shinde RS, Mantur BG, Patil G, Parande MV, Parande AM (2008). "Meningitis due to Rhodotorula glutinis in an HIV infected patient". Indian J Med Microbiol. 26 (4): 375–7. PMID 18974495.
- ↑ Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ (1991). "Infection due to the fungus Acremonium (cephalosporium)". Medicine (Baltimore). 70 (6): 398–409. PMID 1956281.
- ↑ Fuste FJ, Ajello L, Threlkeld R, Henry JE (1973). "Drechslera hawaiiensis: causative agent of a fatal fungal meningo-encephalitis". Sabouraudia. 11 (1): 59–63. PMID 4739938.
- ↑ Rosales CM, Jackson MA, Zwick D (2004). "Malassezia furfur meningitis associated with total parenteral nutrition subdural effusion". Pediatr Dev Pathol. 7 (1): 86–90. doi:10.1007/s10024-003-4030-5. PMID 15255040.
- ↑ Symoens F, Knoop C, Schrooyen M, Denis O, Estenne M, Nolard N; et al. (2006). "Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation". J Heart Lung Transplant. 25 (5): 603–7. doi:10.1016/j.healun.2005.12.011. PMID 16678041.
- ↑ Chin-Hong PV, Sutton DA, Roemer M, Jacobson MA, Aberg JA (2001). "Invasive fungal sinusitis and meningitis due to Arthrographis kalrae in a patient with AIDS". J Clin Microbiol. 39 (2): 804–7. doi:10.1128/JCM.39.2.804-807.2001. PMC 87827. PMID 11158158.
- ↑ Girmenia C, Micozzi A, Venditti M, Meloni G, Iori AP, Bastianello S; et al. (1991). "Fluconazole treatment of Blastoschizomyces capitatus meningitis in an allogeneic bone marrow recipient". Eur J Clin Microbiol Infect Dis. 10 (9): 752–6. PMID 1810730.
- ↑ Naficy AB, Murray HW (1990). "Isolated meningitis caused by Blastoschizomyces capitatus". J Infect Dis. 161 (5): 1041–2. PMID 2324536.
- ↑ Kantarcioğlu AS, Hatemi G, Yücel A, De Hoog GS, Mandel NM (2003). "Paecilomyces variotii central nervous system infection in a patient with cancer". Mycoses. 46 (1–2): 45–50. PMID 12588483.
- ↑ Fagerburg R, Suh B, Buckley HR, Lorber B, Karian J (1981). "Cerebrospinal fluid shunt colonization and obstruction by Paecilomyces variotii. Case report". J Neurosurg. 54 (2): 257–60. doi:10.3171/jns.1981.54.2.0257. PMID 7192726.
- ↑ Kutleša M, Mlinarić-Missoni E, Hatvani L, Voncina D, Simon S, Lepur D; et al. (2012). "Chronic fungal meningitis caused by Aureobasidium proteae". Diagn Microbiol Infect Dis. 73 (3): 271–2. doi:10.1016/j.diagmicrobio.2012.03.007. PMID 22504065.
- ↑ Krcmery V, Mateicka F, Grausova S, Kunova A, Hanzen J (1999). "Invasive infections due to Clavispora lusitaniae". FEMS Immunol Med Microbiol. 23 (1): 75–8. PMID 10030550.
- ↑ MOORE M, RUSSELL WO, SACHS E (1946). "Chronic leptomeningitis and ependymitis caused by Ustilago, probably U. zeae (corn smut)". Am J Pathol. 22: 761–77. PMID 20991975.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy--United States, July-November 2002". MMWR Morb Mortal Wkly Rep. 51 (49): 1109–12. PMID 12530707.
- ↑ 52.0 52.1 Shankar SK, Mahadevan A, Sundaram C, Sarkar C, Chacko G, Lanjewar DN; et al. (2007). "Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario". Neurol India. 55 (3): 198–215. PMID 17921648.
- ↑ 53.0 53.1 53.2 Fraser DW, Ward JI, Ajello L, Plikaytis BD (1979). "Aspergillosis and other systemic mycoses. The growing problem". JAMA. 242 (15): 1631–5. PMID 480580.
- ↑ 54.0 54.1 Invalid
<ref>tag; no text was provided for refs namedpmid8654513 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid12627365 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid11125898 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid10930155 - ↑ 58.0 58.1 Invalid
<ref>tag; no text was provided for refs namedpmid19182676 - ↑ 59.0 59.1 Invalid
<ref>tag; no text was provided for refs namedpmid20375357 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid7231152 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid10066050 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid10987704 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid9810800 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid6834633 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid8381226 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid7195647 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid3706396 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid8160723 - ↑ Cassim N, Schnippel K, Coetzee LM, Glencross DK (2017). "Establishing a cost-per-result of laboratory-based, reflex Cryptococcal antigenaemia screening (CrAg) in HIV+ patients with CD4 counts less than 100 cells/μl using a Lateral Flow Assay (LFA) at a typical busy CD4 laboratory in South Africa". PLoS One. 12 (2): e0171675. doi:10.1371/journal.pone.0171675. PMID 28166254.
- ↑ Greene G, Sriruttan C, Le T, Chiller T, Govender NP (2017). "Looking for fungi in all the right places: screening for cryptococcal disease and other AIDS-related mycoses among patients with advanced HIV disease". Curr Opin HIV AIDS. 12 (2): 139–147. doi:10.1097/COH.0000000000000347. PMID 28134711.
- ↑ 71.0 71.1 Perfect JR, Lang SD, Durack DT (1980). "Chronic cryptococcal meningitis: a new experimental model in rabbits". Am J Pathol. 101 (1): 177–94. PMC 1903580. PMID 7004196.
- ↑ 72.0 72.1 Perfect JR, Durack DT (1985). "Chemotactic activity of cerebrospinal fluid in experimental cryptococcal meningitis". Sabouraudia. 23 (1): 37–45. PMID 3992427.
- ↑ 73.0 73.1 Perfect JR, Durack DT (1985). "Effects of cyclosporine in experimental cryptococcal meningitis". Infect Immun. 50 (1): 22–6. PMC 262129. PMID 3899932.
- ↑ John Marx. Chapter 107. Central Nervous System Infections. Marx: Rosen's Emergency Medicine, 7th ed. Mosby: Elsevier; 2009.
- ↑ Farrugia MK, Fogha EP, Miah AR, Yednock J, Palmer HC, Guilfoose J (2016). "Candida meningitis in an immunocompetent patient detected through (1→3)-beta-d-glucan". Int J Infect Dis. 51: 25–26. doi:10.1016/j.ijid.2016.08.020. PMID 27590564.
- ↑ Nyazika TK, Hagen F, Machiridza T, Kutepa M, Masanganise F, Hendrickx M; et al. (2016). "Cryptococcus neoformans population diversity and clinical outcomes of HIV-associated cryptococcal meningitis patients in Zimbabwe". J Med Microbiol. 65 (11): 1281–1288. doi:10.1099/jmm.0.000354. PMID 27638836.
- ↑ Leonhard SE, Fritz D, van de Beek D, Brouwer MC (2016). "Cryptococcal meningitis complicating sarcoidosis". Medicine (Baltimore). 95 (35): e4587. doi:10.1097/MD.0000000000004587. PMC 5008555. PMID 27583871.
- ↑ Neo WL, Durisala N, Ho EC (2016). "Reversible hearing loss following cryptococcal meningitis: case study". J Laryngol Otol. 130 (7): 691–5. doi:10.1017/S002221511600801X. PMID 27210482.
- ↑ Mody CH, Toews GB, Lipscomb MF (1988). "Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model". Infect Immun. 56 (1): 7–12. PMC 259224. PMID 3275587.
