Osteopontin
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
| Osteopontin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Osteopontin | ||||||||
| Pfam | PF00865 | ||||||||
| InterPro | IPR002038 | ||||||||
| PROSITE | PDOC00689 | ||||||||
| |||||||||
- Not to be confused with Osteocalcin, Osteonectin or Osteoprotegerin (OPG).
Osteopontin (OPN), also known as bone sialoprotein I (BSP-1 or BNSP), early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric),[1] is a protein that in humans is encoded by the SPP1 gene (secreted phosphoprotein 1). The murine ortholog is Spp1. Osteopontin is a SIBLING (glycoprotein) that was first identified in 1986 in osteoblasts.
The prefix osteo- indicates that the protein is expressed in bone, although it is also expressed in other tissues. The suffix -pontin is derived from "pons," the Latin word for bridge, and signifies osteopontin's role as a linking protein. Osteopontin is an extracellular structural protein and therefore an organic component of bone. Synonyms for this protein include sialoprotein I and 44K BPP (bone phosphoprotein).
The gene has 7 exons, spans 5 kilobases in length and in humans it is located on the long arm of chromosome 4 region 22 (4q1322.1). The protein is composed of ~300 amino acids residues and has ~30 carbohydrate residues attached including 10 sialic acid residues, which are attached to the protein during post-translational modification in the Golgi apparatus. The protein is rich in acidic residues: 30-36% are either aspartic or glutamic acid.
Structure
General structure
OPN is a highly negatively charged, extracellular matrix protein that lacks an extensive secondary structure.[2] It is composed of about 300 amino acids (297 in mouse; 314 in human) and is expressed as a 33-kDa nascent protein; there are also functionally important cleavage sites. OPN can go through posttranslational modifications, which increase its apparent molecular weight to about 44 kDa.[3] The OPN gene is composed of 7 exons, 6 of which containing coding sequence.[4][5] The first two exons contain the 5' untranslated region (5' UTR).[6] Exons 2, 3, 4, 5, 6, and 7 code for 17, 13, 27, 14, 108 and 134 amino acids, respectively.[6] All intron-exon boundaries are of the phase 0 type, thus alternative exon splicing maintains the reading frame of the OPN gene.
Isoforms
Full-length OPN (OPN-FL) can be modified by thrombin cleavage, which exposes a cryptic sequence, SVVYGLR on the cleaved form of the protein known as OPN-R (Fig. 1). This thrombin-cleaved OPN (OPN-R) exposes an epitope for integrin receptors of α4β1, α9β1, and α9β4.[7][8] These integrin receptors are present on a number of immune cells such as mast cells,[9] neutrophils,[10] and T cells. It is also expressed by monocytes and macrophages.[11] Upon binding these receptors, cells use several signal transduction pathways to elicit immune responses in these cells. OPN-R can be further cleaved by Carboxypeptidase B (CPB) by removal of C-terminal arginine and become OPN-L. The function of OPN-L is largely unknown.
It appears an intracellular variant of OPN (iOPN) is involved in a number of cellular processes including migration, fusion and motility.[12][13][14][15] Intracellular OPN is generated using an alternative translation start site on the same mRNA species used to generate the extracellular isoform.[16] This alternative translation start site is downstream of the N-terminal endoplasmic reticulum-targeting signal sequence, thus allowing cytoplasmic translation of OPN.
Various human cancers, including breast cancer, have been observed to express splice variants of OPN.[17][18] The cancer-specific splice variants are osteopontin-a, osteopontin-b, and osteopontin-c. Exon 5 is lacking from osteopontin-b, whereas osteopontin-c lacks exon 4.[17] Osteopontin-c has been suggested to facilitate the anchorage-independent phenotype of some human breast cancer cells due to its inability to associate with the extracellular matrix.[17]
Biosynthesis
Osteopontin is biosynthesized by a variety of tissue types including cardiac fibroblasts,[19] preosteoblasts, osteoblasts, osteocytes, odontoblasts, some bone marrow cells, hypertrophic chondrocytes, dendritic cells, macrophages,[20] smooth muscle,[21] skeletal muscle myoblasts,[22] endothelial cells, and extraosseous (non-bone) cells in the inner ear, brain, kidney, deciduum, and placenta. Synthesis of osteopontin is stimulated by calcitriol (1,25-dihydroxy-vitamin D3).
