Tuberculosis: Difference between revisions
Matt Pijoan (talk | contribs) (Created page with "{{InfectiousDisease |description=Tuberculosis overview |historicalPerspective=Tuberculosis historical perspective |pathophysiology=Tuberculosis_pathophysiology }}") |
Matt Pijoan (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
|historicalPerspective=Tuberculosis historical perspective | |historicalPerspective=Tuberculosis historical perspective | ||
|pathophysiology=Tuberculosis_pathophysiology | |pathophysiology=Tuberculosis_pathophysiology | ||
|epidemiology=Tuberculosis_epidemiology_and_demographics | |||
|riskFactors=Tuberculosis_risk_factors | |||
|causedby=Tuberculosis_causes | |||
}} | }} | ||
Revision as of 18:48, 3 July 2012
{{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}} [[Natural Progression::{{{naturalProgression}}}| ]] Classification Classic::Classification Atypical::
Overview
| https://https://www.youtube.com/watch?v=yR51KVF4OX0%7C350}} |
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [3]; João André Alves Silva, M.D. [4]; Ammu Susheela, M.D. [5]
Overview
Tuberculosis (abbreviated as TB or Tuberculosis) is a common infectious disease caused by Mycobacterium tuberculosis. Tuberculosis most commonly involves the lungs as the organism thrives in high oxygen environments, but it can also cause disease in the central nervous system, the lymphatic system, the circulatory system, the genitourinary system, bones, joints and even the skin. Over one-third of the world's population has been exposed to M. tuberculosis, and new infections occur at a rate of one per second. Not all individuals exposed to the bacterium develop clinically overt tuberculosis infection; in fact, asymptomatic, latent TB infection discovered by screening is more common. Approximately, one in ten latent infections progresses to active (symptomatic) TB disease, which, if left untreated, carries mortality rates of up to 50%. Symptoms include shortness of breath, hemoptysis, fever, chills, night sweats, and weight loss. Several treatment regimens are available for the latent and active forms of TB. Classically, a prolonged course of 6-9 months of a single agent (rifampin or isoniazid) is administered to patients with latent TB, while a more aggressive course that consists of 4 major anti-tuberculous agents (rifampin, isoniazid, ethambutol, pyrazinamide) is reserved for patients with active disease.
Historical Perspective
- Tuberculosis has been present in humans for thousands of years.
- The earliest unambiguous detection of Mycobacterium tuberculosis was in the remains of bison, dated 18,000 BC.
- Tuberculosis originated in cattle and then transferred to humans, or diverged from a common ancestor.
- Tuberculosis has had many names including phthisis and Wasting disease.
- Some hypotheses demonstrate that the origin of the genus, Mycobacterium tuberculosis, was more than 150 million years ago.
- TB with its different names and presentations throughout its history was detected on skeletal deformities of Ancient Egyptian mummies, dating back to 2400 BC.
- The first written record of TB, found in India and China, dated back to 3300 and 2300 years ago.
- In the Middle Ages as well as during the Renaissance, TB was referenced to as the “King’s Evil”.
- During this period if time, the contagious nature, pathology and anatomical afflictions were described.
- An English physician named Benjamin Marten, supposed the anticipated origins for this disease by 1720.
- Years later, there were a number of proposed cures but the most significant milestone in the fight against TB was achieved by renowned scientist, Robert Koch.
- Robert Koch discovered the Mycobacterium tuberculosis in 1882.
- In the 19th and early 20th centuries, tuberculosis caused the most widespread public concern, being considered an endemic disease of the urban poor.
- An effective therapy became possible with the development of the antibiotic streptomycin in 1946.
- The drug-resistant strains began to increase in the 1980s.
Classification
- According to exposure, clinical symptoms, and adjunct diagnostic testing tuberculosis is classified into 6 main classes .
- The classification ranges from Class 0, in people without previous exposure to TB and negative tuberculin skin testing and/or interferon-gamma release assays (2 methods of screening for TB), to Class 3 for active TB and Class 5 for suspected TB based on signs and symptoms of the disease.
- The U.S. Citizenship and Immigration Services also made a special classification for immigrants and refugees according to the risk of infection.
TB Classification System
- As per CDC (Centers of Disease Control and Prevention), the clinical classification system for TB used in the United States is based on the pathogenesis of the disease.
- This classification system provides clinicians the opportunity to keep an eye on the development of TB in their patients.
- Health care providers should follow with state and local laws and regulations requiring the reporting of TB disease.
- All persons with Class 3 or Class 5 TB should be reported directly to the local or state health department.