- ↑ Bicanic T, Harrison TS (2004). "Cryptococcal meningitis". Br Med Bull. 72: 99–118. doi:10.1093/bmb/ldh043. PMID 15838017.
| Peritonsillar abscess | |
| ICD-10 | J36 |
|---|---|
| ICD-9 | 475 |
| DiseasesDB | 11141 |
| eMedicine | emerg/417 |
|
WikiDoc Resources for Sandbox:Prince |
|
Articles |
|---|
|
Most recent articles on Sandbox:Prince Most cited articles on Sandbox:Prince |
|
Media |
|
Powerpoint slides on Sandbox:Prince |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sandbox:Prince at Clinical Trials.gov Trial results on Sandbox:Prince Clinical Trials on Sandbox:Prince at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sandbox:Prince NICE Guidance on Sandbox:Prince
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sandbox:Prince Discussion groups on Sandbox:Prince Patient Handouts on Sandbox:Prince Directions to Hospitals Treating Sandbox:Prince Risk calculators and risk factors for Sandbox:Prince
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sandbox:Prince |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Kiran Singh, M.D. [2] Prince Tano Djan, BSc, MBChB [3]
Synonyms and keywords: PTA, tonsillar abscess, intratonsillar abscess
Overview
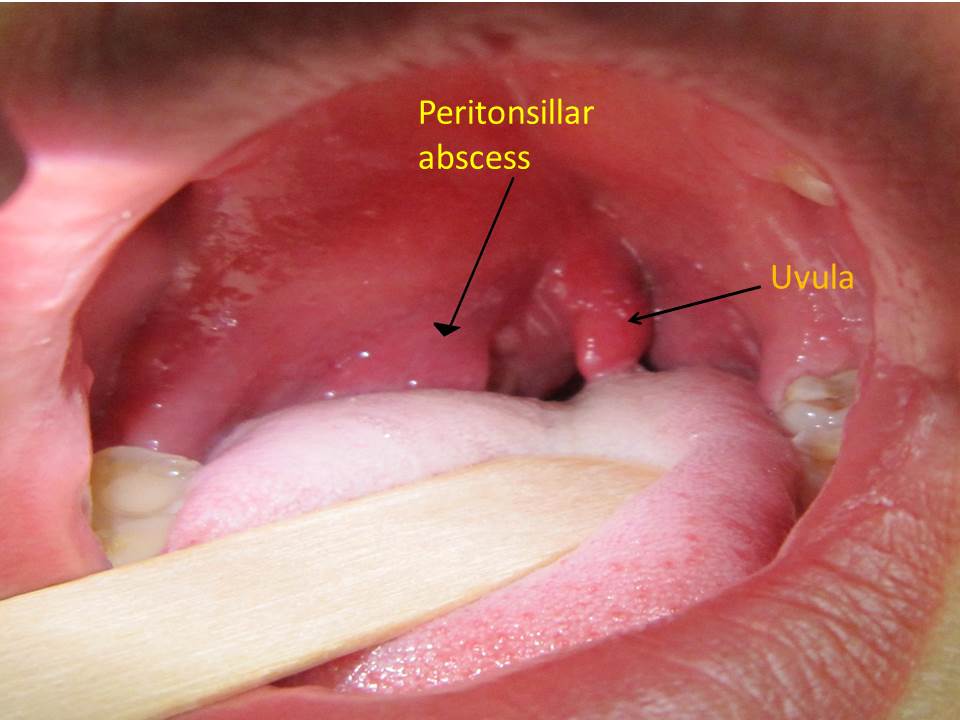
Peritonsillar abscess (PTA), also commonly referred to as quinsy, is defined as a collection of pus located between the tonsillar capsule and the pharyngeal constrictor muscles. It is the most common deep tissue infection of the neck.[1] Historically, it has been thought of as a complication of acute tonsillitis. However, recent studies have proposed additional hypothesis surrounding its pathogenesis making the understanding of the disease a medical dilemma.[2]
Historical perspective
The outline below shows the historical perspective of peritonsillar abscess.[3]
- In second and third century BC, Celcius was the first to document in literature the treatment and pathogenesis of tonsillar pathology.
- In 1700s peritonsillar abscess was first described.
- In the 1930s and 1940s prior to the advent of antibiotics, surgical management was the most common treatment option for peritonsillar abscess. Interval tonsillectomy was mostly done after symptom resolution.
- By 1947, Chaud tonsillectomy or immediate surgical tonsillectomy became the treatment option.
Classification
On the basis of computed tomographical findings, peritonsillar abscess may be classified into 3 broad categories based on the following:
1. Shape of the abscess
On the basis of shape it may be classified as:[4]
- Oval type or
- Cap type
2. Location of the abscess
On the basis of abscess location it may be differentiated into the following:[4]
- Superior or
- Inferior
3. Shape and location
On the basis of shaped and location it may be classified as:[4]
Pathophysiology
Anatomy
A good understanding of the tonsil and its surrounding space is important in the pathogenesis of peritonsillar abscess. The palatine tonsils are found in an anatomical structure called tonsillar fossa. This fossa is bounded anteriorly by palatoglossal muscle, posteriorly by palatopharyngeal muscle, laterally by a fibrous capsule and tonsillar crypts medially. Contents of the tonsillar crypts are expelled by contraction of the tonsillopharyngeus muscle.[5] The tonsils form during the last months of pregnancy and becomes fully formed by 6 to 7 years of age. It then undergoes involution until small size remains in older population. Located within the soft palate is the supratonsillar space occupied by series of 20 to 25 salivary glands described as Weber's glands. The ducts of these glands form a common duct which opens onto the posterior surface of the tonsil after passing through the tonsillar capsule. It is proposed that the secretions from these glands play a rule in food digestion. Peritonsillar abscesses form in the area between the palatine tonsil and its capsule.
Pathogenesis
The pathogenesis of peritonsillar abscess is still not well-understood.[2] There are two proposed theories believed to be involved in the pathogensis of peritonsillar abscess formation.[5][3][6][7]
- 1. It is proposed to arise from an extension of exudative tonsillitis.
Some authorities believe that blockage of drainage from tonsillar crypt in acute tonsillitis results in spread of infection into the peritonsillar space.
- 2. Involvement of Weber's gland account for the abscess formation. Some believe that peritonsillar abscess arises from infectious process involving group of salivary glands called Weber's glands located in the supratonsillar space.
Antigenic response following any disturbance arising from within the tonsillar crypt mucosa allows for lymphocytic interaction. This disruption in the crypt epithelium may be preceded by infectious process. Invasion and proliferation of the tonsillar crypt by infectious pathogens results in localized edema and influx of neutrophils. This is clinically seen as inflamed tonsil with or without exudation.[5] Pus accumulation within tissue behind the supratonsillar space leads to tonsillar bulging, uvula and palate deviation.
Causes
Peritonsillar abscess (PTA) usually arises as a complication of an untreated or partially treated episode of acute tonsillitis. The infection, in these cases, spreads to the peritonsillar area (peritonsillitis). This region comprises of loose connective tissue and is hence susceptible to formation of abscess. Peritonsilar abscess can also occur de novo. Both aerobic and anaerobic bacteria can be causative.[8][8]
Life-threatening causes
Life-threatening conditions may result in death or permanent disability within 24 hours if left untreated. Peritonsillar abscess may become a life-threatening condition and must be treated as such irrespective of the cause.[9][8]
Most common cause
The most frequent pathogen of peritonsillar abscess is Streptococcus pyogenes.[9][8][10][11]
Common causes
Some common causes of peritonsillar abscess include:[9][8]
- Fusobacterium necrophorum
- Streptococcus milleri
- Staphylococci
- Haemophilus
- Fusobacterium
- Prevotella
- Acinetobacter spp.
- Candida albicans
- Peptostreptococcus spp.
- Pseudomonas spp.
- Enterobacter spp.