Regulation
Regulation of the osteopontin gene is incompletely understood. Different cell types may differ in their regulatory mechanisms of the OPN gene. OPN expression in bone predominantly occurs by osteoblasts and osteocyctes (bone-forming cells) as well as osteoclasts (bone-resorbing cells).[23] Runx2 (aka Cbfa1) and osterix (Osx) transcription factors are required for the expression of OPN [24] Runx2 and Osx bind promoters of osteoblast-specific genes such as Col1α1, Bsp, and Opn and upregulate transcription.[25]
Hypocalcemia and hypophosphatemia (instances that stimulate kidney proximal tubule cells to produce calcitriol (1α,25-dihydroxyvitamin D3)) lead to increases in OPN transcription, translation and secretion.[26] This is due to the presence of a high-specificity vitamin D response element (VDRE) in the OPN gene promoter.[27][28][29]
Extracellular inorganic phosphate (ePi) has also been identified as a modulator of OPN expression.[30]
Stimulation of OPN expression also occurs upon exposure of cells to pro-inflammatory cytokines,[31] classical mediators of acute inflammation (e.g. tumour necrosis factor α [TNFα], infterleukin-1β [IL-1β]), angiotensin II, transforming growth factor β (TGFβ) and parathyroid hormone (PTH),[32][33] although a detailed mechanistic understanding of these regulatory pathways are not yet known. Hyperglycemia and hypoxia are also known to increase OPN expression.[32][34][35]
Biological function
Role in biomineralization
OPN belongs to a family of secreted acidic proteins whose members have an abundance of negatively charged amino acids such as Asp and Glu.[36] OPN also has a large number of consensus sequence sites for post-translational phosphorylation of Ser residues to form phosphoserine, providing additional negative charge.[37] Contiguous stretches of high negative charge in OPN have been identified and named the polyAsp motif (poly-aspartic acid) and the ASARM motif (acidic serine- and aspartate-rich motif), with the latter sequence having multiple phosphorylation sites.[38][39][40][41] This overall negative charge of OPN, along with its specific acidic motifs and the fact that OPN is an intrinsically disordered protein[42][43] allowing for open and flexible structures, permit OPN to bind strongly to calcium atoms available at crystal surfaces in various biominerals.[41][44][45] Such binding of OPN to various types of calcium-based biominerals ‒ such as calcium-phosphate mineral in bones and teeth,[46] calcium-carbonate mineral in inner ear otoconia[47] and avian eggshells,[48] and calcium-oxalate mineral in kidney stones[49][50][51] – acts as a mineralization inhibitor to regulate crystal growth.[52]
OPN is a substrate protein for a number of enzymes whose actions may modulate the mineralization-inhibiting function of OPN. PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) is one such enzyme, which extensively degrades OPN, and whose inactivating gene mutations (in X-linked hypophosphatemia, XLH) lead to altered processing of OPN such that inhibitory OPN cannot be degraded and accumulates in the bone (and tooth) extracellular matrix, likely contributing locally to the osteomalacia (soft hypomineralized bones) characteristic of XLH.[53][54][55]
Along with its role in the regulation of normal mineralization within the extracellular matrices of bones and teeth,[56] OPN is also upregulated at sites of pathologic, ectopic calcification[57][58] – such as for example, in urolithiasis and vascular calcification ‒ presumably at least in part to inhibit debilitating mineralization in these soft tissues.
Role in bone remodeling
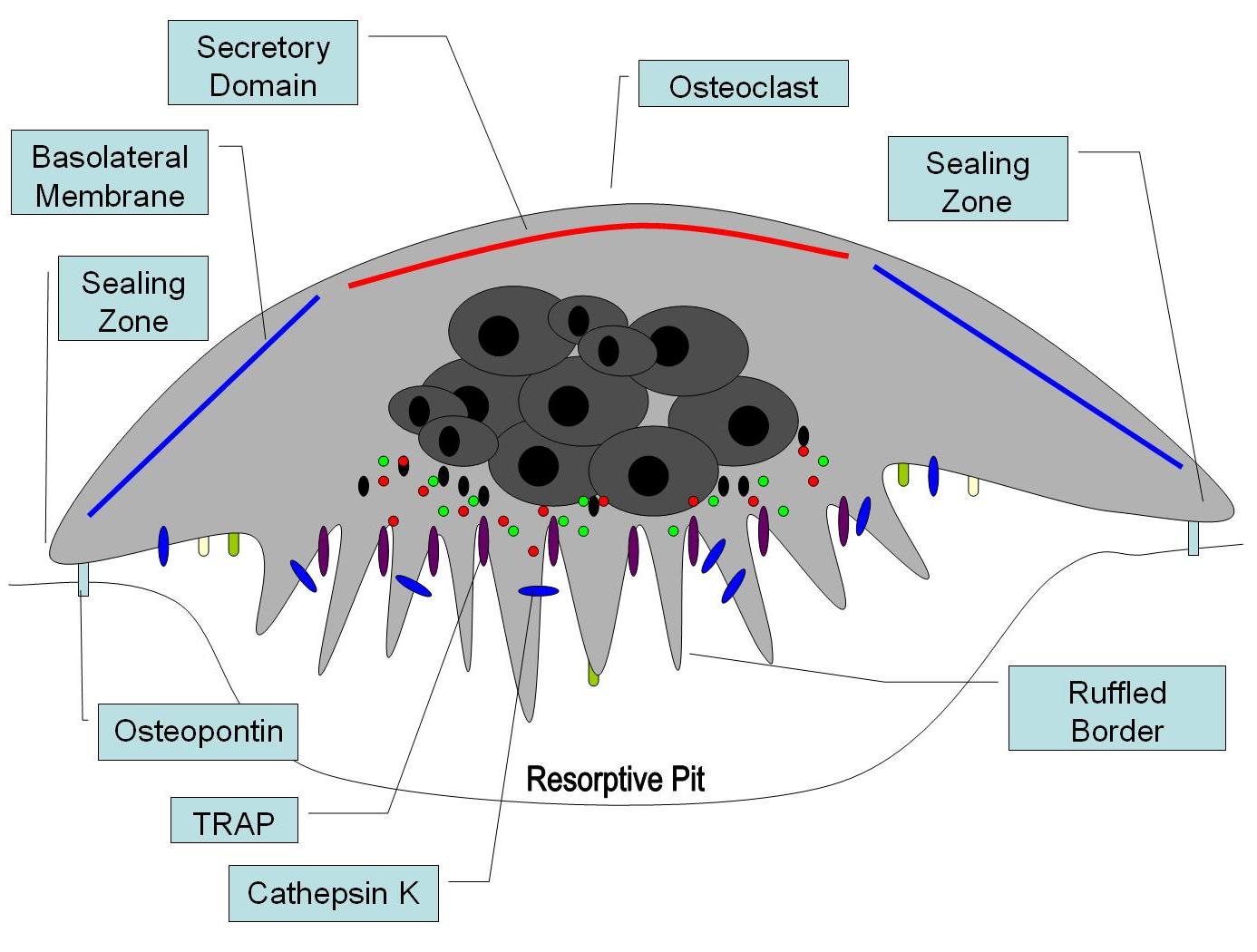
Osteopontin has been implicated as an important factor in bone remodeling.[59] Specifically, research suggests it plays a role in anchoring osteoclasts to the mineral matrix of bones.[9] The organic part of bone is about 20% of the dry weight, and counts in, other than osteopontin, collagen type I, osteocalcin, osteonectin, bone sialo protein, and alkaline phosphatase. Collagen type I counts for 90% of the protein mass. The inorganic part of bone is the mineral hydroxyapatite, Ca10(PO4)6(OH)2. Loss of this mineral may lead to osteoporosis, as the bone is depleted for calcium if this is not supplied in the diet.
