Propranolol (capsule)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2], Sheng Shi, M.D. [3], Rabin Bista, M.B.B.S. [4]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CARDIAC ISCHEMIA AFTER ABRUPT DISCONTINUATION:
See full prescribing information for complete Boxed Warning.
CARDIAC ISCHEMIA AFTER ABRUPT DISCONTINUATION: Following abrupt discontinuation of therapy with beta-blockers, exacerbations of angina pectoris and myocardial infarction have occurred.
When discontinuing chronically administered propranoolol, particularly in patients with ischemic heart disease, gradually reduce the dose over a period of 1-2 weeks and monitor the patients. If angina markedly worsens or acute coronary insufficiency develops, promptly resume therapy, at least temporarily and take other measures appropriate for the management of unstable angina. Warn patients against interruption or discontinuation of therapy without physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abrupt discontinuation of propranolol therapy even in patient treated only for hypertension. |
Overview
Propranolol (capsule) is a beta-adrenergic blocker that is FDA approved for the treatment of hypertension, angina pectoris, migraine, hypertrophic subaortic stenosis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dermatitis, pruritus, diarrhea, dizziness, sleep disorder, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Oral Propranolol
Hypertension
- The usual initial dosage is 80 mg propranolol once daily, whether used alone or added to a diuretic. The dosage may be increased to 120 mg once daily or higher until adequate blood pressure control is achieved. The usual maintenance dosage is 120 to 160 mg once daily. In some instances a dosage of 640 mg may be required. The time needed for full hypertensive response to a given dosage is variable and may range from a few days to several weeks.
Angina Pectoris
- Starting with 80 mg propranolol once daily, dosage should be gradually increased at three- to seven-day intervals until optimal response is obtained. Although individual patients may respond at any dosage level, the average optimal dosage appears to be 160 mg once daily. In angina pectoris, the value and safety of dosage exceeding 320 mg per day have not been established.
- If treatment is to be discontinued, reduce dosage gradually over a period of a few weeks.
Migraine
- The initial oral dose is 80 mg propranolol once daily. The usual effective dose range is 160 to 240 mg once daily. The dosage may be increased gradually to achieve optimal migraine prophylaxis. If a satisfactory response is not obtained within four to six weeks after reaching the maximal dose, propranolol therapy should be discontinued. It may be advisable to withdraw the drug gradually over a period of several weeks depending on the patient's age, comorbidity, and dose of propranolol.
Hypertrophic Subaortic Stenosis
- The usual dosage is 80 to 160 mg propranolol once daily.
Parenteral Propranolol
- The usual dose is 1 mg to 3 mg administered under careful monitoring, such as electrocardiography and central venous pressure. The rate of administration should not exceed 1 mg (1 mL) per minute to diminish the possibility of lowering blood pressure and causing cardiac standstill.
- Sufficient time should be allowed for the drug to reach the site of action even when a slow circulation is present. If necessary, a second dose may be given after two minutes. Thereafter, additional drug should not be given in less than four hours. Additional propranolol hydrochloride should not be given when the desired alteration in heart rate and/or heart rhythm is achieved.
- Transfer to oral therapy as soon as possible.
- The indications for parenteral therapy are listed below.
Cardiac Arrhythmias
- Intravenous administration is usually reserved for life-threatening arrhythmias or those occurring under anesthesia.
Supraventricular arrhythmias
- Intravenous propranolol is indicated for the short-term treatment of supraventricular tachycardia, including Wolff-Parkinson-White syndrome and thyrotoxicosis, to decrease ventricular rate. Use in patients with atrial flutter or atrial fibrillation should be reserved for arrythmias unresponsive to standard therapy or when more prolonged control is required. Reversion to normal sinus rhythm has occasionally been observed, predominantly in patients with sinus or atrial tachycardia.
Ventricular tachycardias
- With the exception of those induced by catecholamines or digitalis, propranolol is not the drug of first choice. In critical situations when cardioversion techniques or other drugs are not indicated or are not effective, propranolol may be considered. If, after consideration of the risks involved, propranolol is used, it should be given intravenously in low dosage and very slowly, as the failing heart requires some sympathetic drive for maintenance of myocardial tone.
- Some patients may respond with complete reversion to normal sinus rhythm, but reduction in ventricular rate is more likely. Ventricular arrhythmias do not respond to propranolol as predictably as do the supraventricular arrhythmias.
- Intravenous propranolol is indicated for the treatment of persistent premature ventricular extrasystoles that impair the well-being of the patient and do not respond to conventional measures.
Tachyarrhythmias of Digitalis Intoxication
- Intravenous propranolol is indicated to control ventricular rate in life-threatening digitalis-induced arrhythmias. Severe bradycardia may occur.
