Hypopituitarism pathophysiology
Template:DiseaseDisorder infobox
|
Hypopituitarism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hypopituitarism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hypopituitarism pathophysiology |
|
Risk calculators and risk factors for Hypopituitarism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Elsaiey, MBBCH [2], Iqra Qamar M.D.[3]
Overview
Hypopituitarism occurrs secondarly to ischemia of the pituitary gland. This ischemia can be due to hemorrhage, tumors, or brain injury. Compression of the blood vessels is one of the mechanisms that cause ischemia to the pituitary gland and leads to hypopituitarism. Pituitary adenomas cause compression of the hypophyseal vessels leading to interruption in the blood supply of the pituitary gland. Traumatic brain injury either primary or secondary also leads to pituitary gland dysfunction.
Pathophysiology
Background on pituitary gland blood supply
- In order to understand the pathophysiology of hypopituitarism, it is necessary to know the blood supply of the pituitary gland, because hypopituitarism occurs mainly by ischemia through different mechanisms like hemorrhage, tumors or brain injury.[1][2]
- The pituitary gland is composed of two parts anterior (adenohypophysis) and posterior (neurohypophysis). Both parts are supplied by the carotid arteries.
- Adenohypophysis: It receives blood supply from the long and short hypophyseal arteries, which arise from the internal carotid artery and the anterior portion of the circle of Willis.
- Neurohypophysis: It receives the blood supply from the inferior and middle hypophyseal arteries.
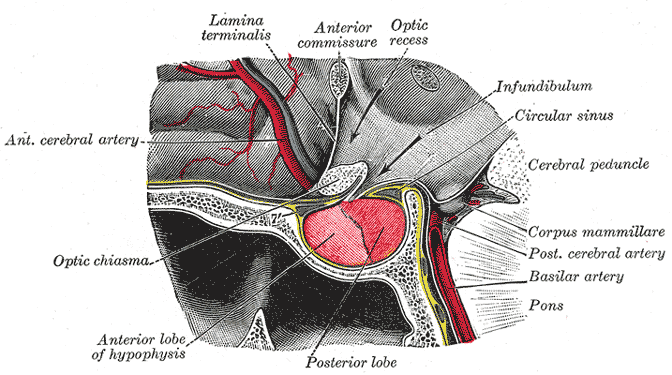
Anterior pituitary (Adenohypophysis)
- Anterior pituitary does not have a direct blood supply and is supplied by the hypophyseal portal system.
- The hypophyseal portal system is a fenestrated set of capillaries that allows rapid exchange of hormones between the hypothalamus and anterior pituitary.
- Occlusion of the blood supply and other vascular abnormalities of the hypophyseal portal system may also cause complications with the exchange of hormones between the hypothalamus and the pituitary gland leading to hypopituitarism.
Posterior pituitary (Neurohypophysis)
- Posterior pituitary has its own blood supply via inferior hypophyseal artery and is less commonly affected as compared to anterior pituitary.
- If posterior pituitary is affected, it can result in neurohypophseal dysfunction and ischemic necrosis of thirst center leading to increased osmotic threshold for thirst onset.[4]
Hypothalamic and pituitary hormones with their action on the target glands