OPN serves to initiate the process by which osteoclasts develop their ruffled borders to begin bone resorption. It is also found in urine, where it inhibits kidney stone formation.
Role in immune functions
As discussed, OPN binds to several integrin receptors including α4β1, α9β1, and α9β4 expressed by leukocytes. These receptors have been well-established to function in cell adhesion, migration, and survival in these cells. Therefore, recent research efforts have focused on the role of OPN in mediating such responses.
Osteopontin (OPN) is expressed in a range of immune cells, including macrophages, neutrophils, dendritic cells, and T and B cells, with varying kinetics. OPN is reported to act as an immune modulator in a variety of manners.[2] Firstly, it has chemotactic properties, which promote cell recruitment to inflammatory sites. It also functions as an adhesion protein, involved in cell attachment and wound healing. In addition, OPN mediates cell activation and cytokine production, as well as promoting cell survival by regulating apoptosis.[2] The following examples are found.[2]
Role in Heart
OPN expression increases under a variety of conditions of the heart, and is associated with increased myocyte apoptosis and myocardial dysfunction.[60]
Chemotaxis
OPN plays an important role in neutrophil recruitment in alcoholic liver disease.[10][61] OPN is important for the migration of neutrophil in vitro.[62] In addition, OPN recruits inflammatory cells to arthritis joints in the collagen-induced arthritis model of rheumatoid arthritis.[63][64] A recent in vitro study in 2008 has found that OPN plays a role in mast cell migration.[65] Here OPN knock-out mast cells were cultured and they observed a decreased level of chemotaxis in these cells compared to wildtype mast cells. OPN was also found to act as a macrophage chemotactic factor.[66] In this study, researchers looked at the accumulation of macrophages in the brain of rhesus monkeys and found that OPN prevented macrophages from leaving the accumulation site, indicating an increased level of chemotaxis.
Cell activation
Activated T cells are promoted by IL-12 to differentiate towards the Th1 type, producing cytokines including IL-12 and IFNγ. OPN inhibits production of the Th2 cytokine IL-10, which leads to enhanced Th1 response. OPN influences cell-mediated immunity and has Th1 cytokine functions. It enhances B cell immunoglobulin production and proliferation.[2] Recent studies in 2008 suggest that OPN also induces mast cell degranulation.[65] The researchers here observed that IgE-mediated anaphylaxis was significantly reduced in OPN knock-out mice compared to wild-type mice. The role of OPN in activation of macrophages has also been implicated in a cancer study, when researchers discovered that OPN-producing tumors were able to induce macrophage activation compared to OPN-deficient tumors.[67]
Apoptosis
OPN is an important anti-apoptotic factor in many circumstances. OPN blocks the activation-induced cell death of macrophages and T cells as well as fibroblasts and endothelial cells exposed to harmful stimuli.[68][69] OPN prevents non-programmed cell death in inflammatory colitis.[70]
Potential clinical application
The fact that OPN interacts with multiple cell surface receptors that are ubiquitously expressed makes it an active player in many physiological and pathological processes including wound healing, bone turnover, tumorigenesis, inflammation, ischemia, and immune responses1. Therefore, manipulation of plasma (or local) OPN levels may be useful in the treatment of autoimmune diseases, cancer metastasis, bone (and tooth) mineralization diseases, osteoporosis, and some forms of stress.[2]
Role in autoimmune diseases
OPN has been implicated in pathogenesis of rheumatoid arthritis. For instance, researchers found that OPN-R, the thrombin-cleaved form of OPN, was elevated in the rheumatoid arthritis joint. However, the role of OPN in rheumatoid arthritis is still unclear. One group found that OPN knock-out mice were protected against arthritis.[71] while others were not able to reproduce this observation.[72] OPN has been found to play a role in other autoimmune diseases including autoimmune hepatitis, allergic airway disease, and multiple sclerosis.[73]
Role in cancers and inflammatory diseases
It has been shown that OPN drives IL-17 production;[74] OPN is overexpressed in a variety of cancers, including lung cancer, breast cancer, colorectal cancer, stomach cancer, ovarian cancer, papillary thyroid carcinoma, melanoma and pleural mesothelioma; OPN contributes both glomerulonephritis and tubulointerstitial nephritis; and OPN is found in atheromatous plaques within arteries. Thus, manipulation of plasma OPN levels may be useful in the treatment of autoimmune diseases, cancer metastasis, osteoporosis and some forms of stress.[2]
Research has implicated osteopontin in excessive scar-forming and a gel has been developed to inhibit its effect.[75]
Role in colitis
Opn is up-regulated in inflammatory bowel disease (IBD).[76] Opn expression is highly up-regulated in intestinal immune and non-immune cells and in the plasma of patients with Crohn’s disease (CD) and ulcerative colitis (UC), as well as in the colon and plasma of mice with experimental colitis.[76][77][78] Increased plasma Opn levels are related to the severity of CD inflammation, and certain Opn gene (Spp1) haplotypes are modifiers of CD susceptibility. Opn has also a proinflammatory role in TNBS- and dextran sulfate sodium (DSS)-induced colitis, which are mouse models for IBD. Opn was found highly expressed by a specific dendritic cell (DC) subset derived from murine mesenteric lymph nodes (MLNs)and is highly proinflammatory for colitis.[79] Dendritic cells are important for the development of intestinal inflammation in humans with IBD and in mice with experimental colitis. Opn expression by this inflammatory MLN DC subset is crucial for their pathogenic action during colitis.[79]