- Klebsiella
Less common causes
Less common causes of peritonsillar abscess include:[9][8]
Differentiating Peritonsillar abscess from Other Diseases
| Disease/Variable | Presentation | Causes | Physical exams findings | Age commonly affected | Imaging finding | Treatment |
|---|---|---|---|---|---|---|
| Peritonsillar abscess | Severe sore throat, otalgia fever, a "hot potato" or muffled voice, drooling, and trismus[1] | Aerobic and anaerobic | Contralateral deflection of the uvula,
the tonsil is displaced inferiorly and medially, tender submandibular and anterior cervical lymph nodes, tonsillar hypertrophy with likely peritonsillar edema. |
The highest occurrence is in adults between 20 to 40 years of age.[1] | On ultrasound peritonsillar abscess appears as focal irregularly marginated hypoechoic area.[12][13][14][15][12][13] | Ampicillin-sulbactam, Clindamycin, Vancomycin or Linezolid |
| Croup | Has cough and stridor but no drooling. Others are Hoarseness, Difficulty breathing, symptoms of the common cold, Runny nose, Fever | Parainfluenza virus | Suprasternal and intercostal indrawing,[16] Inspiratory stridor[17], expiratory wheezing,[17] Sternal wall retractions[18] | Mainly 6 months and 3 years old
rarely, adolescents and adults[19] |
Steeple sign on neck X-ray | Dexamethasone and nebulised epinephrine |
| Epiglottitis | Has stridor and drooling but no cough. Other symptoms include difficulty breathing, fever, chills, difficulty swallowing, hoarseness of voice | H. influenza type b, | Cyanosis, Cervical lymphadenopathy, Inflammed epiglottis | Used to be mostly found in
pediatric age group between 3 to 5 years, however, recent trend favors adults as most commonly affected individuals[20] with a mean age of 44.94 years |
Thumbprint sign on neck x-ray | Airway maintenance, parenteral Cefotaxime or Ceftriaxone in combination with Vancomycin. Adjuvant therapy includes corticosteroids and racemic Epinephrine.[21][22] |
| Pharyngitis | Sore throat, pain on swallowing, fever, headache, abdominal pain, nausea and vomiting | Group A beta-hemolytic | Inflammed pharynx with or without exudate | Mostly in children and young adults,
with 50% of cases identified between the ages of 5 to 24 years.[23] |
_ | Antimicrobial therapy mainly penicillin-based and analgesics. |
| Tonsilitis | Sore throat, pain on swallowing, fever, headache, cough | Most common cause is
viral including adenovirus, coronavirus, and Second most common causes are bacterial; |
Fever, especially 100°F or higher.[25][26]Erythema, edema and Exudate of the tonsils.[27] cervical lymphadenopathy, Dysphonia.[28] | Primarily affects children
between 5 and 15 years old.[29] |
Intraoral or transcutaneous USG may show an abscess making CT scan unnecessary.[4][30][31] | Antimicrobial therapy mainly penicillin-based and analgesics with tonsilectomy in selected cases. |
| Retropharyngeal abscess | Neck pain, stiff neck, torticollis | Polymicrobial infection.
Mostly; Streptococcus pyogenes, Staphylococcus aureus and respiratory anaerobes (example; Fusobacteria, Prevotella, |
Child may be unable to open the mouth widely. May have enlarged
cervical lymph nodes and neck mass. |
Mostly between 2-4 years, but can occur in other age groups.[37][38] | On CT scan, a mass impinging on the posterior pharyngeal wall with rim enhancement is seen[39][40] | Immediate surgical drainage and antimicrobial therapy. emperic therapy involves; ampicillin-sulbactam or clindamycin. |
Epidemiology and Demographics
Prevalence and incidence
The incidence of peritonsillar abscess is highest between November to December and April to May in the northern hemisphere. This has been associated with the highest rates of streptococcal pharyngitis and exudative tonsillitis around that these times.[41][42]
Age
Peritonsillar abscess occur in all age groups. The highest occurrence is in adults between 20 to 40 years of age.[1][43][44]
Race
There is no racial predilection to developing peritonsillar abscess.
Gender
Males are more commonly affected with peritonsillar abscess than female with male to female ratio of approximately 1.4:1. However, equal male to female ratios have been reported in some studies as well.[45][46][47][48][49][50][51]
Developed and developing countries
Peritonsillar abscess has not been found to vary significantly among countries.
Risk Factors
Common risk factors in the development of peritonsillar abscess include:[52][53]
- Smoking
- Previous peritonsillar abscess episodes
- History of recurrent pharyngotonsillitis
- Poor oral hygiene
Screening
There are no screening recommendations for peritonsillar abscess.
Natural History, Complications, and Prognosis
Natural history
Peritonsillar abscess if left untreated may result in extraperitonsillar extension.[54][55]
Complications
The following are some complications that may follow peritonsillar abscess:[1][56][57][58][59]
- Extraperitonsillar spread example parapharyngeal extension, deep neck tissues and posterior mediastinum[54][55][4]
Peritonsillar abscess may spread through the deep fascia of the neck with associated rapid progression to a more serious infection.
- Airway obstruction
- Aspiration pneumonitis or lung abscess secondary to peritonsillar abscess rupture
- Hemorrhage from erosion or septic necrosis into carotid sheath
- Mediastinitis
- Poststreptococcal sequelae (e.g., glomerulonephritis, rheumatic fever) when infection is caused by Group A streptococcus
- Necrotizing fasciitis
Prognosis
The prognosis of peritonsillar abscess is good with early and appropriate treatment.[60][61][62][63]
Diagnosis
History and Symptoms
- Unlike tonsillitis, which is more common in the pediatric age group, peritonsillar abscess has a more even age spread — from children to adults.
- Drooling
- Dysphagia
- Foul smelling breath
- Fever
- Headache
- Hoarseness, muffled voice (also called hot potato voice)
- Odynophagia
- Otalgia (on the side of the abscess)
- Sore throat ( may be severe and unilateral)
- Stridor[64]
- Malaise
Physical Examination
Physical examination findings suggestive of peritonsillar abscess include the following:[1][65][3][66]
Appearance of the Patient
- They are usually acutely-ill looking.
Vital Signs
- High temperature
HEENT
- Muffled voice (also called "hot potato voice")
- Contralateral deflection of the uvula (see image below)
- The tonsil is generally displaced inferiorly and medially
- Facial swelling
- Tonsillar hypertrophy with likely Peritonsillar edema (see image below)
- Trismus
- Drooling
- Rancid or fetor breath
Image below shows edematous and inflamed tonsillar with contralacteral uvula deviation:[67]
Neck
- Tenderness of anterior neck
- Tender submandibular and anterior cervical lymph nodes
Lungs
- May be in obvious respiratory distress with flaring of ala nasi, subcostal and intercostal recessions.
- Increased respiratory rate in both children and adults
- Decreased air-entry depending of degree of airway obstruction
Extremities
Laboratory Findings
Although the diagnosis of peritonsillar abscess may be made without the use of laboratory findings, the following nonspecific laboratory findings may be seen:[2][5][3][6][7]
- Complete blood count with differential
- This usually shows leukocytosis with neutrophilic predominance
- Serum electrolytes
- This is useful too in patients presenting with dehydration
- Gram stain, culture and sensitivity for sample after abscess drainage.
- Emperic therapy should be initiated and modified accordingly when results are ready.
- A routine throat culture for group A streptococcus.
Imaging Findings
The diagnosis of peritonsillar abscess may be made without the use of imaging however, imaging options may help in differentiating peritonsillar abscess from other simialr conditions example, peritonsillar cellulitis, retropharyngeal abscess and epiglottitis.
Ultrasound
This is helpful in differentiating peritonsillar abscess from peritonsillar cellulitis as well as a guide during abscess drainage. The approach may be intraoral or submandibular.[68][14][69][70][71]
On ultrasound the following may be found:[12][13][14][15][12][13]
- Peritonsillar abscess appears as focal irregularly marginated hypoechoic area.
- Irregular hypoechoic areas within the tonsil may represent pockets of developing purulence or necrosis called intratonsillar abscesses.
- Peritonsillar cellulitis appears as enlarged tonsil (arrows) with ill-defined margins and markedly increased echogenicity of surrounding soft tissues that suggests significant inflammatory change/cellulitis.