| Hypothalamic hormone | Axis involved | Mode of action | Pituitary hormone
or target organ |
Action | |
|---|---|---|---|---|---|
| Anterior pituitary hormones | Thyrotropin-releasing hormone | Hypothalmic-pituitary-thyroid (HPT) axis
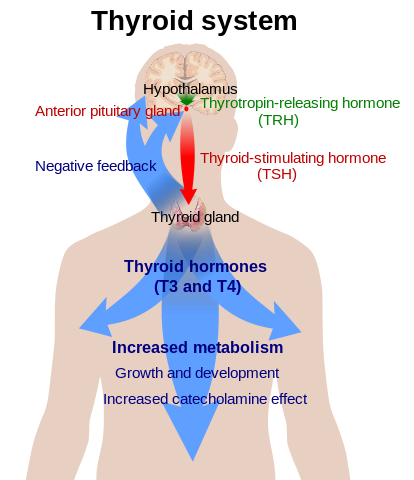 |
Stimulatory | Thyrotropin | Stimulates triiodothyronine and thyroxine production |
| Corticotropin-releasing hormone | Hypothalmic-pituitary-adrenal (HPA) axis
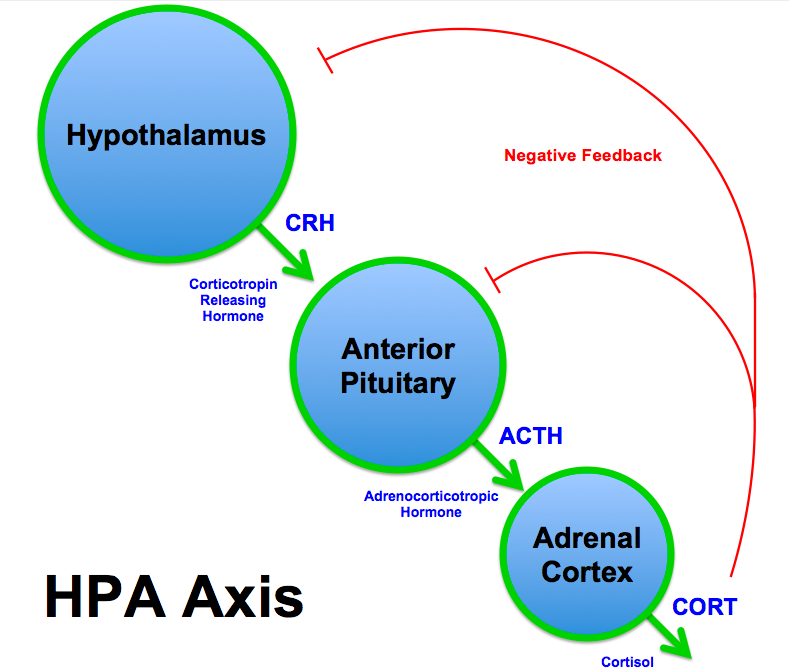 |
Stimulatory | Corticotropin | Stimulates production of cortisol and adrenal androgens | |
| Stimulatory | Prolactin | Stimulates milk production from breasts in females | |||
| Gonadotropin-releasing hormone | Hypothalamus pituitary testicles (HPG) axis
 |
Stimulatory |
| ||
| Dopamine | Inhibitory | Prolactin | _ | ||
| Growth hormone-releasing hormone |
 |
Stimulatory | Growth hormone | Stimulates insulin-like growth factor 1 production | |
| Somatostatin | Inhibitory | Growth hormone | _ | ||
| Posterior pituitary hormones | Vasopressin | Stimulatory | Stimulates free water reabsorption in the collecting ducts | ||
| Oxytocin | Stimulatory | Breast, uterus | Stimulates milk ejection and uterine contraction |
Pathogenesis
- Hypopituitarism occurrs mainly due to the destruction of the pituitary gland cells via ischemia, inflammation or infiltration. However, ischemia is believed to be the cornerstone of the pathogenesis of hypopituitarism.[9][10]
Compression of the blood vessels
- Pituitary adenoma (secretory or non-secretory) is one of the common causes of compression of the hypophyseal vessels resulting in ischemia.[11]
- Large adenomas (more than 1.5 cm) cause greater loss of function than microadenomas. They cause compression (due to a greater mass effect) of the pituitary stalk and the hypophyseal portal vessels, which supply the pituitary gland with blood.
- The tumor growth in the pituitary gland participates in increasing the intrasellar pressure, which causes a decrease in the hypophyseal blood flow leading to a decrease of hormonal delivery from the hypothalamus to the pituitary gland.
- Pituitary gland enlargement due to hypertrophy and hyperplasia of lactotrophic cells in anterior pituitary result in superior hypophyseal artery compression, which may be complicated by decreased portal pressure and vasospasm during delivery, playing an important role in the pathogenesis of Sheehan's syndrome leading to hypopituitarism.[12]
(d) Meningioma
Traumatic brain injury (TBI)
Hypopituitarism may occur via any of the following mechanisms:
(b) Trauma
- The anatomical sitting of the pituitary gland increases its susceptibility to getting injured from trauma.