- A patient should not have a Class 5 classification for more than 3 months.
| Class | Type | Description |
|---|---|---|
| 0 | *No TB exposure *Not infected |
*No history of TB exposure and no evidence of M. tuberculosis infection or disease *Negative reaction to TST or IGRA |
| 1 | *TB exposure *No evidence of infection |
*History of exposure to M. tuberculosis *Negative reaction to TST (Tuberculin skin tests) or IGRA (an interferon gamma release assay blood test) (given at least 8 to 10 weeks after exposure) |
| 2 | *TB infection *No TB disease |
*Positive reaction to TST or IGRA *Negative bacteriological studies (smear and cultures) *No bacteriological or radiographic evidence of active TB disease |
| 3 | *TB clinically active | *Positive culture for M. tuberculosis OR *Positive reaction to TST or IGRA, plus clinical, bacteriological, or radiographic evidence of current active TB |
| 4 | *Previous TB disease (not clinically active) | *May have past medical history of TB disease *Abnormal but stable radiographic findings *Positive reaction to the TST or IGRA *Negative bacteriologic studies (smear and cultures) *No clinical or radiographic evidence of current active TB disease |
| 5 | *TB suspected | *Signs and symptoms of active TB disease, but medical evaluation not complete |
Pathophysiology
- Tuberculosis is a granulomatous infection that is chiefly transmitted through droplets.
- The granuloma encloses mycobacteria and prevents their spreading and facilitates immune immune cell communication.
- Within the granuloma, T lymphocytes (CD4) releases cytokines, such as interferon gamma, that activates local macrophages.
- It is asymptomatic in 90% of immunocompetent individuals.
- In symptomatic patients, it can present as pulmonary or extrapulmonary manifestations. The primary infection may turn into disseminated infection.
- Tuberculosis usually has an impact the progression of HIV if present together. Depending on the age of the patient, tuberculosis may have different clinical manifestations, progression, and prognosis.
Causes
- Mycobacterium tuberculosis is the bacterium responsible for tuberculosis.
- It is an aerobic, non-encapsulated, non-motile, acid-fast bacillus.
- M. tuberculosis is one of the Mycobacterium tuberculosis complex, which also includes bacteria, such as M. bovis and M. africanum.
- The bacterium has a very slow rate of replication, and its genetic variations account for the geographical distribution of different strains, and are involved in drug resistance.
- M. tuberculosis has tropism for different kinds of human cells, with preference for cells of the lung.
- It may infect different species, but human beings are its frequent natural reservoir.
Epidemiology and Demographics
- In 2015, about 10.4 million people developed symptomatic TB and 1.8 million died from the disease.
- There were 9,421 reported cases in the United States in 2014 with an incidence of 3.0 per 100,000 persons.
- Since 1990, the mortality rate was steadily decreasing.
- The prevalence of TB increases with age and it is higher in older men. TB is more prevalent in racial and ethnic minorities than non-Hispanic whites.
- TB is an major cause of death in people coinfected with HIV.
- A third of deaths among these patients is due to TB.
- In 2015, 60% of TB cases worldwide occurred in 6 countries: South Africa, Indonesia, Nigeria, Pakistan, India, and China.
- The WHO has identified 24 other high-burden TB countries including Bangladesh, Congo, Columbia, Lesotho, Cambodia, Korea, Brazil, Ethiopia, Myanmar, Mozambique, Thailand, Angola, Zambia, Vietnam, Kenya, Central Africa, Russia, Liberia, Tanzania, Zimbabwe, Namibia, Philippines, Sierra Leone, Papua New Guinea.
Risk Factors
- The risk factors for the development of tuberculosis include:
- weakened immune system (patients taking immunosuppressive medication or with immunosuppressive diseases, such as HIV or diabetes
- History of contact with infected patients
- Bad hygiene conditions
- Evidence of previous tuberculosis.
- Risk factors for multidrug-resistant TB include:
- Non-adherence to the treatment regimen
- Insufficient medication for that strain of bacteria
- Contact with patients with multidrug-resistant TB.
Screening
- Screening for tuberculosis is generally done by using a mantoux tuberculin skin test, also known as a tuberculin skin test or a PPD.
- The test involves injecting a small amount of a purified protein derivative of the tuberculosis bacterium intradermally and watching for a reaction in the following days.
Natural history, complications and prognosis
- Tuberculosis has been classified as a primary or secondary (post-primary) infection.
- It can have pulmonary and extra pulmonary manifestations as well as severe parenchymal, vascular, pleural, and chest wall complications.
- Pulmonary complications include pleural effusions, cavitations, lymphadenopathy, airway obstruction, pneumonia and bronchiectasis.
- The hematogenous dissemination of infection can lead to miliary tuberculosis.
- The post-primary infection can be due to a recent infection or reactivation of an old infection. Without treatment, 1/3 of patients with active tuberculosis dies within 1 year of the diagnosis, and more than 50% during the first 5 years.
- But with early diagnosis and treatment, it has a good prognosis.
Diagnosis
History and Symptoms
- The general symptoms of tuberculosis include weakness, weight loss, fever, and night sweats.
- Symptoms of pulmonary tuberculosis include cough, chest pain, and hemoptysis.
- Tuberculosis is particularly difficult to diagnose in children, as these may not present with common findings.