Role in allergy and asthma
Osteopontin has recently been associated with allergic inflammation and asthma. Expression of Opn is significantly increased in lung epithelial and subepithelial cells of asthmatic patients in comparison to healthy subjects.[80] Opn expression is also upregulated in lungs of mice with allergic airway inflammation.[80] The secreted form of Opn (Opn-s) plays a proinflammatory role during allergen sensitization (OVA/Alum), as neutralization of Opn-s during that phase results in significantly milder allergic airway inflammation.[80] In contrast, neutralization of Opn-s during antigenic challenge exacerbates allergic airway disease.[80] These effects of Opn-s are mainly mediated by the regulation of Th2-suppressing plasmacytoid dendritic cells (DCs) during primary sensitization and Th2-promoting conventional DCs during secondary antigenic challenge.[80] OPN deficiency was also reported to protect against remodeling and bronchial hyperresponsiveness (BHR), again using a chronic allergen-challenge model of airway remodeling.[81] Furthermore, it was recently demonstrated that OPN expression is upregulated in human asthma, is associated with remodeling changes and its subepithelial expression correlates to disease severity.[82] OPN has also been reported to be increased in the sputum supernatant of smoking asthmatics,[83] as well as the BALF and bronchial tissue of smoking controls and asthmatics.[84]
Role in muscle disease and injury
Evidence is accumulating that suggests that osteopontin plays a number of roles in diseases of skeletal muscle, such as Duchenne muscular dystrophy. Osteopontin has been described as a component of the inflammatory environment of dystrophic and injured muscles,[22][85][86][87] and has also been shown to increase scarring of diaphragm muscles of aged dystrophic mice.[88] A recent study has identified osteopontin as a determinant of disease severity in patients with Duchenne muscular dystrophy.[89] This study found that a mutation in the osteopontin gene promoter, known to cause low levels of osteopontin expression, is associated with a decrease in age to loss of ambulation and muscle strength in patients with Duchenne muscular dystrophy.
Role in hip osteoarthritis
An increase in Plasma OPN levels has been observed in patients with idiopathic hip OA. Furthermore, a correlation between OPN plasma levels and the severity of the disease has been noted.[90]
Role in implantation
OPN is expressed in endometrial cells during implantation. Due to the production of progesterone by the ovaries, OPN is up-regulated immensely to aid in this process. The endometrium must undergo decidualization, the process in which the endometrium undergoes changes to prepare for implantation, which will lead to the attachment of the embryo. The endometrium houses stromal cells that will differentiate to produce an optimal environment for the embryo to attach (decidualization). OPN is a vital protein for stromal cell proliferation and differentiation as well as it binds to the receptor αvβ3 to assist with adhesion. OPN along with decidualization ultimately encourages the successful implantation of the early embryo. A OPN gene knock-out results in attachment instability at the maternal-fetal interface.[91][92]
References
- ↑ "Entrez Gene: SPP1 secreted phosphoprotein 1".
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Wang KX, Denhardt DT (2008). "Osteopontin: role in immune regulation and stress responses". Cytokine Growth Factor Rev. 19 (5–6): 333–45. doi:10.1016/j.cytogfr.2008.08.001. PMID 18952487.
- ↑ Rangaswami H, Bulbule A, Kundu GC (February 2006). "Osteopontin: role in cell signaling and cancer progression". Trends Cell Biol. 16 (2): 79–87. doi:10.1016/j.tcb.2005.12.005. PMID 16406521.
- ↑ Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW (August 1990). "cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN)" (Submitted manuscript). Genomics. 7 (1): 491–502. doi:10.1016/0888-7543(90)90191-V. PMID 1974876.
- ↑ Kiefer MC, Bauer DM, Barr PJ (April 1989). "The cDNA and derived amino acid sequence for human osteopontin". Nucleic Acids Res. 17 (1): 3306. doi:10.1093/nar/17.8.3306. PMC 317745. PMID 2726470.
- ↑ 6.0 6.1 Crosby AH, Edwards SJ, Murray JC, Dixon MJ (May 1995). "Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II". Genomics. 27 (1): 155–160. doi:10.1006/geno.1995.1018. PMID 7665163.
- ↑ Laffón A, García-Vicuña R, Humbría A, Postigo AA, Corbí AL, de Landázuri MO, Sánchez-Madrid F (August 1991). "Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis". J. Clin. Invest. 88 (2): 546–52. doi:10.1172/JCI115338. PMC 295383. PMID 1830891.
- ↑ Seiffge D (December 1996). "Protective effects of monoclonal antibody to VLA-4 on leukocyte adhesion and course of disease in adjuvant arthritis in rats". J. Rheumatol. 23 (12): 2086–91. PMID 8970045.
- ↑ 9.0 9.1 Reinholt FP, Hultenby K, Oldberg A, Heinegård D (June 1990). "Osteopontin--a possible anchor of osteoclasts to bone". Proc. Natl. Acad. Sci. U.S.A. 87 (12): 4473–5. doi:10.1073/pnas.87.12.4473. PMC 54137. PMID 1693772.
- ↑ 10.0 10.1 Banerjee A, Apte UM, Smith R, Ramaiah SK (March 2006). "Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease". J. Pathol. 208 (4): 473–85. doi:10.1002/path.1917. PMID 16440289.
- ↑ Sodek J, Batista Da Silva AP, Zohar R (May 2006). "Osteopontin and mucosal protection". J. Dent. Res. 85 (5): 404–15. doi:10.1177/154405910608500503. PMID 16632752.