Resistant Tachyarrhythmias Due to Excessive Catecholamine Action During Anesthesia
- Intravenous propranolol is indicated to abolish tachyarrhythmias due to excessive catecholamine action during anesthesia when other measures fail. These arrhythmias may arise because of release of endogenous catecholamines or administration of catecholamines. All general inhalation anesthetics produce some degree of myocardial depression. Therefore, when propranolol is used to treat arrhythmias during anesthesia, it should be used with extreme caution, usually with constant monitoring of the ECG and central venous pressure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Thyroid Storm
- Developed by: American Thyroid Association and American Association of Clinical Endocrinologists
- Class of Recommendation: 1 (high recommendation)
- Strength of Evidence: + (poor quality of evidence)
- Dosing Information/Recommendation
- 10–40 mg.[1]
Non–Guideline-Supported Use
Anxiety
Congestive Heart Failure
- Dosing Information
- Initial dose of 10 mg/day followed by 10-mg increments until a dose of 30 mg q8h.[2]
Gastrointestinal Hemorrhage
Percutaneous Coronary Intervention
- Dosing Information
- 15 μg/kg injected through the catheter directly to the coronary artery.[3]
Portal Hypertention
- Dosing Information
- 40–120 mg/day.[4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Propranolol (capsule) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Propranolol in pediatric patients.
Non–Guideline-Supported Use
Congestive Heart Failure
- Dosing Information
- 1,6 mg/kg/day[5]
Tetralogy of Fallot
- Dosing Information
- (Dosage)
Portal Hypertension
Neonatal Thyroid Storm
Contraindications
- Cardiogenic shock
- Sinus bradycardia
- Second-degree AV block
- Third-degree AV block
- Bronchial asthma
- Hypersensitivity
Warnings
|
CARDIAC ISCHEMIA AFTER ABRUPT DISCONTINUATION:
See full prescribing information for complete Boxed Warning.
CARDIAC ISCHEMIA AFTER ABRUPT DISCONTINUATION: Following abrupt discontinuation of therapy with beta-blockers, exacerbations of angina pectoris and myocardial infarction have occurred.
When discontinuing chronically administered propranoolol, particularly in patients with ischemic heart disease, gradually reduce the dose over a period of 1-2 weeks and monitor the patients. If angina markedly worsens or acute coronary insufficiency develops, promptly resume therapy, at least temporarily and take other measures appropriate for the management of unstable angina. Warn patients against interruption or discontinuation of therapy without physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abrupt discontinuation of propranolol therapy even in patient treated only for hypertension. |
Angina Pectoris
- There have been reports of exacerbation of angina and, in some cases, myocardial infarction, following abrupt discontinuance of propranolol therapy. Therefore, when discontinuance of propranolol is planned, the dosage should be gradually reduced over at least a few weeks, and the patient should be cautioned against interruption or cessation of therapy without the physician's advice. If propranolol therapy is interrupted and exacerbation of angina occurs, it usually is advisable to reinstitute propranolol therapy and take other measures appropriate for the management of unstable angina pectoris. Since coronary artery disease may be unrecognized, it may be prudent to follow the above advice in patients considered at risk of having occult atherosclerotic coronary disease who are given propranolol for other indications.
Hypersensitivity and Skin Reactions
- Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, have been associated with the administration of propranolol
- Cutaneous reactions, including Stevens-Johnson Syndrome, toxic epidermal necrolysis, exfoliative dermatitis, erythema multiforme, and urticaria, have been reported with use of propranolol.
Cardiac Failure
- Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta blockade may precipitate more severe failure. Although beta blockers should be avoided in overt congestive heart failure, some have been shown to be highly beneficial when used with close follow-up in patients with a history of failure who are well compensated and are receiving diuretics as needed. Beta-blockers agents do not abolish the inotropic action of digitalis on heart muscle.
- In Patients without a History of heart failure, continued use of beta blockers can, in some cases, lead to cardiac failure.
Nonallergic Bronchospasm (e.g., Chronic Bronchitis, Emphysema)
- In general, patients with bronchospastic lung disease should not receive beta-blockers. Propranolol should be administered with caution in this setting since it may provoke a bronchial asthmatic attack by blocking bronchodilation produced by endogenous and exogenous catecholamine stimulation of beta-receptors.
Major Surgery
- Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
- Beta-adrenergic blockade may prevent the appearance of certain premonitory signs and symptoms (pulse rate and pressure changes) of acute hypoglycemia, especially in labile insulin-dependent diabetics. In these patients, it may be more difficult to adjust the dosage of insulin.
- Propranolol therapy, particularly when given to infants and children, diabetic or not, has been associated with hypoglycemia especially during fasting as in preparation for surgery. Hypoglycemia has been reported in patients taking propranolol after prolonged physical exertion and in patients with renal insufficiency.
Thyrotoxicosis
- Beta-blockade may mask certain clinical signs of hyperthyroidism. Therefore, abrupt withdrawal of propranolol may be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm. Propranolol may change thyroid-function tests, increasing T4 and reverse T3, and decreasing T3.