CT scan
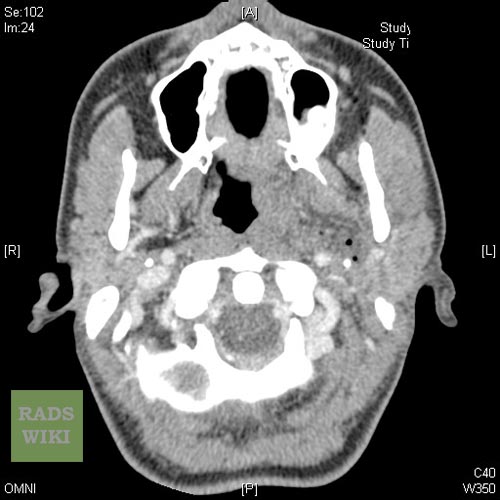
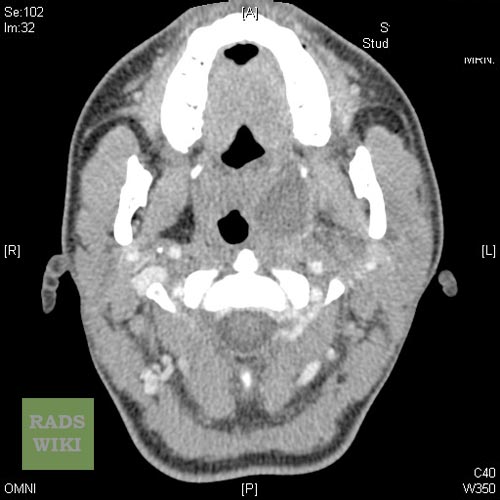
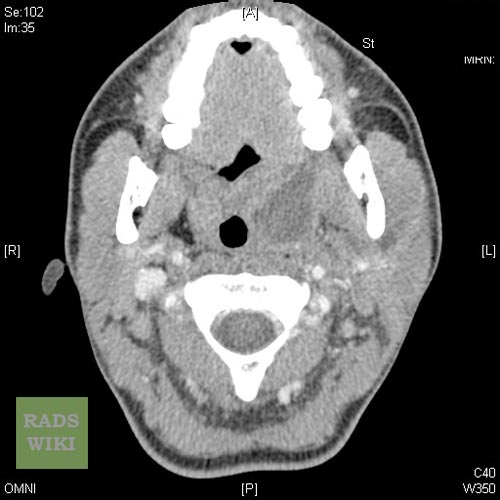
Coronal contrast-enhanced CT scan of the neck may identify the peritonsillar abscess.[14]
Treatment
Medical Therapy
Parenteral therapy is the preferred first line route of administration until the temperature of the patient has settled and clinically improved and then switched to oral therapy to complete a 14-day course.[61]
Antimicrobial Regimen
Below are the antimicrobial regimen available in treating peritonsillar abscess.[67]
- Preferred regimen in adults: Ampicillin-sulbactam 3 g IV 6h
- Preferred regimen in children: Ampicillin-sulbactam 50 mg/kg per dose [maximum single dose 3 g] IV 6h
- Alternative regimen in adults: Clindamycin 600mg IV 6-8h
- Alternative regimen in children: Clindamycin 13 mg/kg per dose [maximum single dose 900 mg] IV 8h
The above alternative therapy are employed in the following situations:
- Patients not improving on Ampicillin-sulbactam or Clindamycin
- Severe infection presenting with;
- Toxic appearance,
- Temperature >39°C,
- Drooling, and/or respiratory distress)
Pathogen-directed antimicrobial therapy
- Resistant Gram-positive cocci
For resistant Gram-positive cocci infections IV Vancomycin or Linezolid is added to the above emperic therapy.
Surgery
Surgical modalities in the management of peritonsillar abscess involve the use of the following:
Indications for tonsillectomy in peritonsillar abscess
- Severe upper respirtaory obstruction
- Previous episodes of severe recurrent pharyngitis or peritonsillar abscess
- Unresolving peritonsillar abscess after antibiotics incision and drainage
Prevention
There are no definite preventive measures for peritonsillar abscess, however, immunization against certain organisms in chikdhood may decrease the burden of peritonsillar abscess resulting from such infections.
- Immunization with the Hib vaccine protects children.[72]
- In the United states, vaccination against Hib in children was initiated in the 1980s. Immunity against Hib has been adequate with an increasing level of immunization among children.
- Post-splenectomy patients are also recommended to be immunized.[72]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Galioto NJ (2008). "Peritonsillar abscess". Am Fam Physician. 77 (2): 199–202. PMID 18246890.
- ↑ 2.0 2.1 2.2 Powell EL, Powell J, Samuel JR, Wilson JA (2013). "A review of the pathogenesis of adult peritonsillar abscess: time for a re-evaluation". J Antimicrob Chemother. 68 (9): 1941–50. doi:10.1093/jac/dkt128. PMID 23612569.
- ↑ 3.0 3.1 3.2 3.3 Passy V (1994). "Pathogenesis of peritonsillar abscess". Laryngoscope. 104 (2): 185–90. doi:10.1288/00005537-199402000-00011. PMID 8302122.
- ↑ 4.0 4.1 4.2 4.3 4.4 Kawabata M, Umakoshi M, Makise T, Miyashita K, Harada M, Nagano H; et al. (2016). "Clinical classification of peritonsillar abscess based on CT and indications for immediate abscess tonsillectomy". Auris Nasus Larynx. 43 (2): 182–6. doi:10.1016/j.anl.2015.09.014. PMID 26527518.
- ↑ 5.0 5.1 5.2 5.3 L. Michaels, H.B. Hellquist Ear, nose and throat histopathology (2nd ed.)Springer-Verlag, London (2001), pp. 281–286
- ↑ 6.0 6.1 Blair AB, Booth R, Baugh R (2015). "A unifying theory of tonsillitis, intratonsillar abscess and peritonsillar abscess". Am J Otolaryngol. 36 (4): 517–20. doi:10.1016/j.amjoto.2015.03.002. PMID 25865201.
- ↑ 7.0 7.1 Herzon FS, Martin AD (2006). "Medical and surgical treatment of peritonsillar, retropharyngeal, and parapharyngeal abscesses". Curr Infect Dis Rep. 8 (3): 196–202. PMID 16643771.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Megalamani SB, Suria G, Manickam U, Balasubramanian D, Jothimahalingam S (2008). "Changing trends in bacteriology of peritonsillar abscess". J Laryngol Otol. 122 (9): 928–30. doi:10.1017/S0022215107001144. PMID 18039418.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Brook I (2004). "Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses". J Oral Maxillofac Surg. 62 (12): 1545–50. PMID 15573356.
- ↑ 10.0 10.1 Snow DG, Campbell JB, Morgan DW (1991). "The microbiology of peritonsillar sepsis". J Laryngol Otol. 105 (7): 553–5. PMID 1875138.
- ↑ 11.0 11.1 Matsuda A, Tanaka H, Kanaya T, Kamata K, Hasegawa M (2002). "Peritonsillar abscess: a study of 724 cases in Japan". Ear Nose Throat J. 81 (6): 384–9. PMID 12092281.
- ↑ 12.0 12.1 12.2 12.3 Lyon M, Blaivas M (2005). "Intraoral ultrasound in the diagnosis and treatment of suspected peritonsillar abscess in the emergency department". Acad Emerg Med. 12 (1): 85–8. doi:10.1197/j.aem.2004.08.045. PMID 15635144.
- ↑ 13.0 13.1 13.2 13.3 Boesen T, Jensen F (1992). "Preoperative ultrasonographic verification of peritonsillar abscesses in patients with severe tonsillitis". Eur Arch Otorhinolaryngol. 249 (3): 131–3. PMID 1642863.
- ↑ 14.0 14.1 14.2 14.3 Bandarkar AN, Adeyiga AO, Fordham MT, Preciado D, Reilly BK (2016). "Tonsil ultrasound: technical approach and spectrum of pediatric peritonsillar infections". Pediatr Radiol. 46 (7): 1059–67. doi:10.1007/s00247-015-3505-7. PMID 26637999.
- ↑ 15.0 15.1 Scott PM, Loftus WK, Kew J, Ahuja A, Yue V, van Hasselt CA (1999). "Diagnosis of peritonsillar infections: a prospective study of ultrasound, computerized tomography and clinical diagnosis". J Laryngol Otol. 113 (3): 229–32. PMID 10435129.
- ↑ Invalid
<ref>tag; no text was provided for refs namedpmid19445760 - ↑ 17.0 17.1 Invalid
<ref>tag; no text was provided for refs namedCherry2008 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid194457602 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid8769531 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid270310102 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid15983574 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid12557859 - ↑ Invalid
<ref>tag; no text was provided for refs named:0 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid3601520 - ↑ Invalid
<ref>tag; no text was provided for refs namedTonsillitis - ↑ Invalid
<ref>tag; no text was provided for refs namedurlTonsillitis - NHS Choices - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid25587367 - ↑ Invalid
<ref>tag; no text was provided for refs namedurlTonsillitis - Symptoms - NHS Choices - ↑ Invalid
<ref>tag; no text was provided for refs namedOroface - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid25946659 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid25945805 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid23520072 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid22481424 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid18948832 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid18427007 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid2235179 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid12777558 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid1876473 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid15667676 - ↑ Invalid
<ref>tag; no text was provided for refs namedpmid12761699 - ↑ Belleza WG, Kalman S (2006). "Otolaryngologic emergencies in the outpatient setting". Med Clin North Am. 90 (2): 329–53. doi:10.1016/j.mcna.2005.12.001. PMID 16448878.
- ↑ Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH, Infectious Diseases Society of America (2002). "Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America". Clin Infect Dis. 35 (2): 113–25. doi:10.1086/340949. PMID 12087516.
- ↑ Steyer TE (2002). "Peritonsillar abscess: diagnosis and treatment". Am Fam Physician. 65 (1): 93–6. PMID 11804446.