- ↑ Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J (July 2000). "Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration". J Cell Physiol. 184 (1): 118–130. doi:10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. PMID 10825241.
- ↑ Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, Sodek J (August 2002). "Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts". J Bone Miner Res. 17 (1): 1486–1497. doi:10.1359/jbmr.2002.17.8.1486. PMID 12162503.
- ↑ Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J (January 2004). "Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin". Journal of Cellular Physiology. 198 (1): 155–167. doi:10.1002/jcp.10394. PMID 14584055.
- ↑ Junaid A, Moon MC, Harding GE, Zahradka P (February 2007). "Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1". Am J Physiol Cell Physiol. 292 (1): 919–926. doi:10.1152/ajpcell.00477.2006. PMID 17005603.
- ↑ Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H (May 2008). "Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells". Proc Natl Acad Sci USA. 105 (1): 7235–7239. doi:10.1073/pnas.0802301105. PMC 2438233. PMID 18480255.
- ↑ 17.0 17.1 17.2 He B, Mirza M, Weber GF (April 2006). "An osteopontin splice variant induces anchorage independence in human breast cancer cells". Oncogene. 25 (1): 2192–2202. doi:10.1038/sj.onc.1209248. PMID 16288209.
- ↑ Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, Weber GF (February 2008). "Osteopontin-c is a selective marker of breast cancer". Int J Cancer. 122 (1): 889–897. doi:10.1002/ijc.23204. PMID 17960616.
- ↑ Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA (November 1996). "Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction". J. Clin. Invest. 98 (10): 2218–27. doi:10.1172/JCI119031. PMC 507670. PMID 8941637.
- ↑ Murry CE, Giachelli CM, Schwartz SM, Vracko R (December 1994). "Macrophages express osteopontin during repair of myocardial necrosis". Am. J. Pathol. 145 (6): 1450–62. PMC 1887495. PMID 7992848.
- ↑ Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K (December 1993). "Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta". J. Clin. Invest. 92 (6): 2814–20. doi:10.1172/JCI116901. PMC 288482. PMID 8254036.
- ↑ 22.0 22.1 Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN (April 2008). "Osteopontin and skeletal muscle myoblasts: Association with muscle regeneration and regulation of myoblast function in vitro". Int. J. Biochem. Cell Biol. 40 (10): 2303–14. doi:10.1016/j.biocel.2008.03.020. PMID 18490187.
- ↑ Merry K, Dodds R, Littlewood A, Gowen M (April 1993). "Expression of Osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone". J Cell Sci. 104 (4): 1013–1020. PMID 8314886.
- ↑ Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (January 2002). "The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation". Cell. 108 (1): 17–29. doi:10.1016/S0092-8674(01)00622-5. PMID 11792318.
- ↑ Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (May 1997). "Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation". Cell. 89 (1): 747–754. doi:10.1016/S0092-8674(00)80257-3. PMID 9182762.
- ↑ Yucha C, Guthrie D (December 2003). "Renal homeostasis of calcium". Nephrol Nurs J. 30 (1): 755–764. PMID 14730782.
- ↑ Prince CW, Butler WT (September 1987). "1,25-Dihydroxyvitamin D3 regulates the biosyntheis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells". Coll Relat Res. 7 (1): 305–313. doi:10.1016/s0174-173x(87)80036-5. PMID 3478171.
- ↑ Oldberg A, Jirskog-Hed B, Axelsson S, Heinegård D (December 1989). "Regulation of bone sialoprotein mRNA by steroid hormones". J Cell Biol. 109 (1): 3183–3186. doi:10.1083/jcb.109.6.3183. PMC 2115918. PMID 2592421.
- ↑ Chang PL, Prince CW (May 1991). "1 alpha,25-Dihydroxyvitamin D3 enhances 12-O-tetradecanoylphorbol-13-acetate- induced tumorigenic transformation and osteopontin expression in mouse JB6 epidermal cells". Cancer Res. 51 (8): 2144–2150. PMID 2009532.
- ↑ Fatherazi S, Matsa-Dunn D, Foster BL, Rutherford RB, Somerman MJ, Presland RB (January 2009). "Phosphate regulates osteopontin gene transcription". J Dent Res. 88 (1): 39–44. doi:10.1177/0022034508328072. PMC 3128439. PMID 19131315.
- ↑ Guo H, Cai CQ, Schroeder RA, Kuo PC (January 2001). "Osteopontin is a negative feedback regulator of nitric oxide synthesis in murine macrophages". J Immunol. 166 (1): 1079–1086. doi:10.4049/jimmunol.166.2.1079. PMID 11145688.
- ↑ 32.0 32.1 Ricardo SD, Franzoni DF, Roesener CD, Crisman JM, Diamond JR (May 2000). "Angiotensinogen and AT(1) antisense inhibition of osteopontin translation in rat proximal tubular cells". Am J Physiol Renal Physiol. 278 (1): 708–716. doi:10.1152/ajprenal.2000.278.5.F708. PMID 10807582.
- ↑ Noda M, Rodan GA (February 1989). "Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone". J Cell Biol. 108 (1): 713–718. doi:10.1083/jcb.108.2.713. PMC 2115413. PMID 2465299.
- ↑ Hullinger TG, Pan Q, Viswanathan HL, Somerman MJ (January 2001). "TGFbeta and BMP-2 activation of the OPN promoter: roles of smad- and hox-binding elements". Exp Cell Res. 262 (1): 69–74. doi:10.1006/excr.2000.5074. PMID 11120606.
- ↑ Sodhi CP, Phadke SA, Batlle D, Sahai A (April 2001). "Hypoxia and high glucose cause exaggerated mesangial cell growth and collagen synthesis: role of osteopontin". Am J Physiol Renal Physiol. 280 (1): 667–674. doi:10.1152/ajprenal.2001.280.4.F667. PMID 11249858.