Wolff-Parkinson-White Syndrome
Beta-blockade in patients with Wolff-Parkinson-White syndrome and tachycardia has been associated with severe bradycardia requiring treatment with a pacemaker. In one case, this result was reported after an initial dose of 5 mg propranolol.
Adverse Reactions
Clinical Trials Experience
Cardiovascular
- Bradycardia
- Congestive heart failure
- Intensification of AV block
- Hypotension
- Paresthesia of hands
- Thrombocytopenic purpura
- Arterial insufficiency, usually of the Raynaud type.
Central Nervous System
- Light-headedness
- Mental depression: Manifested by insomnia, lassitude, weakness, fatigue
- Catatonia
- Visual disturbances
- Hallucinations
- Vivid dreams
- An acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
- For immediate release formulations, fatigue, lethargy, and vivid dreams appear dose related.
Gastrointestinal
- Nausea
- Vomiting
- Epigastric distress
- Abdominal cramping
- Diarrhea
- Constipation
- Mesenteric arterial thrombosis
- Ischemic colitis
Allergic
- Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions
- Pharyngitis
- Agranulocytosis
- Erythematous rash
- Fever combined with aching and sore throat
- Laryngospasm
- Respiratory distress
Respiratory
Hematologic
Autoimmune
- Systemic lupus erythematosus (SLE).
Skin and mucous membranes
- Stevens-Johnson Syndrome
- Toxic epidermal necrolysis
- Dry eyes
- Exfoliative dermatitis
- Erythema multiforme
- Urticaria
- Alopecia
- SLE-like reactions,
- Psoriasisiform rashes.
- Oculomucocutaneous syndrome involving the skin, serous membranes, and conjunctivae reported for a beta-blocker (practolol) have not been associated with propranolol.
Genitourinary
Postmarketing Experience
There is limited information regarding Propranolol (capsule) Postmarketing Experience in the drug label.
Drug Interactions
- All drug interaction studies were conducted with propranolol. There are no data on drug interactions with Inderal LA capsules.
- Interactions with Substrates, Inhibitors or Inducers of Cytochrome P-450 Enzymes
- Because propranolol's metabolism involves multiple pathways in the Cytochrome P-450 system (CYP2D6, 1A2, 2C19), co-administration with drugs that are metabolized by, or affect the activity (induction or inhibition) of one or more of these pathways may lead to clinically relevant drug interactions.
- Substrates or Inhibitors of CYP2D6: Blood levels and/or toxicity of propranolol may be increased by co-administration with substrates or inhibitors of CYP2D6, such as amiodarone, cimetidine, delavudin, fluoxetine, paroxetine, quinidine, and ritonavir. No interactions were observed with either ranitidine or lansoprazole.
- Substrates or Inhibitors of CYP1A2: Blood levels and/or toxicity of propranolol may be increased by co-administration with substrates or inhibitors of CYP1A2, such as imipramine, cimetidine, ciprofloxacin, fluvoxamine, isoniazid, ritonavir, theophylline, zileuton, zolmitriptan, and rizatriptan.
- Substrates or Inhibitors of CYP2C19: Blood levels and/or toxicity of propranolol may be increased by co-administration with substrates or inhibitors of CYP2C19, such as fluconazole, cimetidine, fluoxetine, fluvoxamine, tenioposide, and tolbutamide. No interaction was observed with omeprazole.
- Inducers of Hepatic Drug Metabolism: Blood levels of propranolol may be decreased by co-administration with inducers such as rifampin, ethanol, phenytoin, and phenobarbital. Cigarette smoking also induces hepatic metabolism and has been shown to increase up to 77% the clearance of propranolol, resulting in decreased plasma concentrations.
Cardiovascular Drugs
Antiarrhythmics
- The AUC of propafenone is increased by more than 200% by co-administration of propranolol.
- The metabolism of propranolol is reduced by co-administration of quinidine, leading to a two to three fold increased blood concentration and greater degrees of clinical beta-blockade.
- The metabolism of lidocaine is inhibited by co-administration of propranolol, resulting in a 25% increase in lidocaine concentrations.
Calcium Channel Blockers
- The mean Cmax and AUC of propranolol are increased respectively, by 50% and 30% by co-administration of nisoldipine and by 80% and 47%, by co-administration of nicardipine.
- The mean Cmax and AUC of nifedipine are increased by 64% and 79%, respectively, by co-administration of propranolol.
- Propranolol does not affect the pharmacokinetics of verapamil and norverapamil. Verapamil does not affect the pharmacokinetics of propranolol.
Non-Cardiovascular Drugs
Migraine Drugs
- Administration of zolmitriptan or rizatriptan with propranolol resulted in increased concentrations of zolmitriptan (AUC increased by 56% and Cmax by 37%) or rizatriptan (the AUC and Cmax were increased by 67% and 75%, respectively).
