Tuberculosis medical therapy
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis medical therapy On the Web |
|
American Roentgen Ray Society Images of Tuberculosis medical therapy |
|
Risk calculators and risk factors for Tuberculosis medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor-in-Chief: Somal Khan
Overview
Tuberculosis, or TB is a bacterial infection that kills 3 million people worldwide, more people than any other infection in the world. Approximately one-third of the world is infected, and 15 million people in the US. Active tuberculosis kills 60% of the time if not treated, but treatment cures 90% of patients. Most people are infected with TB have latent TB. This means that the bacteria is controlled by the body's immune system. People with latent TB do not have symptoms and cannot transmit TB to other people. However, later if the infected person has a weakened immune system (AIDS, young children, elderly, sick with other diseases, etc.), the bacteria can break out leading to active TB, or TB disease.
If their is a high probability of infection, presumptively treat the patient even if the stain is negative, while waiting for the culture results. The patient should be brought back in few weeks. Patients usually feel better a few weeks post-treatment. In the U.S., all TB is tested for drug resistance. Isoniazid (INH) resistant TB can be treated in same way as non-MDR TB
Responsibility for Successful Treatment
The overall goals for treatment of tuberculosis are: 1) to cure the individual patient, and 2) to minimize the transmission of Mycobacterium tuberculosis to other persons. Thus, successful treatment of tuberculosis has benefits both for the individual patient and the community in which the patient resides. For this reason the prescribing physician, be he/she in the public or private sector, is carrying out a public health function with responsibility not only for prescribing an appropriate regimen but also for successful completion of therapy. Prescribing physician responsibility for treatment completion is a fundamental principle in tuberculosis control. However, given a clear understanding of roles and responsibilities, oversight of treatment may be shared between a public health program and a private physician.
Organization and Supervision of Treatment
Treatment of patients with tuberculosis is most successful within a comprehensive framework that addresses both clinical and social issues of relevance to the patient. It is essential that treatment be tailored and supervision be based on each patient's clinical and social circumstances (patient-centered care). Patients may be managed in the private sector, by public health departments, or jointly, but in all cases the health department is ultimately responsible for ensuring that adequate, appropriate diagnostic and treatment services are available, and for monitoring the results of therapy.
It is strongly recommended that patient-centered care be the initial management strategy, regardless of the source of supervision. This strategy should always include an adherence plan that emphasizes directly observed therapy (DOT), in which patients are observed to ingest each dose of antituberculosis medications, to maximize the likelihood of completion of therapy. Programs utilizing DOT as the central element in a comprehensive, patient-centered approach to case management (enhanced DOT) have higher rates of treatment completion than less intensive strategies. Each patient's management plan should be individualized to incorporate measures that facilitate adherence to the drug regimen. Such measures may include, for example, social service support, treatment incentives and enablers, housing assistance, referral for treatment of substance abuse, and coordination of tuberculosis services with those of other providers.
Acute Pharmacotherapies
- Typical treatment for active pulmonary disease:
- RIPE for 6 months; all for first 2 months, then just INH & rifampin for last 4 months
- Rifampin little upfront activity, more active vs. latent TB; good at sterilizing tissues
- INH rapidly kills TB (always give with vitamin B6 because of risk peripheral neuropathy)
- Prazoloacridine (Pza) has upfront ability to kill bacteriain people with disease, only drug which can shorten duration of treatment from 9 to 6 months
- Ethambutol does nothing but protect vs. resistance to other drugs
- RIPE for 6 months; all for first 2 months, then just INH & rifampin for last 4 months
- First-Line Drugs
- Isoniazid
- Rifampin
- Rifabutin
- Rifapentine
- Pyrazinamide
- Ethambutol
- Fixed-dose combination preparations: Two combined preparations, INH and RIF (Rifamate®) and INH, RIF, and PZA (Rifater®).