- ↑ Khayr W, Taepke J (2005). "Management of peritonsillar abscess: needle aspiration versus incision and drainage versus tonsillectomy". Am J Ther. 12 (4): 344–50. PMID 16041198.
- ↑ Ong YK, Goh YH, Lee YL (2004). "Peritonsillar infections: local experience". Singapore Med J. 45 (3): 105–9. PMID 15029410.
- ↑ Marom T, Cinamon U, Itskoviz D, Roth Y (2010). "Changing trends of peritonsillar abscess". Am J Otolaryngol. 31 (3): 162–7. doi:10.1016/j.amjoto.2008.12.003. PMID 20015734.
- ↑ Klug TE (2014). "Incidence and microbiology of peritonsillar abscess: the influence of season, age, and gender". Eur J Clin Microbiol Infect Dis. 33 (7): 1163–7. doi:10.1007/s10096-014-2052-8. PMID 24474247.
- ↑ Gavriel H, Lazarovitch T, Pomortsev A, Eviatar E (2009). "Variations in the microbiology of peritonsillar abscess". Eur J Clin Microbiol Infect Dis. 28 (1): 27–31. doi:10.1007/s10096-008-0583-6. PMID 18612664.
- ↑ Sunnergren O, Swanberg J, Mölstad S (2008). "Incidence, microbiology and clinical history of peritonsillar abscesses". Scand J Infect Dis. 40 (9): 752–5. doi:10.1080/00365540802040562. PMID 19086341.
- ↑ Hidaka H, Kuriyama S, Yano H, Tsuji I, Kobayashi T (2011). "Precipitating factors in the pathogenesis of peritonsillar abscess and bacteriological significance of the Streptococcus milleri group". Eur J Clin Microbiol Infect Dis. 30 (4): 527–32. doi:10.1007/s10096-010-1114-9. PMID 21086007.
- ↑ Costales-Marcos M, López-Álvarez F, Núñez-Batalla F, Moreno-Galindo C, Alvarez Marcos C, Llorente-Pendás JL (2012). "[Peritonsillar infections: prospective study of 100 consecutive cases]". Acta Otorrinolaringol Esp. 63 (3): 212–7. doi:10.1016/j.otorri.2012.01.001. PMID 22425204.
- ↑ Lehnerdt G, Senska K, Fischer M, Jahnke K (2005). "[Smoking promotes the formation of peritonsillar abscesses]". Laryngorhinootologie. 84 (9): 676–9. doi:10.1055/s-2005-870289. PMID 16142623.
- ↑ Dilkes MG, Dilkes JE, Ghufoor K (1992). "Smoking and quinsy". Lancet. 339 (8808): 1552. PMID 1351238.
- ↑ 54.0 54.1 Coughlin AM, Baugh RF, Pine HS (2014). "Lingual tonsil abscess with parapharyngeal extension: a case report". Ear Nose Throat J. 93 (9): E7–8. PMID 25255362.
- ↑ 55.0 55.1 Deeva YV (2015). "[SURGICAL TREATMENT OF TONSILLAR NECK PHLEGMON]". Klin Khir (7): 47–8. PMID 26591220.
- ↑ Goldenberg D, Golz A, Joachims HZ (1997). "Retropharyngeal abscess: a clinical review". J Laryngol Otol. 111 (6): 546–50. PMID 9231089.
- ↑ Stevens HE (1990). "Vascular complication of neck space infection: case report and literature review". J Otolaryngol. 19 (3): 206–10. PMID 2355414.
- ↑ Greinwald JH, Wilson JF, Haggerty PG (1995). "Peritonsillar abscess: an unlikely cause of necrotizing fasciitis". Ann Otol Rhinol Laryngol. 104 (2): 133–7. doi:10.1177/000348949510400209. PMID 7857015.
- ↑ Wenig BL, Shikowitz MJ, Abramson AL (1984). "Necrotizing fasciitis as a lethal complication of peritonsillar abscess". Laryngoscope. 94 (12 Pt 1): 1576–9. PMID 6594557.
- ↑ Powell J, Wilson JA (2012). "An evidence-based review of peritonsillar abscess". Clin Otolaryngol. 37 (2): 136–45. doi:10.1111/j.1749-4486.2012.02452.x. PMID 22321140.
- ↑ 61.0 61.1 Apostolopoulos NJ, Nikolopoulos TP, Bairamis TN (1995). "Peritonsillar abscess in children. Is incision and drainage an effective management?". Int J Pediatr Otorhinolaryngol. 31 (2–3): 129–35. PMID 7782170.
- ↑ Johnson RF, Stewart MG, Wright CC (2003). "An evidence-based review of the treatment of peritonsillar abscess". Otolaryngol Head Neck Surg. 128 (3): 332–43. doi:10.1067/mhn.2003.93. PMID 12646835.
- ↑ Herzon FS (1995). "Harris P. Mosher Award thesis. Peritonsillar abscess: incidence, current management practices, and a proposal for treatment guidelines". Laryngoscope. 105 (8 Pt 3 Suppl 74): 1–17. PMID 7630308.
- ↑ Ferri, Fred (2015). Ferri's clinical advisor 2015 : 5 books in 1. Philadelphia, PA: Elsevier/Mosby. ISBN 978-0323083751.
- ↑ Ferri, Fred (2015). Ferri's clinical advisor 2015 : 5 books in 1. Philadelphia, PA: Elsevier/Mosby. ISBN 978-0323083751.
- ↑ Nwe TT, Singh B (2000). "Management of pain in peritonsillar abscess". J Laryngol Otol. 114 (10): 765–7. PMID 11127146.
- ↑ 67.0 67.1 DescriptionEnglish: A right sided peritonsilar abscess Date 13 May 2011 Source Own work Author James Heilman,MD wikimedia commons https://commons.wikimedia.org/wiki/File:PeritonsilarAbsess.jpg
- ↑ Costantino TG, Satz WA, Dehnkamp W, Goett H (2012). "Randomized trial comparing intraoral ultrasound to landmark-based needle aspiration in patients with suspected peritonsillar abscess". Acad Emerg Med. 19 (6): 626–31. doi:10.1111/j.1553-2712.2012.01380.x. PMID 22687177.
- ↑ Buckley AR, Moss EH, Blokmanis A (1994). "Diagnosis of peritonsillar abscess: value of intraoral sonography". AJR Am J Roentgenol. 162 (4): 961–4. doi:10.2214/ajr.162.4.8141026. PMID 8141026.
- ↑ Strong EB, Woodward PJ, Johnson LP (1995). "Intraoral ultrasound evaluation of peritonsillar abscess". Laryngoscope. 105 (8 Pt 1): 779–82. doi:10.1288/00005537-199508000-00002. PMID 7630286.
- ↑ Blaivas M, Theodoro D, Duggal S (2003). "Ultrasound-guided drainage of peritonsillar abscess by the emergency physician". Am J Emerg Med. 21 (2): 155–8. doi:10.1053/ajem.2003.50029. PMID 12671820.
- ↑ 72.0 72.1 Mathoera RB, Wever PC, van Dorsten FR, Balter SG, de Jager CP (2008). "Epiglottitis in the adult patient". Neth J Med. 66 (9): 373–7. PMID 18931398.
External links
Template:Respiratory pathology
ka:პერიტონზილური აბსცესი nl:Peritonsillair abces fi:Kurkkupaise
Physical Examination
Physical examination findings suggestive of peritonsillar abscess include the following:[1][2][3][4]
Appearance of the Patient
- They are usually acutely-ill looking.
Vital Signs
- High temperature
HEENT
- Muffled voice (also called "hot potato voice")
- Contralateral deflection of the uvula
- The tonsil is generally displaced inferiorly and medially
- Facial swelling
- Tonsillar hypertrophy with likely peritonsillar edema.
- Trismus
- Drooling
- Rancid or fetor breath
Neck
- Tenderness of anterior neck
- Tender submandibular and anterior cervical lymph nodes
Lungs
- May be in obvious respiratory distress with flaring of ala nasi, subcostal and intercostal recessions.