Theophylline
- Co-administration of theophylline with propranolol decreases theophylline oral clearance by 30% to 52%.
Benzodiazepines
- Propranolol can inhibit the metabolism of diazepam, resulting in increased concentrations of diazepam and its metabolites. Diazepam does not alter the pharmacokinetics of propranolol.
- The pharmacokinetics of oxazepam, triazolam, lorazepam, and alprazolam are not affected by co-administration of propranolol.
Neuroleptic Drugs
- Co-administration of long-acting propranolol at doses greater than or equal to 160 mg/day resulted in increased thioridazine plasma concentrations ranging from 55% to 369% and increased thioridazine metabolite (mesoridazine) concentrations ranging from 33% to 209%.
- Co-administration of chlorpromazine with propranolol resulted in a 70% increase in propranolol plasma level.
Anti-Ulcer Drugs
- Co-administration of propranolol with cimetidine, a non-specific CYP450 inhibitor, increased propranolol AUC and Cmax by 46% and 35%, respectively. Co-administration with aluminum hydroxide gel (1200 mg) may result in a decrease in propranolol concentrations.
- Co-administration of metoclopramide with the long-acting propranolol did not have a significant effect on propranolol's pharmacokinetics.
Lipid Lowering Drugs
- Co-administration of cholestyramine or colestipol with propranolol resulted in up to 50% decrease in propranolol concentrations.
- Co-administration of propranolol with lovastatin or pravastatin, decreased 18% to 23% the AUC of both, but did not alter their pharmacodynamics. Propranolol did not have an effect on the pharmacokinetics of fluvastatin.
Warfarin
- Concomitant administration of propranolol and warfarin has been shown to increase warfarin bioavailability and increase prothrombin time.
Use in Specific Populations
Pregnancy
- In a series of reproductive and developmental toxicology studies, propranolol was given to rats by gavage or in the diet throughout pregnancy and lactation. At doses of 150 mg/kg/day, but not at doses of 80 mg/kg/day (equivalent to the MRHD on a body surface area basis), treatment was associated with embryotoxicity (reduced litter size and increased resorption rates) as well as neonatal toxicity (deaths). Propranolol hydrochloride also was administered (in the feed) to rabbits (throughout pregnancy and lactation) at doses as high as 150 mg/kg/day (about 5 times the maximum recommended human oral daily dose). No evidence of embryo or neonatal toxicity was noted.
- There are no adequate and well-controlled studies in pregnant women. Intrauterine growth retardation, small placentas, and congenital abnormalities have been reported in neonates whose mothers received propranolol during pregnancy. Neonates whose mothers are receiving propranolol at parturition have exhibited bradycardia, hypoglycemia and/or respiratory depression. Adequate facilities for monitoring such infants at birth should be available. Inderal LA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Propranolol (capsule) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Propranolol (capsule) during labor and delivery.
Nursing Mothers
Propranolol is excreted in human milk. Caution should be exercised when propranolol is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Propranolol (capsule) in pediatric settings.
Geriatic Use
- Clinical studies of propranolol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of the decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Gender
- In a study of 9 healthy women and 12 healthy men, neither the administration of testosterone nor the regular course of the menstrual cycle affected the plasma binding of the propranolol enantiomers. In contrast, there was a significant, although non-enantioselective diminution of the binding of propranolol after treatment with ethinyl estradiol. These findings are inconsistent with another study, in which administration of testosterone cypionate confirmed the stimulatory role of this hormone on propranolol metabolism and concluded that the clearance of propranolol in men is dependent on circulating concentrations of testosterone. In women, none of the metabolic clearances for propranolol showed any significant association with either estradiol or testosterone.
Race
- A study conducted in 12 Caucasian and 13 African-American male subjects taking propranolol, showed that at steady state, the clearance of R(+)- and S(-)-propranolol were about 76% and 53% higher in African-Americans than in Caucasians, respectively.
- Chinese subjects had a greater proportion (18% to 45% higher) of unbound propranolol in plasma compared to Caucasians, which was associated with a lower plasma concentration of alpha-1-acid glycoprotein.
Renal Impairment
- The pharmacokinetics of propranolol have not been investigated in patients with renal insufficiency.
- In a study conducted in 5 patients with chronic renal failure, 6 patients on regular dialysis, and 5 healthy subjects, who received a single oral dose of 40 mg of propranolol, the peak plasma concentrations (Cmax) of propranolol in the chronic renal failure group were 2 to 3-fold higher (161±41 ng/mL) than those observed in the dialysis patients (47±9 ng/mL) and in the healthy subjects (26±1 ng/mL). Propranolol plasma clearance was also reduced in the patients with chronic renal failure.