- Second-Line Drugs
Recommended Treatment Regimens
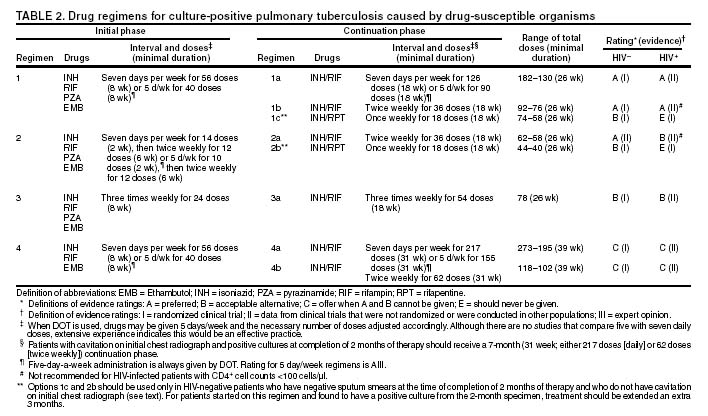
The recommended treatment regimens are, in large part, based on evidence from clinical trials and are rated on the basis of a system developed by the United States Public Health Service (USPHS) and the Infectious Diseases Society of America (IDSA).
There are four recommended regimens for treating patients with tuberculosis caused by drug-susceptible organisms. Although these regimens are broadly applicable, there are modifications that should be made under specified circumstances, described subsequently. Each regimen has an initial phase of 2 months followed by a choice of several options for the continuation phase of either 4 or 7 months.
Because of the relatively high proportion of adult patients with tuberculosis caused by organisms that are resistant to isoniazid, four drugs are necessary in the initial phase for the 6-month regimen to be maximally effective. Thus, in most circumstances, the treatment regimen for all adults with previously untreated tuberculosis should consist of a 2-month initial phase of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB). If (when) drug susceptibility test results are known and the organisms are fully susceptible, EMB need not be included. For children whose visual acuity cannot be monitored, EMB is usually not recommended except when there is an increased likelihood of the disease being caused by INH-resistant organisms or when the child has "adult-type" (upper lobe infiltration, cavity formation) tuberculosis. If PZA cannot be included in the initial phase of treatment, or if the isolate is resistant to PZA alone (an unusual circumstance), the initial phase should consist of INH, RIF, and EMB given daily for 2 months. Examples of circumstances in which PZA may be withheld include severe liver disease, gout, and, perhaps, pregnancy.
The initial phase may be given daily for 2 weeks and then twice weekly for 6 weeks, or three times weekly. For patients receiving daily therapy, EMB can be discontinued as soon as the results of drug susceptibility studies demonstrate that the isolate is susceptible to INH and RIF. When the patient is receiving less than daily drug administration, expert opinion suggests that EMB can be discontinued safely in less than 2 months (i.e., when susceptibility test results are known), but there is no evidence to support this approach.
Although clinical trials have shown that the efficacy of streptomycin (SM) is approximately equal to that of EMB in the initial phase of treatment, the increasing frequency of resistance to SM globally has made the drug less useful. Thus, SM is not recommended as being interchangeable with EMB unless the organism is known to be susceptible to the drug or the patient is from a population in which SM resistance is unlikely.
The continuation phase of treatment is given for either 4 or 7 months. The 4-month continuation phase should be used in the large majority of patients. The 7-month continuation phase is recommended only for three groups: patients with cavitary pulmonary tuberculosis caused by drug-susceptible organisms and whose sputum culture obtained at the time of completion of 2 months of treatment is positive; patients whose initial phase of treatment did not include PZA; and patients being treated with once weekly INH and rifapentine and whose sputum culture obtained at the time of completion of the initial phase is positive. The continuation phase may be given daily, two times weekly by DOT, or three times weekly by DOT. For human immunodeficiency virus (HIV)-seronegative patients with noncavitary pulmonary tuberculosis (as determined by standard chest radiography), and negative sputum smears at completion of 2 months of treatment, the continuation phase may consist of rifapentine and INH given once weekly for 4 months by DOT. If the culture at completion of the initial phase of treatment is positive, the once weekly INH and rifapentine continuation phase should be extended to 7 months. All of the 6-month regimens, except the INH--rifapentine once weekly continuation phase for persons with HIV infection, are rated as AI or AII, or BI or BII, in both HIV-infected and uninfected patients. The once-weekly continuation phase is contraindicated (Rating EI) in patients with HIV infection because of an unacceptable rate of failure/relapse, often with rifamycin-resistant organisms. For the same reason twice weekly treatment, either as part of the initial phase or continuation phase is not recommended for HIV-infected patients with CD4+ cell counts <100 cells/µl. These patients should receive either daily or three times weekly (continuation phase) treatment.