- Increased respiratory rate in both children and adults
- Decreased air-entry depending of degree of airway obstruction
Extremities
- Cyanosis
| Variable | Croup | Epiglottitis | Pharyngitis | Tonsilitis | Retropharyngeal abscess | |
|---|---|---|---|---|---|---|
| Presentation | Cough | ✔ | — | Sore throat, pain on swallowing, fever, headache, abdominal pain, nausea and vomiting | Sore throat, pain on swallowing, fever, headache, cough | Neck pain, stiff neck, torticollis |
| Stridor | ✔ | ✔ | ||||
| Drooling | — | ✔ | ||||
| Others are Hoarseness, Difficulty breathing, symptoms of the common cold, Runny nose, Fever | Other symptoms include difficulty breathing, fever, chills, difficulty swallowing, hoarseness of voice | |||||
| Causes | Parainfluenza virus | H. influenza type b, beta-hemolytic streptococci, Staphylococcus aureus, fungi and viruses. | Group A beta-hemolytic streptococcus. | Most common cause is viral including adenovirus, rhinovirus, influenza, coronavirus, and respiratory syncytial virus. Second most common causes are bacterial; Group A streptococcal bacteria,[5] | Polymicrobial infection. Mostly; Streptococcus pyogenes, Staphylococcus aureus and respiratory anaerobes (example; Fusobacteria, Prevotella, and Veillonella species)[6][7][8][9][10][11] | |
| Physical exams findings | Suprasternal and intercostal indrawing,[12] Inspiratory stridor[13], expiratory wheezing,[13] Sternal wall retractions[14] | Cyanosis, Cervical lymphadenopathy, Inflammed epiglottis | Inflammed pharynx with or without exudate | Fever, especially 100°F or higher.[15][16]Erythema, edema and Exudate of the tonsils.[17] cervical lymphadenopathy, Dysphonia.[18] | Child may be unable to open the mouth widely. May have enlarged
cervical lymph nodes and neck mass. | |
| Age commonly affected | Mainly 6 months and 3 years old
rarely, adolescents and adults[19] |
Used to be mostly found in
pediatric age group between 3 to 5 years, however, recent trend favors adults as most commonly affected individuals[20] with a mean age of 44.94 years. |
Mostly in children and young adults,
with 50% of cases identified between the ages of 5 to 24 years.[21] |
Primarily affects children
between 5 and 15 years old.[22] |
Mostly between 2-4 years, but can occur in other age groups.[23][24] | |
| Imaging finding | Steeple sign on neck X-ray | Thumbprint sign on neck x-ray | — | Intraoral or transcutaneous USG may show an abscess making CT scan unnecessary.[25][26][27] | On CT scan, a mass impinging on the posterior pharyngeal wall with rim enhancement is seen[28][29] | |
| Treatment | Dexamethasone and nebulised epinephrine | Airway maintenance, parenteral Cefotaxime or Ceftriaxone in combination with Vancomycin. Adjuvant therapy includes corticosteroids and racemic Epinephrine.[30][31] | Antimicrobial therapy mainly penicillin-based and analgesics. | Antimicrobial therapy mainly penicillin-based and analgesics with tonsilectomy in selected cases. | Immediate surgical drainage and antimicrobial therapy. emperic therapy involves; ampicillin-sulbactam or clindamycin. | |
-
Peritonsillar abscess Image courtesy of RadsWiki and copylefted
-
Peritonsillar abscess Image courtesy of RadsWiki and copylefted
-
Peritonsillar abscess Image courtesy of RadsWiki and copylefted
-
Peritonsillar abscess Image courtesy of RadsWiki and copylefted
Treatment
Treatment is, as for all abscesses, through surgical incision and drainage of the pus, thereby relieving the pain of the stretched tissues. The drainage can often be achieved in the Outpatient Department using a guarded No. 11 blade in an awake and co-operative patient. Sometimes, a needle aspiration can suffice. Antibiotics are also given to treat the infection.
Peritonsillar abscesses are widely considered one of the most painful complications, primarily the surgical draining of the abscess itself. The patient is operated on awake, surgically slicing open the tonsil and draining the abscess.
Complications
- Parapharyngeal abscess
- Extension of abscess in other deep neck spaces leading to airway compromise
- Septicaemia
Alternaria spp[32]
Rhodotorula spp [33]
Acremonium spp.[34]
Dreschlera spp[35]
Malassezia spp[36]
Scedosporium spp[37]
Arthrographis spp[38]
Blastoschizomyces (11, 12),
Paecilomyces (13, 14),
Aureobasidium (15),
Clavispora (16), Ustilago (17),
Exophiala (Wangiella) (18),
and Exserohilum (19, 20).
On the other hand, most cases of fungal CNS infections are caused by only a few important species,
The common causes of fungal meningistis may be classified into two subgroups. This inlcudes:
Primary fungal pathogens of humans
All of these may cause CNS infections. This group includes: C. neoformans (22, 23),
Coccidioides immitis (24, 25, 26),
Blastomyces dermatitidis (27, 28),
Paracoccidioides brasiliensis (29, 30),
Sporothrix schenckii (31, 32),
H. capsulatum (33, 34),
Pseudallescheria boydii (Scedosporium apiospermum) (35, 36),
dematiaceous fungi (37, 38, 39).
The second group is considered opportunists, which take advantage of significant immune defects in the host. This group includes
Candida species (40, 41, 42),
Aspergillus species (43, 44, 45),
mucormycosis (46, 47), and
Trichosporon species (48, 49).
Title:Fungal Meningitis
Author / Creator:Horan ; Perfect, Jennifer, John L. R.
Language: English
Is Part Of: Infections of the Central Nervous System
Identifier: ISBN: 978-1-4698-8366-3
Source: Gale Virtual Reference Library (GVRL)
| According to severity of the disease | |||
| Mild |
| ||
| Moderate |
| ||
| Severe |
| ||
| According to the duration of disease[39] | |||
| Acute |
| ||
| Subacute |
| ||
| Chronic |
| ||
| Recurrent |
| ||
| Variable | Empyema Thoracis | Lung abscess | Pleural effusion | Pneumonia | Lung cancer | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presentation | Variable presentation
but may follow long standing pneumonia |
Usually has history of aspiration pneumonia, alcoholics, drug abusers, seizure disorder, have undergone recent general anesthesia, or have a nasogastric or endotracheal tube. | Usually follows pneumonia as a complication | presents with fever, pleuritc chest pain, cough | mostly asymptomatic but may
have cough productive with hemoptysis and chronic history of smoking | ||||||||||||||||
| Causes | In general any bacteria
can cause an empyema, however different bacteria are associated with different rates of empyema formation.[1] Common causes include bacteroides, fusobacterium, |
Lung abscess is commonly caused by bacterial infections and these include bacteroides, peptostreptococcus and prevotella mostly after aspiration | Common causes of transudative pleural effusion include;[1][2][3][4][5] left ventricular failure, Nephrotic syndrome, and cirrhosis, while common causes of exudative pleural effusions[6] are bacterial pneumonia and malignancy | Pneumonia can result from a variety of causes, including infection with bacteria, viruses, fungi, parasites, and chemical injury to the lungs | Direct cause of lung cancers
is DNA mutations that often result in either activation of proto-oncogenes (e.g. K-RAS) or the inactivation of tumors suppressor genes (e.g. TP53) or both. The risk of these genetic mutations may be increased following exposure to environmental components example smoking | ||||||||||||||||
| Laboratory findings | The pleural fluid typically has a low pH (<7.20),
low glucose (<60 mg/dL), and contains infectious organisms. Therefore, the diagnosis relies on the presence of pus or organisms on gram stain. A positive bacteria culture from pleural fluid is not needed to make diagnosis of empyema.[40][41] |
Raised inflammatory markers ( eg high ESR, CRP) are usual but not specific | The most widely used criteria is to differentiate between exudate and transudate using the light's criteria. Fluid is exudate when:
|
Laboratory findings are non specific example leukocytosis, sputum samples for gram staining and culture. Other tests include urine antigen test, PCR, C-reactive protein and procalcitonin | The laboratory findings are
non specific including: hypercarbia, hypoxia, and tumor cells in sputum and pleural effusion cytology. | ||||||||||||||||
| Physical examination | On examination, the following
findings may be seen:[42][43][44] Lateral chest wall swelling and tenderness, clubbing of the fingernails, dull percussion note, r educed breath sounds on the affected side of the chest, egophony, coarse crackles, increased tactile fremitus, mediastinal shift to opposite side with large empyema |
Chest examination shows features of consolidation such as localised dullness on percussion, bronchial breath sound etc.