- Studies have reported a delayed absorption rate and a reduced half-life of propranolol in patients with renal failure of varying severity. Despite this shorter plasma half-life, propranolol peak plasma levels were 3-4 times higher and total plasma levels of metabolites were up to 3 times higher in these patients than in subjects with normal renal function.
- Chronic renal failure has been associated with a decrease in drug metabolism via down regulation of hepatic cytochrome P450 activity resulting in a lower "first-pass" clearance.
- Propranolol is not significantly dialyzable
Hepatic Impairment
- The pharmacokinetics of propranolol have not been investigated in patients with hepatic insufficiency. Propranolol is extensively metabolized by the liver. In a study conducted in 6 patients with cirrhosis and 7 healthy subjects receiving 160 mg of a long-acting preparation of propranolol once a day for 7 days, the steady-state propranolol concentration in patients with cirrhosis was increased 2.5-fold in comparison to controls. In the patients with cirrhosis, the half-life obtained after a single intravenous dose of 10 mg propranolol increased to 7.2 hours compared to 2.9 hours in control
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Propranolol (capsule) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Propranolol (capsule) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral/Intravenous
Monitoring
Resistant Tachyarrhythmias Due to Excessive Catecholamine Action During Anesthesi
- When propranolol is used to treat arrhythmias during anesthesia, it should be used with extreme caution, usually with constant monitoring of the ECG and central venous pressure.
IV Compatibility
There is limited information regarding the compatibility of Propranolol (capsule) and IV administrations.
Overdosage
- Propranolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed:
General
- If ingestion is or may have been recent, evacuate gastric contents, taking care to prevent pulmonary aspiration.
Supportive Therapy
- Hypotension and bradycardia have been reported following propranolol overdose and should be treated appropriately. Glucagon can exert potent inotropic and chronotropic effects and may be particularly useful for the treatment of hypotension or depressed myocardial function after a propranolol overdose. Glucagon should be administered as 50-150 mcg/kg intravenously followed by continuous drip of 1-5 mg/hour for positive chronotropic effect. Isoproterenol, dopamine or phosphodiesterase inhibitors may also be useful. Epinephrine, however, may provoke uncontrolled hypertension. Bradycardia can be treated with atropine or isoproterenol. Serious bradycardia may require temporary cardiac pacing. The electrocardiogram, pulse, blood pressure, neurobehavioral status and intake and output balance must be monitored. Isoproterenol and aminophylline may be used for bronchospasm.
Pharmacology
| |
1 : 1 mixture (racemate)Propranolol
| |
| Systematic (IUPAC) name | |
| (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 259.34 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 26% |
| Metabolism | hepatic (extensive) |
| Half life | 4–5 hours |
| Excretion | renal <1% |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, anal, IV |
Mechanism of Action
- The mechanism of the antihypertensive effect of propranolol has not been established. Among the factors that may be involved in contributing to the antihypertensive action include: (1) decreased cardiac output, (2) inhibition of renin release by the kidneys, and (3) diminution of tonic sympathetic nerve outflow from vasomotor centers in the brain. Although total peripheral resistance may increase initially, it readjusts to or below the pretreatment level with chronic use of propranolol. Effects of propranolol on plasma volume appear to be minor and somewhat variable.
- In angina pectoris, propranolol generally reduces the oxygen requirement of the heart at any given level of effort by blocking the catecholamine-induced increases in the heart rate, systolic blood pressure, and the velocity and extent of myocardial contraction. Propranolol may increase oxygen requirements by increasing left ventricular fiber length, end diastolic blood pressure, and systolic ejection period. The net physiologic effect of beta-blockade is usually advantageous and is manifested during exercise by delayed onset of pain and increased work capacity.
- Propranolol exerts its antiarrhythmic effects in concentrations associated with beta-blockade, and this appears to be its principal antiarrhythmic mechanism of action. In dosages greater than required for beta-blockade, propranolol also exerts a quinidine-like or anesthetic-like membrane action which affects the cardiac action potential. The significance of the membrane action in the treatment of arrhythmias is uncertain.
- The mechanism of the anti-migraine effect of propranolol has not been established. Beta-adrenergic receptors have been demonstrated in the pial vessels of the brain.
Structure
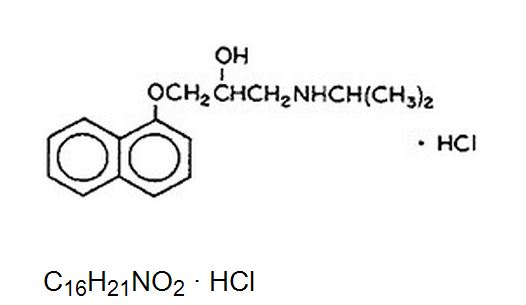
- Propranolol hydrochloride is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,(±)-. Its molecular and structural formulae are:

- Propranolol hydrochloride is a stable, white, crystalline solid which is readily soluble in water and ethanol. Its molecular weight is 295.80.