- It is believed that tissue necrosis results in the release of sequestered antigens, precipitating autoimmunity of the pituitary gland and hypopituitarism in Sheehan's syndrome.[13][14][15]
(c) Brain injury due to other events like hypotension or hypoxia
Genetics
Hypopituitarism is caused by a mutation in any one of the following genes.[16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]
| Isolated
hormonal abnormalities |
Gene | Inheritance
[Autosomal Recessive (AR), Autosomal Dominant (AD), X-linked (XL)] |
Phenotype |
|---|---|---|---|
| GH1 | AD, AR | Isolated GH deficiency | |
| GHRHR | AR | Isolated GH deficiency | |
| TSHB | AR | Isolated TSH deficiency | |
| TRHR | AR | Isolated TSH deficiency | |
| TPIT | AR | Isolated ACTH deficiency | |
| GnRHR | AR | HH | |
| PC1 | AR | ACTH deficiency, hypoglycemia, HH, obesity | |
| POMC | AR | ACTH deficiency, obesity, red hair | |
| DAX1 | XL | Adrenal hypoplasia congenital and HH | |
| CRH | AR | CRH deficiency | |
| KAL1 | XL | Kallman syndrome, renal agenesis, synkinesia | |
| FGFR1 | AD, AR | Kallman syndrome, cleft lip and palate, facial dysmorphism | |
| Leptin | AR | HH, obesity | |
| Leptin-R | AR | HH, obesity | |
| GPR54 | AR | HH | |
| Kisspeptin | AR | HH | |
| FSHB | AR | Primary amenorrhea, defective spermatogenesis | |
| LHB | AR | Delayed puberty | |
| PROK2 | AD | Kallman syndrome, severe sleep disorder, obesity | |
| PROKR2 | AD, AR | Kallman syndrome | |
| AVP-NPII | AR, AD | Diabetes insipidus | |
| Combined pituitary hormone deficiency | POU1F1 | AR, AD | GH, TSH, and prolactin deficiencies |
| PROP1 | AR | GH, TSH, LH, FSH, prolactin, and evolving ACTH deficiencies | |
| Specific syndromes | HESX1 | AR, AD | Septo-optic dysplasia |
| LHX3 | AR | GH, TSH, LH, FSH, prolactin deficiencies, limited neck rotation | |
| LHX4 | AD | GH, TSH, ACTH deficiencies with cerebellar abnormalities | |
| SOX3 | XL | Hypopituitarism and mental retardation | |
| GLI2 | AD | Holoprosencephaly and multiple midline defects | |
| SOX2 | AD | Anophthalmia, hypopituitarism, oesophageal atresia | |
| GLI3 | AD | Pallister-Hall syndrome | |
| PITX2 | AD | Rieger syndrome |
Gross pathology
- The predominant finding is hemorrhage with or without necrosis.
- Pale, necrotic material is particularly found when there is a long interval between the acute clinical event and surgery.
Microscopic pathology
On microscopy, the following findings may be observed:
- Ischemic necrosis leading to scarring of neurohypophysis
- Scarring of paraventricular and supraoptic nuclei
- Electron microscopic shows evidence of abnormal fenestration of tumor vessels (pituitary adenoma) with fragmented basal membranes that may predispose the patient to hemorrhage

References
- ↑ Dusick JR, Wang C, Cohan P, Swerdloff R, Kelly DF (2012). "Pathophysiology of hypopituitarism in the setting of brain injury". Pituitary. 15 (1): 2–9. doi:10.1007/s11102-008-0130-6. PMC 4170072. PMID 18481181.
- ↑ Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E (2007). "Hypopituitarism". Lancet. 369 (9571): 1461–70. doi:10.1016/S0140-6736(07)60673-4. PMID 17467517.
- ↑ Henry Gray (1918) Anatomy of the Human Body, Bartleby.com: Gray's Anatomy, Plate 721, Public Domain, <https://commons.wikimedia.org/w/index.php?curid=541543>
- ↑ Atmaca H, Tanriverdi F, Gokce C, Unluhizarci K, Kelestimur F (2007). "Posterior pituitary function in Sheehan's syndrome". Eur. J. Endocrinol. 156 (5): 563–7. doi:10.1530/EJE-06-0727. PMID 17468192.
- ↑ All used images are in public domain. Public Domain<https://commons.wikimedia.org/w/index.php?curid=8567011>
- ↑ CC BY-SA 3.0 <https://commons.wikimedia.org/w/index.php?curid=23363130>
- ↑ Acraciaderivative work: Boghog2 - Testosterona-ciclo.png, CC BY-SA 3.0, <https://commons.wikimedia.org/w/index.php?curid=11186183>
- ↑ Used with permission. All used images are in public domain., Public Domain,<https://commons.wikimedia.org/w/index.php?curid=6699074>
- ↑ Arafah BM (2002). "Medical management of hypopituitarism in patients with pituitary adenomas". Pituitary. 5 (2): 109–17. PMID 12675508.
- ↑ Vance, Mary Lee (1994). "Hypopituitarism". New England Journal of Medicine. 330 (23): 1651–1662. doi:10.1056/NEJM199406093302306. ISSN 0028-4793.
- ↑ Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR (2000). "The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas". J Clin Endocrinol Metab. 85 (5): 1789–93. doi:10.1210/jcem.85.5.6611. PMID 10843153.