Deciding To Initiate Treatment
The decision to initiate combination antituberculosis chemotherapy should be based on epidemiologic information; clinical, pathological, and radiographic findings; and the results of microscopic examination of acid-fast bacilli (AFB)--stained sputum (smears) (as well as other appropriately collected diagnostic specimens) and cultures for mycobacteria. A purified protein derivative (PPD)-tuberculin skin test may be done at the time of initial evaluation, but a negative PPD-tuberculin skin test does not exclude the diagnosis of active tuberculosis. However, a positive PPD-tuberculin skin test supports the diagnosis of culture-negative pulmonary tuberculosis, as well as latent tuberculosis infection in persons with stable abnormal chest radiographs consistent with inactive tuberculosis.
If the suspicion of tuberculosis is high or the patient is seriously ill with a disorder, either pulmonary or extrapulmonary, that is thought possibly to be tuberculosis, combination chemotherapy using one of the recommended regimens should be initiated promptly, often before AFB smear results are known and usually before mycobacterial culture results have been obtained. A positive AFB smear provides strong inferential evidence for the diagnosis of tuberculosis. If the diagnosis is confirmed by isolation of M. tuberculosis or a positive nucleic acid amplification test, treatment can be continued to complete a standard course of therapy. When the initial AFB smears and cultures are negative, a diagnosis other than tuberculosis should be considered and appropriate evaluations undertaken. If no other diagnosis is established and the PPD-tuberculin skin test is positive (in this circumstance a reaction of 5 mm or greater induration is considered positive), empirical combination chemotherapy should be initiated. If there is a clinical or radiographic response within 2 months of initiation of therapy and no other diagnosis has been established, a diagnosis of culture-negative pulmonary tuberculosis can be made and treatment continued with an additional 2 months of INH and RIF to complete a total of 4 months of treatment, an adequate regimen for culture-negative pulmonary tuberculosis. If there is no clinical or radiographic response by 2 months, treatment can be stopped and other diagnoses including inactive tuberculosis considered.
If AFB smears are negative and suspicion for active tuberculosis is low, treatment can be deferred until the results of mycobacterial cultures are known and a comparison chest radiograph is available (usually within 2 months). In low-suspicion patients not initially being treated, if cultures are negative, the PPD-tuberculin skin test is positive (5 mm or greater induration), and the chest radiograph is unchanged after 2 months, one of the three regimens recommended for the treatment of latent tuberculosis infection could be used. These include INH for a total of 9 months, RIF with or without INH for a total of 4 months, or RIF and PZA for a total of 2 months. Because of reports of an increased rate of hepatotoxicity with the RIF--PZA regimen, it should be reserved for patients who are not likely to complete a longer course of treatment, can be monitored closely, and do not have contraindications to the use of this egimen.
Baseline and Follow-Up Evaluations
Patients suspected of having tuberculosis should have appropriate specimens collected for microscopic examination and mycobacterial culture. When the lung is the site of disease, three sputum specimens should be obtained. Sputum induction with hypertonic saline may be necessary to obtain specimens and bronchoscopy (both performed under appropriate infection control measures) may be considered for patients who are unable to produce sputum, depending on the clinical circumstances. Susceptibility testing for INH, RIF, and EMB should be performed on a positive initial culture, regardless of the source of the specimen. Second-line drug susceptibility testing should be done only in reference laboratories and be limited to specimens from patients who have had prior therapy, who are contacts of patients with drug-resistant tuberculosis, who have demonstrated resistance to rifampin or to other first-line drugs, or who have positive cultures after more than 3 months of treatment.