- ↑ Fisher LW, Fedarko NS (2003). "Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins". Connect. Tissue Res. 44 Suppl 1: 33–40. doi:10.1080/03008200390152061. PMID 12952171.
- ↑ Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sørensen ES (August 2005). "Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications". Biochem. J. 390 (Pt 1): 285–92. doi:10.1042/BJ20050341. PMC 1184582. PMID 15869464.
- ↑ David V, Martin A, Hedge AM, Drezner MK, Rowe PS (March 2011). "ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate". Am. J. Physiol. Renal Physiol. 300 (3): F783–91. doi:10.1152/ajprenal.00304.2010. PMC 3064126. PMID 21177780.
- ↑ Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS (April 2008). "Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP". Endocrinology. 149 (4): 1757–72. doi:10.1210/en.2007-1205. PMC 2276704. PMID 18162525.
- ↑ Addison WN, Nakano Y, Loisel T, Crine P, McKee MD (October 2008). "MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM". J. Bone Miner. Res. 23 (10): 1638–49. doi:10.1359/jbmr.080601. PMID 18597632.
- ↑ 41.0 41.1 Addison WN, Masica DL, Gray JJ, McKee MD (April 2010). "Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage". J. Bone Miner. Res. 25 (4): 695–705. doi:10.1359/jbmr.090832. PMID 19775205.
- ↑ Kurzbach D, Platzer G, Schwarz TC, Henen MA, Konrat R, Hinderberger D (July 2013). "Cooperative Unfolding of Compact Conformations of the Intrinsically Disordered Protein Osteopontin". Biochemistry. 52 (31): 5167–75. doi:10.1021/bi400502c. PMC 3737600. PMID 23848319.
- ↑ Kalmar L, Homola D, Varga G, Tompa P (September 2012). "Structural disorder in proteins brings order to crystal growth in biomineralization". Bone. 51 (3): 528–34. doi:10.1016/j.bone.2012.05.009. PMID 22634174.
- ↑ Azzopardi PV, O'Young J, Lajoie G, Karttunen M, Goldberg HA, Hunter GK (2010). "Roles of electrostatics and conformation in protein-crystal interactions". PLoS ONE. 5 (2): e9330. doi:10.1371/journal.pone.0009330. PMC 2824833. PMID 20174473.
- ↑ Hunter GK, O'Young J, Grohe B, Karttunen M, Goldberg HA (December 2010). "The flexible polyelectrolyte hypothesis of protein-biomineral interaction". Langmuir. 26 (24): 18639–46. doi:10.1021/la100401r. PMID 20527831.
- ↑ McKee MD, Nanci A (May 1995). "Postembedding colloidal-gold immunocytochemistry of noncollagenous extracellular matrix proteins in mineralized tissues". Microsc. Res. Tech. 31 (1): 44–62. doi:10.1002/jemt.1070310105. PMID 7626799.
- ↑ Takemura T, Sakagami M, Nakase T, Kubo T, Kitamura Y, Nomura S (September 1994). "Localization of osteopontin in the otoconial organs of adult rats". Hear. Res. 79 (1–2): 99–104. doi:10.1016/0378-5955(94)90131-7. PMID 7806488.
- ↑ Hincke MT, Nys Y, Gautron J, Mann K, Rodriguez-Navarro AB, McKee MD (2012). "The eggshell: structure, composition and mineralization". Front. Biosci. 17: 1266–80. doi:10.2741/3985. PMID 22201802.
- ↑ McKee MD, Nanci A, Khan SR (December 1995). "Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi". J. Bone Miner. Res. 10 (12): 1913–29. doi:10.1002/jbmr.5650101211. PMID 8619372.
- ↑ O'Young J, Chirico S, Al Tarhuni N, Grohe B, Karttunen M, Goldberg HA, Hunter GK (2009). "Phosphorylation of osteopontin peptides mediates adsorption to and incorporation into calcium oxalate crystals". Cells Tissues Organs (Print). 189 (1–4): 51–5. doi:10.1159/000151724. PMID 18728346.
- ↑ Chien YC, Masica DL, Gray JJ, Nguyen S, Vali H, McKee MD (August 2009). "Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning". J. Biol. Chem. 284 (35): 23491–501. doi:10.1074/jbc.M109.021899. PMC 2749123. PMID 19581305.
- ↑ Sodek J, Ganss B, McKee MD (2000). "Osteopontin". Crit. Rev. Oral Biol. Med. 11 (3): 279–303. doi:10.1177/10454411000110030101. PMID 11021631.
- ↑ McKee MD, Hoac B, Addison WN, Barros NM, Millán JL, Chaussain C (October 2013). "Extracellular matrix mineralization in periodontal tissues: Noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia". Periodontology 2000. 63 (1): 102–22. doi:10.1111/prd.12029. PMC 3766584. PMID 23931057.
- ↑ Boukpessi T, Hoac B, Coyac BR, Leger T, Garcia C, Wicart P, Whyte MP, Glorieux FH, Linglart A, Chaussain C, McKee MD (February 2017). "Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia". Bone. 95: 151–161. doi:10.1016/j.bone.2016.11.019. PMID 27884786.
- ↑ Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD (March 2013). "Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia". Journal of Bone and Mineral Research. 28 (3): 688–99. doi:10.1002/jbmr.1766. PMID 22991293.
- ↑ McKee MD, Addison WN, Kaartinen MT (2005). "Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton". Cells Tissues Organs (Print). 181 (3–4): 176–88. doi:10.1159/000091379. PMID 16612083.
- ↑ Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM (December 2002). "Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification". Am. J. Pathol. 161 (6): 2035–46. doi:10.1016/S0002-9440(10)64482-3. PMC 1850905. PMID 12466120.