- ↑ Scheithauer BW, Sano T, Kovacs KT, Young WF, Ryan N, Randall RV (1990). "The pituitary gland in pregnancy: a clinicopathologic and immunohistochemical study of 69 cases". Mayo Clin. Proc. 65 (4): 461–74. PMID 2159093.
- ↑ Goswami R, Kochupillai N, Crock PA, Jaleel A, Gupta N (2002). "Pituitary autoimmunity in patients with Sheehan's syndrome". J. Clin. Endocrinol. Metab. 87 (9): 4137–41. doi:10.1210/jc.2001-020242. PMID 12213861.
- ↑ "AUTOANTIBODIES IN SHEEHAN'S SYNDROME - ScienceDirect".
- ↑ Falorni A, Minarelli V, Bartoloni E, Alunno A, Gerli R (2014). "Diagnosis and classification of autoimmune hypophysitis". Autoimmun Rev. 13 (4–5): 412–6. doi:10.1016/j.autrev.2014.01.021. PMID 24434361.
- ↑ Carvalho LR, Woods KS, Mendonca BB, Marcal N, Zamparini AL, Stifani S, Brickman JM, Arnhold IJ, Dattani MT (2003). "A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction". J. Clin. Invest. 112 (8): 1192–201. doi:10.1172/JCI18589. PMC 213489. PMID 14561704.
- ↑ Sobrier ML, Maghnie M, Vié-Luton MP, Secco A, di Iorgi N, Lorini R, Amselem S (2006). "Novel HESX1 mutations associated with a life-threatening neonatal phenotype, pituitary aplasia, but normally located posterior pituitary and no optic nerve abnormalities". J. Clin. Endocrinol. Metab. 91 (11): 4528–36. doi:10.1210/jc.2006-0426. PMID 16940453.
- ↑ Netchine I, Sobrier ML, Krude H, Schnabel D, Maghnie M, Marcos E, Duriez B, Cacheux V, Moers A, Goossens M, Grüters A, Amselem S (2000). "Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency". Nat. Genet. 25 (2): 182–6. doi:10.1038/76041. PMID 10835633. Vancouver style error: initials (help)
- ↑ Machinis K, Pantel J, Netchine I, Léger J, Camand OJ, Sobrier ML, Dastot-Le Moal F, Duquesnoy P, Abitbol M, Czernichow P, Amselem S (2001). "Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4". Am. J. Hum. Genet. 69 (5): 961–8. PMC 1274372. PMID 11567216.
- ↑ Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O'Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, Rosenfeld MG (1998). "Mutations in PROP1 cause familial combined pituitary hormone deficiency". Nat. Genet. 18 (2): 147–9. doi:10.1038/ng0298-147. PMID 9462743.
- ↑ Pellegrini-Bouiller I, Bélicar P, Barlier A, Gunz G, Charvet JP, Jaquet P, Brue T, Vialettes B, Enjalbert A (1996). "A new mutation of the gene encoding the transcription factor Pit-1 is responsible for combined pituitary hormone deficiency". J. Clin. Endocrinol. Metab. 81 (8): 2790–6. doi:10.1210/jcem.81.8.8768831. PMID 8768831.
- ↑ Pfäffle RW, DiMattia GE, Parks JS, Brown MR, Wit JM, Jansen M, Van der Nat H, Van den Brande JL, Rosenfeld MG, Ingraham HA (1992). "Mutation of the POU-specific domain of Pit-1 and hypopituitarism without pituitary hypoplasia". Science. 257 (5073): 1118–21. PMID 1509263.
- ↑ Turton JP, Reynaud R, Mehta A, Torpiano J, Saveanu A, Woods KS, Tiulpakov A, Zdravkovic V, Hamilton J, Attard-Montalto S, Parascandalo R, Vella C, Clayton PE, Shalet S, Barton J, Brue T, Dattani MT (2005). "Novel mutations within the POU1F1 gene associated with variable combined pituitary hormone deficiency". J. Clin. Endocrinol. Metab. 90 (8): 4762–70. doi:10.1210/jc.2005-0570. PMID 15928241.
- ↑ Bhangoo AP, Hunter CS, Savage JJ, Anhalt H, Pavlakis S, Walvoord EC, Ten S, Rhodes SJ (2006). "Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency". J. Clin. Endocrinol. Metab. 91 (3): 747–53. doi:10.1210/jc.2005-2360. PMID 16394081.
- ↑ Cogan JD, Wu W, Phillips JA, Arnhold IJ, Agapito A, Fofanova OV, Osorio MG, Bircan I, Moreno A, Mendonca BB (1998). "The PROP1 2-base pair deletion is a common cause of combined pituitary hormone deficiency". J. Clin. Endocrinol. Metab. 83 (9): 3346–9. doi:10.1210/jcem.83.9.5142. PMID 9745452.