It is recommended that all patients with tuberculosis have counseling and testing for HIV infection, at least by the time treatment is initiated, if not earlier. For patients with HIV infection, a CD4+ lymphocyte count should be obtained. Patients with risk factors for hepatitis B or C viruses (e.g., injection drug use, foreign birth in Asia or Africa, HIV infection) should have serologic tests for these viruses. For all adult patients baseline measurements of serum amino transferases (aspartate aminotransferase [AST], alanine aminotransferase [ALT]), bilirubin, alkaline phosphatase, and serum creatinine and a platelet count should be obtained. Testing of visual acuity and red-green color discrimination should be obtained when EMB is to be used.
During treatment of patients with pulmonary tuberculosis, a sputum specimen for microscopic examination and culture should be obtained at a minimum of monthly intervals until two consecutive specimens are negative on culture. More frequent AFB smears may be useful to assess the early response to treatment and to provide an indication of infectiousness. For patients with extrapulmonary tuberculosis the frequency and kinds of evaluations will depend on the site involved. In addition, it is critical that patients have clinical evaluations at least monthly to identify possible adverse effects of the antituberculosis medications and to assess adherence. Generally, patients do not require follow-up after completion of therapy but should be instructed to seek care promptly if signs or symptoms recur.
Routine measurements of hepatic and renal function and platelet count are not necessary during treatment unless patients have baseline abnormalities or are at increased risk of hepatotoxicity (e.g., hepatitis B or C virus infection, alcohol abuse).
Identification and Management of Patients at Increased Risk of Treatment Failure and Relapse
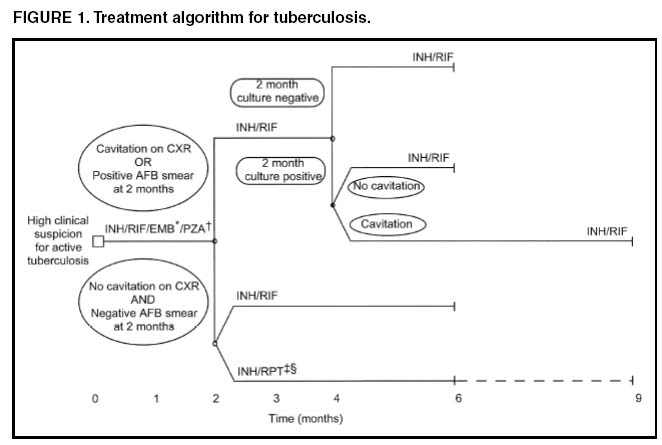
The presence of cavitation on the initial chest radiograph combined with having a positive sputum culture at the time the initial phase of treatment is completed has been shown in clinical trials to identify patients at high risk for adverse outcomes (treatment failure, usually defined by positive cultures after 4 months of treatment, or relapse, defined by recurrent tuberculosis at any time after completion of treatment and apparent cure). For this reason it is particularly important to conduct a microbiological evaluation 2 months after initiation of treatment (Figure 1). Approximately 80% of patients with pulmonary tuberculosis caused by drug-susceptible organisms who are started on standard four-drug therapy will have negative sputum cultures at this time. Patients with positive cultures after 2 months of treatment should undergo careful evaluation to determine the cause. For patients who have positive cultures after 2 months of treatment and have not been receiving DOT, the most common reason is nonadherence to the regimen. Other possibilities, especially for patients receiving DOT, include extensive cavitary disease at the time of diagnosis, drug resistance, malabsorption of drugs, laboratory error, and biological variation in response.
In USPHS Study 22, nearly 21% of patients in the control arm of the study (a continuation phase of twice weekly INH and RIF) who had both cavitation on the initial chest radiograph and a positive culture at the 2-month juncture relapsed. Patients who had only one of these factors (either cavitation or a positive 2-month culture) had relapse rates of 5--6% compared with 2% for patients who had neither risk factor. In view of this evidence, it is recommended that, for patients who have cavitation on the initial chest radiograph and whose 2-month culture is positive, the minimum duration of treatment should be 9 months (a total of 84--273 doses depending on whether the drugs are given daily or intermittently) (Figure 1 and Table 2). The recommendation to lengthen the continuation phase of treatment is based on expert opinion and on the results of a study of the optimal treatment duration for patients with silicotuberculosis showing that extending treatment from 6 to 8 months greatly reduced the rate of relapse (Rating AIII). The recommendation is also supported by the results of a trial in which the once weekly INH--rifapentine continuation phase was extended to 7 months for patients at high risk of relapse. The rate of relapse was reduced significantly compared with historical control subjects from another trial in which the continuation phase was 4 months.