- Propranolol hydrochloride is formulated to provide a sustained release of propranolol hydrochloride. Propranolol hydrochloride is available as 60 mg, 80 mg, 120 mg, and 160 mg capsules for oral administration.
- The inactive ingredients contained in propranolol hydrochloride capsules are: cellulose, ethylcellulose, gelatin capsules, hypromellose, and titanium dioxide. In addition, propranolol hydrochloride 60 mg, 80 mg, and 120 mg capsules contain D&C Red No. 28 and FD&C Blue No. 1; propranolol hydrochloride 160 mg capsules contain FD&C Blue No. 1.
These capsules comply with USP Dissolution Test 1.
Pharmacodynamics
- Propranolol is a nonselective, beta-adrenergic receptor-blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor-stimulating agents for available receptor sites. When access to beta-receptor sites is blocked by propranolol, the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation are decreased proportionately. At dosages greater than required for beta-blockade, propranolol also exerts a quinidine-like or anesthetic-like membrane action, which affects the cardiac action potential. The significance of the membrane action in the treatment of arrhythmias is uncertain.
Pharmacokinetics
Absorption
- Propranolol is highly lipophilic and almost completely absorbed after oral administration. However, it undergoes high first pass metabolism by the liver and on average, only about 25% of propranolol reaches the systemic circulation. Inderal LA Capsules (60, 80, 120, and 160 mg) release propranolol HCl at a controlled and predictable rate. Peak blood levels following dosing with Inderal LA occur at about 6 hours.
- The effect of food on Inderal LA bioavailability has not been investigated.
Distribution
- Approximately 90% of circulating propranolol is bound to plasma proteins (albumin and alpha-1-acid glycoprotein). The binding is enantiomer-selective. The S(-)-enantiomer is preferentially bound to alpha-1-glycoprotein and the R(+)-enantiomer preferentially bound to albumin. The volume of distribution of propranolol is approximately 4 liters/kg.
- Propranolol crosses the blood-brain barrier and the placenta, and is distributed into breast milk.
Metabolism and Elimination
- Propranolol is extensively metabolized with most metabolites appearing in the urine. Propranolol is metabolized through three primary routes: aromatic hydroxylation (mainly 4-hydroxylation), N-dealkylation followed by further side-chain oxidation, and direct glucuronidation. It has been estimated that the percentage contributions of these routes to total metabolism are 42%, 41% and 17%, respectively, but with considerable variability between individuals. The four major metabolites are propranolol glucuronide, naphthyloxylactic acid and glucuronic acid, and sulfate conjugates of 4-hydroxy propranolol.
- In-vitro studies have indicated that the aromatic hydroxylation of propranolol is catalyzed mainly by polymorphic CYP2D6. Side-chain oxidation is mediated mainly by CYP1A2 and to some extent by CYP2D6. 4-hydroxy propranolol is a weak inhibitor of CYP2D6.
- Propranolol is also a substrate of CYP2C19 and a substrate for the intestinal efflux transporter, p-glycoprotein (p-gp). Studies suggest however that p-gp is not dose-limiting for intestinal absorption of propranolol in the usual therapeutic dose range.
- In healthy subjects, no difference was observed between CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs) with respect to oral clearance or elimination half-life. Partial clearance of 4-hydroxy propranolol was significantly higher and naphthyloxyactic acid was significantly lower in EMs than PMs.
- When measured at steady state over a 24-hour period the areas under the propranolol plasma concentration-time curve (AUCs) for the Inderal LA capsules are approximately 60% to 65% of the AUCs for a comparable divided daily dose of Inderal Tablets. The lower AUCs for the Inderal LA capsules are due to greater hepatic metabolism of propranolol, resulting from the slower rate of absorption of propranolol. Over a twenty-four (24) hour period, blood levels are fairly constant for about twelve (12) hours, then decline exponentially. The apparent plasma half-life is about 10 hours.
Enantiomers
- Propranolol is a racemic mixture of two enantiomers, R(+) and S(-). The S(-)-enantiomer is approximately 100 times as potent as the R(+)-enantiomer in blocking beta adrenergic receptors. In normal subjects receiving oral doses of racemic propranolol, S(-)-enantiomer concentrations exceeded those of the R(+)-enantiomer by 40-90% as a result of stereoselective hepatic metabolism. Clearance of the pharmacologically active S(-)-propranolol is lower than R(+)-propranolol after intravenous and oral doses.
Nonclinical Toxicology
- Carcinogenesis
- Mutagenesis
- Impairment of Fertility
- In dietary administration studies in which mice and rats were treated with propranolol hydrochloride for up to 18 months at doses of up to 150 mg/kg/day, there was no evidence of drug-related tumorigenesis. On a body surface area basis, this dose in the mouse and rat is, respectively, about equal to and about twice the maximum recommended human oral daily dose (MRHD) of 640 mg propranolol hydrochloride. In a study in which both male and female rats were exposed to propranolol hydrochloride in their diets at concentrations of up to 0.05% (about 50 mg/kg body weight and less than the MRHD), from 60 days prior to mating and throughout pregnancy and lactation for two generations, there were no effects on fertility.