- ↑ Flück C, Deladoey J, Rutishauser K, Eblé A, Marti U, Wu W, Mullis PE (1998). "Phenotypic variability in familial combined pituitary hormone deficiency caused by a PROP1 gene mutation resulting in the substitution of Arg-->Cys at codon 120 (R120C)". J. Clin. Endocrinol. Metab. 83 (10): 3727–34. doi:10.1210/jcem.83.10.5172. PMID 9768691.
- ↑ Rosenbloom AL, Almonte AS, Brown MR, Fisher DA, Baumbach L, Parks JS (1999). "Clinical and biochemical phenotype of familial anterior hypopituitarism from mutation of the PROP1 gene". J. Clin. Endocrinol. Metab. 84 (1): 50–7. doi:10.1210/jcem.84.1.5366. PMID 9920061.
- ↑ Pernasetti F, Toledo SP, Vasilyev VV, Hayashida CY, Cogan JD, Ferrari C, Lourenço DM, Mellon PL (2000). "Impaired adrenocorticotropin-adrenal axis in combined pituitary hormone deficiency caused by a two-base pair deletion (301-302delAG) in the prophet of Pit-1 gene". J. Clin. Endocrinol. Metab. 85 (1): 390–7. doi:10.1210/jcem.85.1.6324. PMID 10634415.
- ↑ Lee JK, Zhu YS, Cordero JJ, Cai LQ, Labour I, Herrera C, Imperato-McGinley J (2004). "Long-term growth hormone therapy in adulthood results in significant linear growth in siblings with a PROP-1 gene mutation". J. Clin. Endocrinol. Metab. 89 (10): 4850–6. doi:10.1210/jc.2003-031816. PMID 15472175.
- ↑ Yamamoto M, Iguchi G, Takeno R, Okimura Y, Sano T, Takahashi M, Nishizawa H, Handayaningshi AE, Fukuoka H, Tobita M, Saitoh T, Tojo K, Mokubo A, Morinobu A, Iida K, Kaji H, Seino S, Chihara K, Takahashi Y (2011). "Adult combined GH, prolactin, and TSH deficiency associated with circulating PIT-1 antibody in humans". J. Clin. Invest. 121 (1): 113–9. doi:10.1172/JCI44073. PMC 3007153. PMID 21123951.
- ↑ Vallette-Kasic S, Brue T, Pulichino AM, Gueydan M, Barlier A, David M, Nicolino M, Malpuech G, Déchelotte P, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch CJ, Sippell WG, Berberoglu M, Atasay B, de Zegher F, Beckers D, Kyllo J, Donohoue P, Fassnacht M, Hahner S, Allolio B, Noordam C, Dunkel L, Hero M, Pigeon B, Weill J, Yigit S, Brauner R, Heinrich JJ, Cummings E, Riddell C, Enjalbert A, Drouin J (2005). "Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death, explained by TPIT gene mutations". J. Clin. Endocrinol. Metab. 90 (3): 1323–31. doi:10.1210/jc.2004-1300. PMID 15613420.
- ↑ Yang Y, Guo QH, Wang BA, Dou JT, Lv ZH, Ba JM, Lu JM, Pan CY, Mu YM (2013). "Pituitary stalk interruption syndrome in 58 Chinese patients: clinical features and genetic analysis". Clin. Endocrinol. (Oxf). 79 (1): 86–92. doi:10.1111/cen.12116. PMID 23199197.
- ↑ Wang W, Wang S, Jiang Y, Yan F, Su T, Zhou W, Jiang L, Zhang Y, Ning G (2015). "Relationship between pituitary stalk (PS) visibility and the severity of hormone deficiencies: PS interruption syndrome revisited". Clin. Endocrinol. (Oxf). 83 (3): 369–76. doi:10.1111/cen.12788. PMID 25845766.
- ↑ Mendonca BB, Osorio MG, Latronico AC, Estefan V, Lo LS, Arnhold IJ (1999). "Longitudinal hormonal and pituitary imaging changes in two females with combined pituitary hormone deficiency due to deletion of A301,G302 in the PROP1 gene". J. Clin. Endocrinol. Metab. 84 (3): 942–5. doi:10.1210/jcem.84.3.5537. PMID 10084575.
- ↑ De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A (2005). "Primary empty sella". J. Clin. Endocrinol. Metab. 90 (9): 5471–7. doi:10.1210/jc.2005-0288. PMID 15972577.
- ↑ <http://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Common