For patients who have either cavitation on the initial film or a positive culture after completing the initial phase of treatment (i.e., at 2 months), the rates of relapse were 5--6%. In this group decisions to prolong the continuation phase should be made on an individual basis.
Completion of Treatment
A full course of therapy (completion of treatment) is determined more accurately by the total number of doses taken, not solely by the duration of therapy. For example, the "6-month" daily regimen (given 7 days/week; see below) should consist of at least 182 doses of INH and RIF, and 56 doses of PZA. Thus, 6 months is the minimum duration of treatment and accurately indicates the amount of time the drugs are given only if there are no interruptions in drug administration. In some cases, either because of drug toxicity or nonadherence to the treatment regimen, the specified number of doses cannot be administered within the targeted period. In such cases the goal is to deliver the specified number of doses within a recommended maximum time. For example, for a 6-month daily regimen the 182 doses should be administered within 9 months of beginning treatment. If treatment is not completed within this period, the patient should be assessed to determine the appropriate action to take---continuing treatment for a longer duration or restarting treatment from the beginning, either of which may require more restrictive measures to be used to ensure completion.
Clinical experience suggests that patients being managed by DOT administered 5 days/week have a rate of successful therapy equivalent to those being given drugs 7 days/week. Thus, "daily therapy" may be interpreted to mean DOT given 5 days/week and the required number of doses adjusted accordingly. For example, for the 6-month "daily" regimen given 5 days/week the planned total number of doses is 130. As an option, patients might be given the medications to take without DOT on weekends.
Interruptions in treatment may have a significant effect on the duration of therapy. Reinstitution of treatment must take into account the bacillary load of the patient, the point in time when the interruption occurred, and the duration of the interruption. In general, the earlier in treatment and the longer the duration of the interruption, the more serious the effect and the greater the need to restart therapy from the beginning.
Treatment in Special Situations
HIV infection
Recommendations for the treatment of tuberculosis in HIV-infected adults are, with a few exceptions, the same as those for HIV-uninfected adults. The INH--rifapentine once weekly continuation phase is contraindicated in HIV-infected patients because of an unacceptably high rate of relapse, frequently with organisms that have acquired resistance to rifamycins. The development of acquired rifampin resistance has also been noted among HIV-infected patients with advanced immunosuppression treated with twice weekly rifampin- or rifabutin-based regimens. Consequently, patients with CD4+ cell counts <100/µl should receive daily or three times weekly treatment. DOT and other adherence-promoting strategies are especially important for patients with HIV-related tuberculosis.
Management of HIV-related tuberculosis is complex and requires expertise in the management of both HIV disease and tuberculosis. Because HIV-infected patients are often taking numerous medications, some of which interact with antituberculosis medications, it is strongly encouraged that experts in the treatment of HIV-related tuberculosis be consulted. A particular concern is the interaction of rifamycins with antiretroviral agents and other antiinfective drugs. Rifampin can be used for the treatment of tuberculosis with certain combinations of antiretroviral agents. Rifabutin, which has fewer problematic drug interactions, may also be used in place of rifampin and appears to be equally effective although the doses of rifabutin and antiretroviral agents may require adjustment. As new antiretroviral agents and more pharmacokinetic data become available, these recommendations are likely to be modified.
On occasion, patients with HIV-related tuberculosis may experience a temporary exacerbation of symptoms, signs, or radiographic manifestations of tuberculosis while receiving antituberculosis treatment. This clinical or radiographic worsening (paradoxical reaction) occurs in HIV-infected patients with active tuberculosis and is thought to be the result of immune reconstitution as a consequence of effective antiretroviral therapy. Symptoms and signs may include high fevers, lymphadenopathy, expanding central nervous system lesions, and worsening of chest radiographic findings. The diagnosis of a paradoxical reaction should be made only after a thorough evaluation has excluded other etiologies, particularly tuberculosis treatment failure. Nonsteroidal antiinflammatory agents may be useful for symptomatic relief. For severe paradoxical reactions, prednisone (1--2 mg/kg per day for 1--2 weeks, then in gradually decreasing doses) may be used, although there are no data from controlled trials to support this approach.