Dental decay is common especially in alcoholics and children. Clubbing is present in one third of patients. |
Bulging of the intercostal spaces,
decreased chest expansion bronchovesicular breath sounds of decreased intensity, egophony, dullness to percussion, decreased or absent fremitus. |
Physical examination increased respiratory rate, low oxygen saturation, difficulty breathing, bronchial breathe sounds, increased tactile fremitus crackling sounds, or increased whispered pectoriloquy. | Physical examination findings are non specific and may include decreased/absent breath sounds, pallor, low-grade fever, tachypnea and cachezia. | ||||||||||||||||
| CXR | Chest X ray of empyema shows air-fluid level continuos homogenous pattern from the mediastinum to the chest wall forming obtuse angle with the lung parenchyma.[45] |
Chest xray shows often unilateral cavity containing an air-fluid level and consolidation of lung parenchyema. |
A homogenous opacification is noted at the affected side. The costophrenic angle is obliterated with a meniscus. | CXR shows areas of diffused opacities. | CXR may show lung mass, widening of the mediastinum, atelectasis, or pleural effusion. | ||||||||||||||||
| Chest ultrasound | Ultrasound in empyema is positive
for suspended microbubble sign, air fluid level, curtains sign and loss of gliding sign.[46] |
Ultrasound in lung abscess is negative for suspended microbubble sign, curtains sign and loss of gliding sign but air fluid level may be seen,.[47] | Ultrasonography is helpful in making diagnosis of pleural effusion particularly in differentiating effusion from masses.[48] The extended thoracic spine sign on sonography has high sensitivity and specificity for diagnosing pleural effusion.[49] Chest or upper abdominal ultrasound may show subpulmonic effusion as shown below.[50][51][52] | Not reqiured unless complicated with empyema | USG is helpful in guiding biopsy, staging and estimating prognosis. It may show hypo- and hyperechogenic masses.[53][54][55] | ||||||||||||||||
| CT scan | Seen as a lung mass whose cavity
is regular with smooth and regular lumen, well-defined boundary and shape changes with change in patient's position.[56] Mass may resolve on antibiotics The split pleura sign is present[57] (most reliable sign to differentiate empyema from lung abscess)[58] |
Lung mass whose cavity is rregular with undulated lumen, irregular-poorly defined boundary and shape does not change with change in patient's position.[59] Mass may resolve on antibiotics | In most cases CT imaging may not provide additional information that would influence the clinical decision-making process.[60][61] [62] CT scan shows heterogeneous opacification of the affected side and cardiomediastinal shift to the opposite site in unilateral effusion.[63] |
|
Seen as a spiculated irregular solid mass that does not resolve on antibiotics | ||||||||||||||||
|
|
|||||||||||||||||||||
- ↑ Galioto NJ (2008). "Peritonsillar abscess". Am Fam Physician. 77 (2): 199–202. PMID 18246890.
- ↑ Ferri, Fred (2015). Ferri's clinical advisor 2015 : 5 books in 1. Philadelphia, PA: Elsevier/Mosby. ISBN 978-0323083751.
- ↑ Passy V (1994). "Pathogenesis of peritonsillar abscess". Laryngoscope. 104 (2): 185–90. doi:10.1288/00005537-199402000-00011. PMID 8302122.
- ↑ Nwe TT, Singh B (2000). "Management of pain in peritonsillar abscess". J Laryngol Otol. 114 (10): 765–7. PMID 11127146.
- ↑ Putto A (1987). "Febrile exudative tonsillitis: viral or streptococcal?". Pediatrics. 80 (1): 6–12. PMID 3601520.
- ↑ Cheng J, Elden L (2013). "Children with deep space neck infections: our experience with 178 children". Otolaryngol Head Neck Surg. 148 (6): 1037–42. doi:10.1177/0194599813482292. PMID 23520072.
- ↑ Abdel-Haq N, Quezada M, Asmar BI (2012). "Retropharyngeal abscess in children: the rising incidence of methicillin-resistant Staphylococcus aureus". Pediatr Infect Dis J. 31 (7): 696–9. doi:10.1097/INF.0b013e318256fff0. PMID 22481424.
- ↑ Inman JC, Rowe M, Ghostine M, Fleck T (2008). "Pediatric neck abscesses: changing organisms and empiric therapies". Laryngoscope. 118 (12): 2111–4. doi:10.1097/MLG.0b013e318182a4fb. PMID 18948832.
- ↑ Brook I (2004). "Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses". J Oral Maxillofac Surg. 62 (12): 1545–50. PMID 15573356.
- ↑ Wright CT, Stocks RM, Armstrong DL, Arnold SR, Gould HJ (2008). "Pediatric mediastinitis as a complication of methicillin-resistant Staphylococcus aureus retropharyngeal abscess". Arch Otolaryngol Head Neck Surg. 134 (4): 408–13. doi:10.1001/archotol.134.4.408. PMID 18427007.
- ↑ Asmar BI (1990). "Bacteriology of retropharyngeal abscess in children". Pediatr Infect Dis J. 9 (8): 595–7. PMID 2235179.
- ↑ Johnson D (2009). "Croup". BMJ Clin Evid. 2009. PMC 2907784. PMID 19445760.
- ↑ 13.0 13.1 Cherry, James D. (2008). "Croup". New England Journal of Medicine. 358 (4): 384–391. doi:10.1056/NEJMcp072022. ISSN 0028-4793.
- ↑ Johnson D (2009). "Croup". BMJ Clin Evid. 2009. PMC 2907784. PMID 19445760.
- ↑ Tonsillitis. Medline Plus. https://www.nlm.nih.gov/medlineplus/ency/article/001043.htm. Accessed May 2nd, 2016.
- ↑ "Tonsillitis - NHS Choices".
- ↑ Stelter K (2014). "Tonsillitis and sore throat in children". GMS Curr Top Otorhinolaryngol Head Neck Surg. 13: Doc07. doi:10.3205/cto000110. PMC 4273168. PMID 25587367.
- ↑ "Tonsillitis - Symptoms - NHS Choices".
- ↑ Tong MC, Chu MC, Leighton SE, van Hasselt CA (1996). "Adult croup". Chest. 109 (6): 1659–62. PMID 8769531.
- ↑ Lichtor JL, Roche Rodriguez M, Aaronson NL, Spock T, Goodman TR, Baum ED (2016). "Epiglottitis: It Hasn't Gone Away". Anesthesiology. 124 (6): 1404–7. doi:10.1097/ALN.0000000000001125. PMID 27031010.
- ↑ Bennett, John (2015). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1455748013.
- ↑ Sharav, Yair; Benoliel, Rafael (2008). Orofacial Pain and Headache. Elsevier. ISBN 0723434123.
- ↑ Craig FW, Schunk JE (2003). "Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management". Pediatrics. 111 (6 Pt 1): 1394–8. PMID 12777558.
- ↑ Coulthard M, Isaacs D (1991). "Neonatal retropharyngeal abscess". Pediatr Infect Dis J. 10 (7): 547–9. PMID 1876473.
- ↑ Kawabata M, Umakoshi M, Makise T, Miyashita K, Harada M, Nagano H; et al. (2016). "Clinical classification of peritonsillar abscess based on CT and indications for immediate abscess tonsillectomy". Auris Nasus Larynx. 43 (2): 182–6. doi:10.1016/j.anl.2015.09.014. PMID 26527518.
- ↑ Nogan S, Jandali D, Cipolla M, DeSilva B (2015). "The use of ultrasound imaging in evaluation of peritonsillar infections". Laryngoscope. 125 (11): 2604–7. doi:10.1002/lary.25313. PMID 25946659.
- ↑ Fordham MT, Rock AN, Bandarkar A, Preciado D, Levy M, Cohen J; et al. (2015). "Transcervical ultrasonography in the diagnosis of pediatric peritonsillar abscess". Laryngoscope. 125 (12): 2799–804. doi:10.1002/lary.25354. PMID 25945805.
- ↑ Philpott CM, Selvadurai D, Banerjee AR (2004). "Paediatric retropharyngeal abscess". J Laryngol Otol. 118 (12): 919–26. PMID 15667676.
- ↑ Vural C, Gungor A, Comerci S (2003). "Accuracy of computerized tomography in deep neck infections in the pediatric population". Am J Otolaryngol. 24 (3): 143–8. PMID 12761699.
- ↑ Nickas BJ (2005). "A 60-year-old man with stridor, drooling, and "tripoding" following a nasal polypectomy". J Emerg Nurs. 31 (3): 234–5, quiz 321. doi:10.1016/j.jen.2004.10.015. PMID 15983574.
- ↑ Wick F, Ballmer PE, Haller A (2002). "Acute epiglottis in adults". Swiss Med Wkly. 132 (37–38): 541–7. PMID 12557859.