- Based on differing results from Ames Tests performed by different laboratories, there is equivocal evidence for a genotoxic effect of propranolol in bacteria (S. typhimurium strain TA 1538).
Clinical Studies
Hypertension
- In a double-blind, parallel dose-response study in patients with mild-to-moderate hypertension (n=434), doses of propranolol from 80 to 640 mg were taken once daily at approximately 10 p.m. propranolol significantly lowered sitting systolic blood pressure and diastolic blood pressure when measurements were taken approximately 16 hours later. The placebo-subtracted diastolic blood pressure effect for the 80 and 120 mg doses was -3.0 and -4.0 mm Hg, respectively. Higher doses of propranolol (160, 640 mg) had no additional blood pressure lowering effect when compared with 120 mg. The antihypertensive effects of propranolol were seen in the elderly (≥65 years old) and men and women. There were too few non-white patients to assess the efficacy of propranolol in these patients.
How Supplied
Capsules
Inderal® LA Capsules (propranolol hydrochloride)
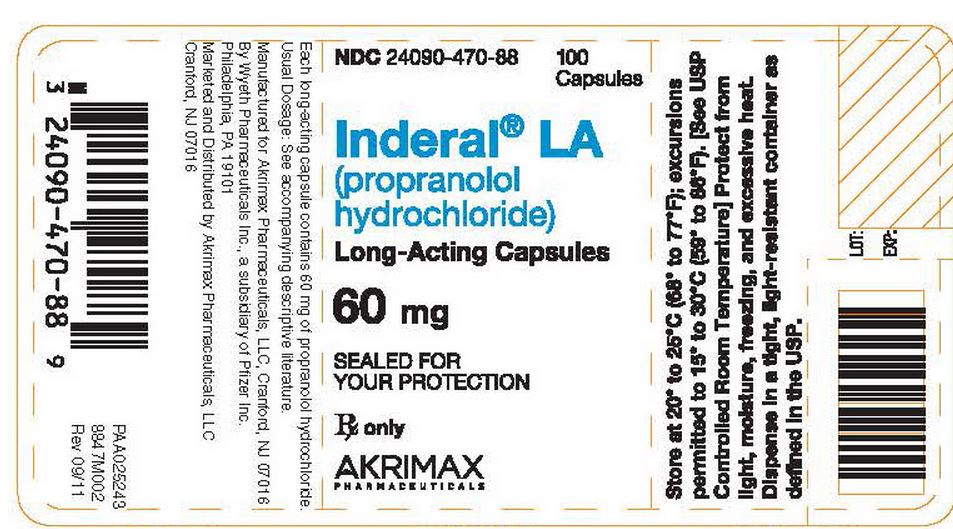
- Each white/light-blue capsule, identified by 3 narrow bands, 1 wide band, and "INDERAL LA 60," contains 60 mg of propranolol hydrochloride in bottles of 100 (NDC 24090-470-88).
- Each light-blue capsule, identified by 3 narrow bands, 1 wide band, and "INDERAL LA 80," contains 80 mg of propranolol hydrochloride in bottles of 100 (NDC 24090-471-88).
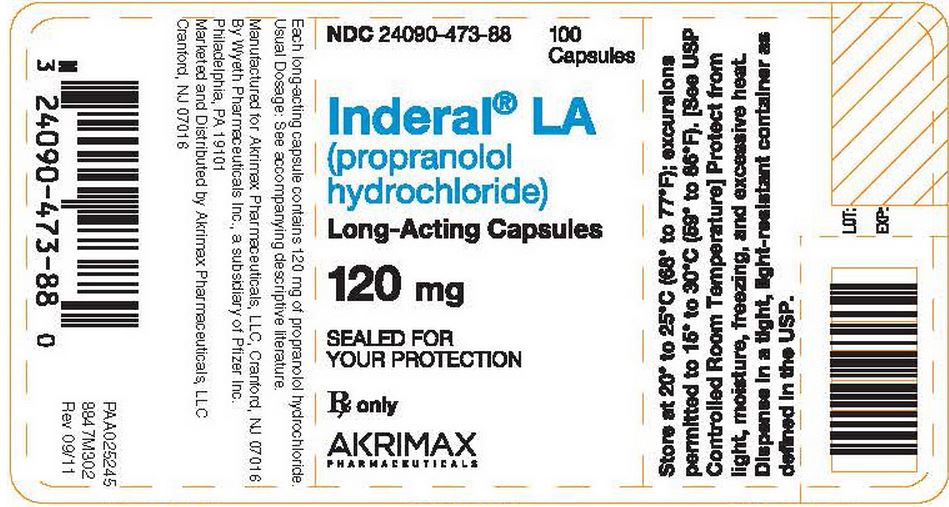
- Each light-blue/dark-blue capsule, identified by 3 narrow bands, 1 wide band, and "INDERAL LA 120," contains 120 mg of propranolol hydrochloride in bottles of 100 (NDC 24090-473-88).