Children
Because of the high risk of disseminated tuberculosis in infants and children younger than 4 years of age, treatment should be started as soon as the diagnosis of tuberculosis is suspected. In general, the regimens recommended for adults are also the regimens of choice for infants, children, and adolescents with tuberculosis, with the exception that ethambutol is not used routinely in children. Because there is a lower bacillary burden in childhood-type tuberculosis there is less concern with the development of acquired drug resistance. However, children and adolescents may develop "adult-type" tuberculosis with upper lobe infiltration, cavitation, and sputum production. In such situations an initial phase of four drugs should be given until susceptibility is proven. When clinical or epidemiologic circumstances suggest an increased probability of INH resistance, EMB can be used safely at a dose of 15--20 mg/kg per day, even in children too young for routine eye testing. Streptomycin, kanamycin, or amikacin also can be used as the fourth drug, when necessary.
Most studies of treatment in children have used 6 months of INH and RIF supplemented during the first 2 months with PZA. This three-drug combination has a success rate of greater than 95% and an adverse drug reaction rate of less than 2%. Most treatment studies of intermittent dosing in children have used daily drug administration for the first 2 weeks to 2 months. DOT should always be used in treating children.
Because it is difficult to isolate M. tuberculosis from a child with pulmonary tuberculosis, it is frequently necessary to rely on the results of drug susceptibility tests of the organisms isolated from the presumed source case to guide the choice of drugs for the child. In cases of suspected drug-resistant tuberculosis in a child or when a source case isolate is not available, specimens for microbiological evaluation should be obtained via early morning gastric aspiration, bronchoalveolar lavage, or biopsy.
In general, extrapulmonary tuberculosis in children can be treated with the same regimens as pulmonary disease. Exceptions are disseminated tuberculosis and tuberculous meningitis, for which there are inadequate data to support 6-month therapy; thus 9--12 months of treatment is recommended.
The optimal treatment of pulmonary tuberculosis in children and adolescents with HIV infection is unknown. The American Academy of Pediatrics recommends that initial therapy should always include at least three drugs, and the total duration of therapy should be at least 9 months, although there are no data to support this recommendation.
Extrapulmonary tuberculosis
The basic principles that underlie the treatment of pulmonary tuberculosis also apply to extrapulmonary forms of the disease. Although relatively few studies have examined treatment of extrapulmonary tuberculosis, increasing evidence suggests that 6- to 9-month regimens that include INH and RIF are effective. Thus, a 6-month course of therapy is recommended for treating tuberculosis involving any site with the exception of the meninges, for which a 9- 12-month regimen is recommended. Prolongation of therapy also should be considered for patients with tuberculosis in any site that is slow to respond. The addition of corticosteroids is recommended for patients with tuberculous pericarditis and tuberculous meningitis.
Culture-negative pulmonary tuberculosis and radiographic evidence of prior pulmonary tuberculosis
Failure to isolate M. tuberculosis from persons suspected of having pulmonary tuberculosis on the basis of clinical features and chest radiographic examination does not exclude a diagnosis of active tuberculosis. Alternative diagnoses should be considered carefully and further appropriate diagnostic studies undertaken in persons with apparent culture-negative tuberculosis. The general approach to management is shown in Figure 2. A diagnosis of tuberculosis can be strongly inferred by the clinical and radiographic response to antituberculosis treatment. Careful reevaluation should be performed after 2 months of therapy to determine whether there has been a response attributable to antituberculosis treatment. If either clinical or radiographic improvement is noted and no other etiology is identified, treatment should be continued for active tuberculosis. Treatment regimens in this circumstance include one of the standard 6-month chemotherapy regimens or INH, RIF, PZA, and EMB for 2 months followed by INH and RIF for an additional 2 months (4 months total). However, HIV-infected patients with culture-negative pulmonary tuberculosis should be treated for a minimum of 6 months.