- ↑ OHASHI Y (1960). "On a rare disease due to Alternaria tenuis Nees (alternariasis)". Tohoku J Exp Med. 72: 78–82. PMID 13730495.
- ↑ Shinde RS, Mantur BG, Patil G, Parande MV, Parande AM (2008). "Meningitis due to Rhodotorula glutinis in an HIV infected patient". Indian J Med Microbiol. 26 (4): 375–7. PMID 18974495.
- ↑ Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ (1991). "Infection due to the fungus Acremonium (cephalosporium)". Medicine (Baltimore). 70 (6): 398–409. PMID 1956281.
- ↑ Fuste FJ, Ajello L, Threlkeld R, Henry JE (1973). "Drechslera hawaiiensis: causative agent of a fatal fungal meningo-encephalitis". Sabouraudia. 11 (1): 59–63. PMID 4739938.
- ↑ Rosales CM, Jackson MA, Zwick D (2004). "Malassezia furfur meningitis associated with total parenteral nutrition subdural effusion". Pediatr Dev Pathol. 7 (1): 86–90. doi:10.1007/s10024-003-4030-5. PMID 15255040.
- ↑ Symoens F, Knoop C, Schrooyen M, Denis O, Estenne M, Nolard N; et al. (2006). "Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation". J Heart Lung Transplant. 25 (5): 603–7. doi:10.1016/j.healun.2005.12.011. PMID 16678041.
- ↑ Chin-Hong PV, Sutton DA, Roemer M, Jacobson MA, Aberg JA (2001). "Invasive fungal sinusitis and meningitis due to Arthrographis kalrae in a patient with AIDS". J Clin Microbiol. 39 (2): 804–7. doi:10.1128/JCM.39.2.804-807.2001. PMC 87827. PMID 11158158.
- ↑ Zheng H, Chen Q, Xie Z, Wang D, Li M, Zhang X; et al. (2016). "A retrospective research of HIV-negative cryptococcal meningoencephalitis patients with acute/subacute onset". Eur J Clin Microbiol Infect Dis. 35 (2): 299–303. doi:10.1007/s10096-015-2545-0. PMID 26792138.
- ↑ Mavroudis C, Ganzel BL, Cox SK, Polk HC (1987). "Experimental aerobic-anaerobic thoracic empyema in the guinea pig". Ann Thorac Surg. 43 (3): 298–302. PMID 3548615.
- ↑ Perez VP, Caierão J, Fischer GB, Dias CA, d'Azevedo PA (2016). "Pleural effusion with negative culture: a challenge for pneumococcal diagnosis in children". Diagn Microbiol Infect Dis. 86 (2): 200–4. doi:10.1016/j.diagmicrobio.2016.07.022. PMID 27527890.
- ↑ Atay S, Banki F, Floyd C (2016). "Empyema necessitans caused by actinomycosis: A case report". Int J Surg Case Rep. 23: 182–5. doi:10.1016/j.ijscr.2016.04.005. PMC 5022073. PMID 27180228.
- ↑ Gomes MM, Alves M, Correia JB, Santos L (2013). "Empyema necessitans: very late complication of pulmonary tuberculosis". BMJ Case Rep. 2013. doi:10.1136/bcr-2013-202072. PMC 3863066. PMID 24326441.
- ↑ Kuan YC, How SH, Yeen WC, Ng TH, Fauzi AR (2011). "Empyema thoracis complicated by pneumothorax necessitans manifesting as lobulated, localized subcutaneous emphysematous swellings". Ann Thorac Surg. 91 (6): 1969–71. doi:10.1016/j.athoracsur.2010.11.075. PMID 21619994.
- ↑ Moffett BK, Panchabhai TS, Nakamatsu R, Arnold FW, Peyrani P, Wiemken T; et al. (2016). "Comparing posteroanterior with lateral and anteroposterior chest radiography in the initial detection of parapneumonic effusions". Am J Emerg Med. 34 (12): 2402–2407. doi:10.1016/j.ajem.2016.09.021. PMID 27793503.
- ↑ Lin FC, Chou CW, Chang SC (2004). "Differentiating pyopneumothorax and peripheral lung abscess: chest ultrasonography". Am J Med Sci. 327 (6): 330–5. PMID 15201646.
- ↑ Lin FC, Chou CW, Chang SC (2004). "Differentiating pyopneumothorax and peripheral lung abscess: chest ultrasonography". Am J Med Sci. 327 (6): 330–5. PMID 15201646.
- ↑ Invalid
<ref>tag; no text was provided for refs namedpmid21345104 - ↑ Dickman E, Terentiev V, Likourezos A, Derman A, Haines L (2015). "Extension of the Thoracic Spine Sign: A New Sonographic Marker of Pleural Effusion". J Ultrasound Med. 34 (9): 1555–61. doi:10.7863/ultra.15.14.06013. PMID 26269297.
- ↑ Almeida FA, Eiger G (2008). "Subpulmonic effusion". Intern Med J. 38 (3): 216–7. doi:10.1111/j.1445-5994.2007.01619.x. PMID 18290818.
- ↑ Connell DG, Crothers G, Cooperberg PL (1982). "The subpulmonic pleural effusion: sonographic aspects". J Can Assoc Radiol. 33 (2): 101–3. PMID 7107669.
- ↑ Halvorsen RA, Thompson WM (1986). "Ascites or pleural effusion? CT and ultrasound differentiation". Crit Rev Diagn Imaging. 26 (3): 201–40. PMID 3536306.
- ↑ Mroz RM, Korniluk M, Swidzinska E, Dzieciol J, Czaban J, Panek B; et al. (2010). "Lung mass in a 28-year-old male: a case report of a rare tumor". Eur J Med Res. 15 Suppl 2: 95–7. PMC 4360372. PMID 21147631.
- ↑ Torun E, Fidan A, Cağlayan B, Salepçi T, Mayadağli A, Salepçi B (2008). "[Prognostic factors in small cell lung cancer]". Tuberk Toraks. 56 (1): 22–9. PMID 18330751.
- ↑ Filon E, Kodur E, Cygan M (1989). "[Ultrasonographic examination of the adrenal glands for detection of lung cancer metastasis]". Nowotwory. 39 (3–4): 157–61. PMID 2700089.
- ↑ Baber CE, Hedlund LW, Oddson TA, Putman CE (1980). "Differentiating empyemas and peripheral pulmonary abscesses: the value of computed tomography". Radiology. 135 (3): 755–8. doi:10.1148/radiology.135.3.7384467. PMID 7384467.
- ↑ Stark DD, Federle MP, Goodman PC, Podrasky AE, Webb WR (1983). "Differentiating lung abscess and empyema: radiography and computed tomography". AJR Am J Roentgenol. 141 (1): 163–7. doi:10.2214/ajr.141.1.163. PMID 6602513.
- ↑ Kraus GJ (2007). "The split pleura sign". Radiology. 243 (1): 297–8. doi:10.1148/radiol.2431041658. PMID 17392263.
- ↑ Baber CE, Hedlund LW, Oddson TA, Putman CE (1980). "Differentiating empyemas and peripheral pulmonary abscesses: the value of computed tomography". Radiology. 135 (3): 755–8. doi:10.1148/radiology.135.3.7384467. PMID 7384467.
- ↑ Corcoran JP, Acton L, Ahmed A, Hallifax RJ, Psallidas I, Wrightson JM; et al. (2016). "Diagnostic value of radiological imaging pre- and post-drainage of pleural effusions". Respirology. 21 (2): 392–5. doi:10.1111/resp.12675. PMID 26545413.
- ↑ Federle MP, Mark AS, Guillaumin ES (1986). "CT of subpulmonic pleural effusions and atelectasis: criteria for differentiation from subphrenic fluid". AJR Am J Roentgenol. 146 (4): 685–9. doi:10.2214/ajr.146.4.685. PMID 3485341.
- ↑ Halvorsen RA, Thompson WM (1986). "Ascites or pleural effusion? CT and ultrasound differentiation". Crit Rev Diagn Imaging. 26 (3): 201–40. PMID 3536306.
- ↑ Wolverson MK, Crepps LF, Sundaram M, Heiberg E, Vas WG, Shields JB (1983). "Hyperdensity of recent hemorrhage at body computed tomography: incidence and morphologic variation". Radiology. 148 (3): 779–84. doi:10.1148/radiology.148.3.6878700. PMID 6878700.