- ↑ Giachelli CM (March 1999). "Ectopic calcification: gathering hard facts about soft tissue mineralization". Am. J. Pathol. 154 (3): 671–5. doi:10.1016/S0002-9440(10)65313-8. PMC 1866412. PMID 10079244.
- ↑ Choi ST, Kim JH, Kang EJ, Lee SW, Park MC, Park YB, Lee SK (December 2008). "Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis". Rheumatology (Oxford). 47 (12): 1775–9. doi:10.1093/rheumatology/ken385. PMID 18854347.
- ↑ Singh M, Dalal S, Singh K (2014). "Osteopontin: At the cross-roads of myocyte survival and myocardial function". Life Sci. 118 (1): 1–6. doi:10.1016/j.lfs.2014.09.014. PMC 4254317. PMID 25265596.
- ↑ Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK (August 2005). "Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis". Toxicol. Appl. Pharmacol. 207 (1): 25–38. doi:10.1016/j.taap.2004.12.018. PMID 15885730.
- ↑ Koh A, da Silva AP, Bansal AK, Bansal M, Sun C, Lee H, Glogauer M, Sodek J, Zohar R (December 2007). "Role of osteopontin in neutrophil function". Immunology. 122 (4): 466–75. doi:10.1111/j.1365-2567.2007.02682.x. PMC 2266047. PMID 17680800.
- ↑ Ohshima S, Kobayashi H, Yamaguchi N, Nishioka K, Umeshita-Sasai M, Mima T, Nomura S, Kon S, Inobe M, Uede T, Saeki Y (April 2002). "Expression of osteopontin at sites of bone erosion in a murine experimental arthritis model of collagen-induced arthritis: possible involvement of osteopontin in bone destruction in arthritis". Arthritis Rheum. 46 (4): 1094–101. doi:10.1002/art.10143. PMID 11953989.
- ↑ Sakata M, Tsuruha JI, Masuko-Hongo K, Nakamura H, Matsui T, Sudo A, Nishioka K, Kato T (July 2001). "Autoantibodies to osteopontin in patients with osteoarthritis and rheumatoid arthritis". J. Rheumatol. 28 (7): 1492–5. PMID 11469452.
- ↑ 65.0 65.1 Nagasaka A, Matsue H, Matsushima H, Aoki R, Nakamura Y, Kambe N, Kon S, Uede T, Shimada S (February 2008). "Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells". Eur. J. Immunol. 38 (2): 489–99. doi:10.1002/eji.200737057. PMID 18200503.
- ↑ Burdo TH, Wood MR, Fox HS (June 2007). "Osteopontin prevents monocyte recirculation and apoptosis". J. Leukoc. Biol. 81 (6): 1504–11. doi:10.1189/jlb.1106711. PMC 2490714. PMID 17369493.
- ↑ Crawford HC, Matrisian LM, Liaw L (November 1998). "Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo". Cancer Res. 58 (22): 5206–15. PMID 9823334.
- ↑ Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS (May 2001). "Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival". J. Clin. Invest. 107 (9): 1055–61. doi:10.1172/JCI12980. PMC 209291. PMID 11342566.
- ↑ Standal T, Borset M, Sundan A (September 2004). "Role of osteopontin in adhesion, migration, cell survival and bone remodeling". Exp. Oncol. 26 (3): 179–84. PMID 15494684.
- ↑ Da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R (September 2006). "Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death". J. Cell. Physiol. 208 (3): 629–39. doi:10.1002/jcp.20701. PMID 16741956.
- ↑ Yumoto K, Ishijima M, Rittling SR, Tsuji K, Tsuchiya Y, Kon S, Nifuji A, Uede T, Denhardt DT, Noda M (April 2002). "Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice". Proc. Natl. Acad. Sci. U.S.A. 99 (7): 4556–61. doi:10.1073/pnas.052523599. PMC 123686. PMID 11930008.
- ↑ Jacobs JP, Pettit AR, Shinohara ML, Jansson M, Cantor H, Gravallese EM, Mathis D, Benoist C (August 2004). "Lack of requirement of osteopontin for inflammation, bone erosion, and cartilage damage in the K/BxN model of autoantibody-mediated arthritis". Arthritis Rheum. 50 (8): 2685–94. doi:10.1002/art.20381. PMID 15334485.
- ↑ Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L (November 2001). "The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease". Science. 294 (5547): 1731–5. doi:10.1126/science.1062960. PMID 11721059.
- ↑ Steinman L (February 2007). "A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage". Nat. Med. 13 (2): 139–45. doi:10.1038/nm1551. PMID 17290272.
- ↑ "Gel 'to speed up wound healing'". Health. BBC NEWS. 2008-01-22. Retrieved 2009-01-26.
- ↑ 76.0 76.1 Gassler N, Autschbach F, Gauer S, Bohn J, Sido B, Otto HF, Geiger H, Obermüller N (November 2002). "Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum". Scand J Gastroenterol. 37 (11): 1286–95. doi:10.1080/003655202761020560. PMID 12465727.
- ↑ Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, Fukushima T, Uede T, Hibi T (September 2005). "Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response". Gut. 54 (9): 1254–62. doi:10.1136/gut.2004.048298. PMC 1774642. PMID 16099792.
- ↑ Mishima R, Takeshima F, Sawai T, Ohba K, Ohnita K, Isomoto H, Omagari K, Mizuta Y, Ozono Y, Kohno S (February 2007). "High plasma osteopontin levels in patients with inflammatory bowel disease". J Clin Gastroenterol. 41 (2): 167–72. doi:10.1097/MCG.0b013e31802d6268. PMID 17245215.
- ↑ 79.0 79.1 Kourepini E, Aggelakopoulou M, Alissafi T, Paschalidis N, Simoes DC, Panoutsakopoulou V (March 2014). "Osteopontin expression by CD103- dendritic cells drives intestinal inflammation". Proc Natl Acad Sci U S A. 111 (9): E856–E865. doi:10.1073/pnas.1316447111. PMC 3948306. PMID 24550510.