Persons with a positive tuberculin skin test who have radiographic evidence of prior tuberculosis (e.g., upper lobe fibronodular infiltrations) but who have not received adequate therapy are at increased risk for the subsequent development of tuberculosis. Unless previous radiographs are available showing that the abnormality is stable, it is recommended that sputum examination (using sputum induction if necessary) be performed to assess the possibility of active tuberculosis being present. Also, if the patient has symptoms of tuberculosis related to an extrapulmonary site, an appropriate evaluation should be undertaken. Once active tuberculosis has been excluded (i.e., by negative cultures and a stable chest radiograph), the treatment regimens are those used for latent tuberculosis infection: INH for 9 months, RIF (with or without INH) for 4 months, or RIF and PZA for 2 months (for patients who are unlikely to complete a longer course and who can be monitored closely).
Renal insufficiency and end-stage renal disease
For patients undergoing hemodialysis, administration of all drugs after dialysis is preferred to facilitate DOT and to avoid premature removal of drugs such as PZA and cycloserine. To avoid toxicity it is important to monitor serum drug concentrations in persons with renal failure who are taking cycloserine or EMB. There is little information concerning the effects of peritoneal dialysis on clearance of antituberculosis drugs.
Liver disease
INH, RIF, and PZA all can cause hepatitis that may result in additional liver damage in patients with preexisting liver disease. However, because of the effectiveness of these drugs (particularly INH and RIF), they should be used if at all possible, even in the presence of preexisting liver disease. If serum AST is more than three times normal before the initiation of treatment (and the abnormalities are not thought to be caused by tuberculosis), several treatment options exist. One option is to treat with RIF, EMB, and PZA for 6 months, avoiding INH. A second option is to treat with INH and RIF for 9 months, supplemented by EMB until INH and RIF susceptibility are demonstrated, thereby avoiding PZA. For patients with severe liver disease a regimen with only one hepatotoxic agent, generally RIF plus EMB, could be given for 12 months, preferably with another agent, such as a fluoroquinolone, for the first 2 months; however, there are no data to support this recommendation.
In all patients with preexisting liver disease, frequent clinical and laboratory monitoring should be performed to detect drug-induced hepatic injury.
Pregnancy and breastfeeding
Because of the risk of tuberculosis to the fetus, treatment of tuberculosis in pregnant women should be initiated whenever the probability of maternal disease is moderate to high. The initial treatment regimen should consist of INH, RIF, and EMB. Although all of these drugs cross the placenta, they do not appear to have teratogenic effects. Streptomycin is the only antituberculosis drug documented to have harmful effects on the human fetus (congenital deafness) and should not be used. Although detailed teratogenicity data are not available, PZA can probably be used safely during pregnancy and is recommended by the World Health Organization (WHO) and the International Union against Tuberculosis and Lung Disease (IUATLD). If PZA is not included in the initial treatment regimen, the minimum duration of therapy is 9 months.
Breastfeeding should not be discouraged for women being treated with the first-line antituberculosis agents because the small concentrations of these drugs in breast milk do not produce toxicity in the nursing newborn. Conversely, drugs in breast milk should not be considered to serve as effective treatment for tuberculosis or for latent tuberculosis infection in a nursing infant. Pyridoxine supplementation (25 mg/day) is recommended for all women taking INH who are either pregnant or breastfeeding. The amount of pyridoxine in multivitamins is variable but generally less than the needed amount.
Treatment of Tuberculosis in Low-Income Countries: Recommendations of the WHO and Guidelines from the IUATLD
To place the current guidelines in an international context it is necessary to have an understanding of the approaches to treatment of tuberculosis in high-incidence, low-income countries. It is important to recognize that the American Thoracic Society/CDC/Infectious Diseases Society of America (ATS/CDC/IDSA) recommendations cannot be assumed to be applicable under all epidemiologic and economic circumstances. The incidence of tuberculosis and the resources with which to confront the disease to an important extent determine the approaches used. Given the increasing proportion of patients in low-incidence countries who were born in high-incidence countries, it is also important for persons managing these cases to be familiar with the approaches used in the countries of origin.