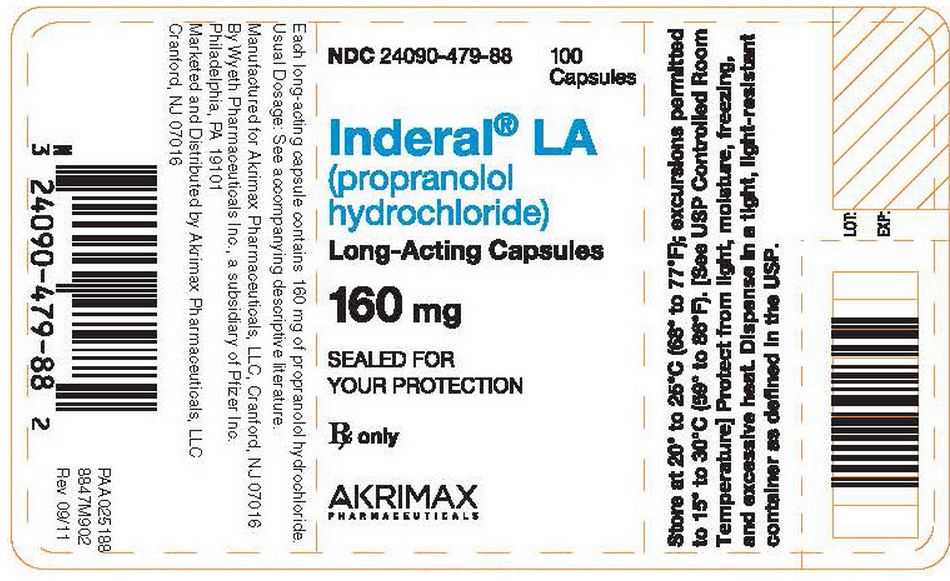
- Each dark-blue capsule, identified by 3 narrow bands, 1 wide band, and "INDERAL LA 160," contains 160 mg of propranolol hydrochloride in bottles of 100 (NDC 24090-479-88).
Injection
- Propranolol Hydrochloride Injection, USP, 1 mg/mL is supplied as follows:
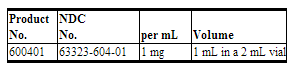
- Packaged in tens.
- Vial stoppers do not contain natural rubber latex.
Storage
Capsules
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]
- Protect from light, moisture, freezing, and excessive heat.
- Dispense in a tight, light-resistant container as defined in the USP.
Injection
- Store at 20° to 25°C (68° to 77°F).
- Protect from freezing and excessive heat.
Images
Drug Images
{{#ask: Page Name::Propranolol (capsule) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Propranolol (capsule) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not interrupt or discontinue using propranolol without a physician’s advice.
- Patients with heart failure should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
- Inform diabetic patients that certain signs of hypoglycemia may be masked. Patients should report any changes in blood sugar levels to their physician.
Precautions with Alcohol
Alcohol-Propranolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Inderal
- Inderal LA
- InnoPran XL
- Propranolol HCl Intensol
- Inderal XL
- Hemangeol
Look-Alike Drug Names
- Inderal - Adderall
- Propranolol - Prasugrel
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I; et al. (2011). "Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists". Thyroid. 21 (6): 593–646. doi:10.1089/thy.2010.0417. PMID 21510801.
- ↑ Aronow WS, Ahn C, Kronzon I (1997). "Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors". Am J Cardiol. 80 (2): 207–9. PMID 9230162.
- ↑ Wang FW, Osman A, Otero J, Stouffer GA, Waxman S, Afzal A; et al. (2003). "Distal myocardial protection during percutaneous coronary intervention with an intracoronary beta-blocker". Circulation. 107 (23): 2914–9. doi:10.1161/01.CIR.0000072787.25131.03. PMID 12771007.
- ↑ Venon WD, Baronio M, Leone N, Rolfo E, Fadda M, Barletti C; et al. (2003). "Effects of long-term Irbesartan in reducing portal pressure in cirrhotic patients: comparison with propranolol in a randomised controlled study". J Hepatol. 38 (4): 455–60. PMID 12663237.
- ↑ Buchhorn R, Hulpke-Wette M, Hilgers R, Bartmus D, Wessel A, Bürsch J (2001). "Propranolol treatment of congestive heart failure in infants with congenital heart disease: The CHF-PRO-INFANT Trial. Congestive heart failure in infants treated with propanol". Int J Cardiol. 79 (2–3): 167–73. PMID 11461738.