- ↑ 80.0 80.1 80.2 80.3 80.4 Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, Lambrecht BN, Lloyd CM, Panoutsakopoulou V (May 2007). "Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets". Nat. Med. 13 (5): 570–9. doi:10.1038/nm1580. PMC 3384679. PMID 17435770.
- ↑ Simoes DC, Xanthou G, Petrochilou K, Panoutsakopoulou V, Roussos C, Gratziou C (May 2009). "Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma". Am J Respir Crit Care Med. 179 (10): 894–902. doi:10.1164/rccm.200807-1081OC. PMID 19234104.
- ↑ Samitas K, Zervas E, Vittorakis S, Semitekolou M, Alissafi T, Bossios A, Gogos H, Economidou E, Lötvall J, Xanthou G, Panoutsakopoulou V, Gaga M (2010). "Osteopontin expression and relation to disease severity in human asthma". Eur. Respir. J. 37 (2): 331–41. doi:10.1183/09031936.00017810. PMID 20562127.
- ↑ Hillas G, Loukides S, Kostikas K, Simoes D, Petta V, Konstantellou E, Emmanouil P, Papiris S, Koulouris N, Bakakos P (Jan 2013). "Increased levels of osteopontin in sputum supernatant of smoking asthmatics". Cytokine. 61 (1): 251–5. doi:10.1016/j.cyto.2012.10.002. PMID 23098767.
- ↑ Samitas K, Zervas E, Xanthou G, Panoutsakopoulou V, Gaga M (Feb 2013). "Osteopontin is increased in the bronchoalveolar lavage fluid and bronchial tissue of smoking asthmatics". Cytokine. 61 (3): 713–5. doi:10.1016/j.cyto.2012.12.028. PMID 23384656.
- ↑ Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH (May 2002). "A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice". Hum Mol Genet. 11 (3): 263–72. doi:10.1093/hmg/11.3.263. PMID 11823445.
- ↑ Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM (2002). "Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle". Proc Natl Acad Sci U S A. 99 (23): 15000–15005. doi:10.1073/pnas.192571199. PMC 137534. PMID 12415109.
- ↑ Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, Motoyoshi K, Kamakura K, Miyagoe-Suzuki Y, Takeda S (2003). "Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin". Am J Pathol. 163 (1): 203–215. doi:10.1016/S0002-9440(10)63644-9. PMC 1868192. PMID 12819025.
- ↑ Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ (2009). "Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta". J Clin Invest. 119 (6): 1583–1594. doi:10.1172/JCI37662. PMC 2689112. PMID 19451692.
- ↑ Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM (2011). "SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy". Neurology. 76 (3): 219–226. doi:10.1212/WNL.0b013e318207afeb. PMC 3034396. PMID 21178099.
- ↑ El Deeb S, Abdelnaby R, Khachab A, Bläsius K, Tingart M, Rath B (July 2016). "Osteopontin as a biochemical marker and severity indicator for idiopathic hip osteoarthritis". Hip International : The Journal of Clinical and Experimental Research on Hip Pathology and Therapy. 26 (4): 397–403. doi:10.5301/hipint.5000361. PMID 27229171.
- ↑ Kang YJ, Forbes K, Carver J, Aplin JD (2014). "The role of the osteopontin-integrin αvβ3 interaction at implantation: functional analysis using three different in vitro models". Human Reproduction (Oxford, England). 29 (4): 739–49. doi:10.1093/humrep/det433. PMID 24442579.
- ↑ Johnson GA, Burghardt RC, Bazer FW, Spencer TE (2003). "Osteopontin: roles in implantation and placentation". Biology of Reproduction. 69 (5): 1458–71. doi:10.1095/biolreprod.103.020651. PMID 12890718.
Additional images
Further reading
- Fujisawa R (2002). "[Recent advances in research on bone matrix proteins]". Nippon Rinsho. 60. Suppl 3: 72–8. PMID 11979972.
- Denhardt DT, Mistretta D, Chambers AF, Krishna S, Porter JF, Raghuram S, Rittling SR (2003). "Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter". Clin. Exp. Metastasis. 20 (1): 77–84. doi:10.1023/A:1022550721404. PMID 12650610.
- Yeatman TJ, Chambers AF (2003). "Osteopontin and colon cancer progression". Clin. Exp. Metastasis. 20 (1): 85–90. doi:10.1023/A:1022502805474. PMID 12650611.
- O'Regan A (2004). "The role of osteopontin in lung disease". Cytokine Growth Factor Rev. 14 (6): 479–88. doi:10.1016/S1359-6101(03)00055-8. PMID 14563350.
- Wai PY, Kuo PC (2004). "The role of Osteopontin in tumor metastasis". J. Surg. Res. 121 (2): 228–41. doi:10.1016/j.jss.2004.03.028. PMID 15501463.
- Konno S, Hizawa N, Nishimura M, Huang SK (2007). "Osteopontin: a potential biomarker for successful bee venom immunotherapy and a potential molecule for inhibiting IgE-mediated allergic responses". Allergology International. 55 (4): 355–9. doi:10.2332/allergolint.55.355. PMID 17130676.
- Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mänsson H (2007). "The role of osteopontin in tumor progression and metastasis in breast cancer". Cancer Epidemiol. Biomarkers Prev. 16 (6): 1087–97. doi:10.1158/1055-9965.EPI-06-1008. PMID 17548669.
- Ramaiah SK, Rittling S (2007). "Role of osteopontin in regulating hepatic inflammatory responses and toxic liver injury". Expert Opinion on Drug Metabolism & Toxicology. 3 (4): 519–26. doi:10.1517/17425225.3.4.519. PMID 17696803.
External links
- Osteopontin at the US National Library of Medicine Medical Subject Headings (MeSH)