The major international recommendations and guidelines for treating tuberculosis are those of the WHO and of the IUATLD. The WHO document was developed by an expert committee whereas the IUATLD document is a distillation of IUATLD practice, validated in the field.
The WHO and IUATLD documents target, in general, countries in which mycobacterial culture, drug susceptibility testing, radiographic facilities, and second-line drugs are not widely available as a routine. A number of differences exist between these new ATS/CDC/IDSA recommendations, and the current tuberculosis treatment recommendations of the WHO and guidelines of the IUATLD. Both international sets of recommendations are built around a national case management strategy called "DOTS," the acronym for "directly observed therapy, short course," in which direct observation of therapy (DOT) is only one of five key elements. The five components of DOTS are 1) government commitment to sustained tuberculosis control activities, 2) case detection by sputum smear microscopy among symptomatic patients self-reporting to health services, 3) a standardized treatment regimen of 6--8 months for at least all confirmed sputum smear--positive cases, with DOT for at least the initial 2 months, 4) a regular, uninterrupted supply of all essential antituberculosis drugs, and 5) a standardized recording and reporting system that enables assessment of treatment results for each patient and of the tuberculosis control program overall.
A number of other differences exist as well
The WHO and the IUATLD recommend diagnosis and classification of tuberculosis cases and assessment of response based on sputum AFB smears. Culture and susceptibility testing for new patients is not recommended because of cost, limited applicability, and lack of facilities. Chest radiography is recommended by both the WHO and IUATLD only for patients with negative sputum smears and is not recommended at all for follow-up. Both 6- and 8-month treatment regimens are recommended by the WHO. The IUATLD recommends an 8-month regimen with thioacetazone in the continuation phase for HIV-negative patients. For patients suspected of having or known to have HIV infection, ethambutol is substituted for thioacetazone The WHO and the IUATLD recommend a standardized 8-month regimen for patients who have relapsed, had interrupted treatment, or have failed treatment. Patients who have failed supervised retreatment are considered "chronic" cases and are highly likely to have tuberculosis caused by MDR organisms. Susceptibility testing and a tailored regimen using second-line drugs based on the test results are recommended by the WHO, if testing and second-line drugs are available. The IUATLD recommendations do not address the issue. Neither baseline nor follow-up biochemical testing is recommended by the WHO and the IUATLD. It is recommended that patients be taught to recognize the symptoms associated with drug toxicity and to report them promptly.
A Research Agenda for Tuberculosis Treatment
New antituberculosis drugs are needed for three main reasons: 1) to shorten or otherwise simplify treatment of tuberculosis caused by drug-susceptible organisms, 2) to improve treatment of drug-resistant tuberculosis, and 3) to provide more efficient and effective treatment of latent tuberculosis infection. No truly novel compounds that are likely to have a significant impact on tuberculosis treatment are close to clinical trials. However, further work to optimize the effectiveness of once-a-week rifapentine regimens using higher doses of the drug and using rifapentine in combination with moxifloxacin is warranted, on the basis of experimental data.
New categories of drugs that have shown promise for use in treating tuberculosis include the nitroimidazopyrans and the oxazolidinones. Experimental data also suggest that a drug to inhibit an enzyme, isocitrate lyase, thought to be necessary for maintaining the latent state, might be useful for treatment of latent tuberculosis infection.
A number of other interventions that might lead to improved treatment outcome have been suggested, although none has undergone rigorous clinical testing. These include various drug delivery systems, cytokine inhibitors, administration of "protective" cytokines such as interferon-g and interleukin-2, and nutritional supplements, especially vitamin A and zinc.
Research is also needed to identify factors that are predictive of a greater or lesser risk of relapse to determine optimal length of treatment. Identification of such factors would enable more efficient targeting of resources to supervise treatment. In addition, identification of behavioral factors that identify patients at greater or lesser likelihood of being adherent to therapy would also enable more efficient use of DOT.