Physical Examination
- A physical examination can give an overview about the general condition and other factors that may influence the tuberculosis response to treatment, such as HIV infection or other diseases.
- The most common physical findings include fever, decreased breath sounds, tachypnea and tachycardia.
- Physical findings will depend on the location of the tuberculosis infection.
Laboratory findings
- Routine laboratory exams are usually in the normal ranges.
- The presence of acid-fast-bacilli (AFB) on a sputum smear or another specimen often indicates TB disease and a positive culture for M. tuberculosis confirms the diagnosis.
- Other laboratory tests include peritoneal fluid or CSF analysis, urinalysis, and Interferon-Gamma release assays.
Electrocardiogram
- Echocardiography or Ultrasound can be helpful in patients who develop pericardial effusion secondary to TB. In rare occasions TB may lead to congestive heart failure, in which case echocardiograph may also help in the diagnosis.
- Common findings in CHF on the echocardiogram include: hypokinesia; valvular insufficiency; and enlargement of all heart chambers.
Chest X-Ray
- A chest X-ray is one of the important diagnostic tools in tuberculosis.
- A chest radiograph may be used to rule out the possibility of pulmonary TB in a person who are symptomatic or had a positive reaction to a tuberculin test or QFT-G and no symptoms of the disease.
- The findings on chest x-ray can be divided into parenchymal and pleural.
- The early parenchymal findings can be infiltrated, and cavity.
- A healed tuberculotic lesion can present as fibrosis, and calcification.
- Pleural lesions in form of pleural effusion can also be seen.
- An advanced tuberculosis lesion can present a combination of these early lesions and termed fibrocavitary lesions.
CT
- The majority of patients with pulmonary tuberculosis will have abnormal findings in a chest CT, which include micronodules, interlobular septal thickening, cavitation and consolidation.
- CT scan is more sensitive than an X-ray to detect lymphadenopathies.
MRI
- MRI is used for the assessment of extrapulmonary tuberculosis, such as CNS tuberculosis, Pott's disease, and parotid gland tuberculosis.
Echocardiography or Ultrasound
- Echocardiography or Ultrasound can be helpful in patients who develop pericardial effusion secondary to TB.
- In rare occasions TB may lead to congestive heart failure, in which case echocardiograph may also help in the diagnosis.
- Common findings in CHF on the echocardiogram include: hypokinesia; valvular insufficiency; and enlargement of all heart chambers.
Other Imaging findings
- The abreugraphy is a smaller variant of the chest X-ray that allows the identification of lung abnormalities that may suggest the diagnosis of TB.
- With the decrease of incidence of TB, the abreugraphy is no longer recommended in most countries for low-risk populations.
- However, depending on the screening resources of each country, it may be used for the screening of high-risk groups, such as HIV-positive patients and alcoholics.
Other Diagnostic Studies
- Because of difficulties with the Tuberculin skin test, many laboratory methods of diagnosis are emerging.
Treatment
Medical Therapy
- If there is a high probability of infection, presumptively treat the patient even if the stain is negative, while waiting for the culture results.
- The patient should be brought back in a few weeks.
- Patients usually feel better a few weeks post-treatment.
- Patients must be monitored for adverse effects and treatment failure.
- In the U.S., all TB is tested for drug resistance.
Special conditions
- Medical therapy for tuberculosis in special conditions include HIV co-infection and extra pulmonary manifestations.
- Different approaches are taken for patients taking ART and those who do not take ART.
- Although WHO recommends the same drug regimen for pulmonary and extrapulmonary manifestations, various stages of skeletal tuberculosis are managed differently.
- For patients with renal or liver diseases, the first line of drugs are substituted with second-line drugs to prevent complications.
Drug-resistant
- Drug-resistant tuberculosis is caused by M. tuberculosis organisms that are resistant to at least one first-line anti-TB drug.
- Multidrug-resistant TB (MDR TB) is resistant to more than one anti-TB drug and at least isoniazid (INH) and rifampin (RIF).
- Treatment should be started with an empirical treatment of at least 4 drugs based on expert advice as soon as drug-resistant TB disease is suspected.
Children
- Tuberculosis in children aged 15 years or younger is a public health problem of special significance because it is a marker for recent transmission of TB. *Infants and young children are more likely to develop life-threatening forms of tuberculosis, such as miliary TB or TB meningitis.
- Screening in children is very important, as the clinical manifestations are usually poor or non-specific.
- History of close contact with tuberculosis patients has an important role in the diagnosis of TB in children.
- The treatment is similar to adults, with adjusted dosing according to the child's weight.
Surgery
- Surgery may be necessary, especially to drain abscesses , empyema, venticular shunt in tubercular meningitis, surgical resection of tissues affected in abdominal tuberculosis, stabilize the spine in case of Pott's disease, lobectomy, pneumonectomy, pericardiocentesis or surgical repair of pericardium.
Primary Prevention
- Primary prevention in tuberculosis is targeted to avoid disease transmission and infection of healthy individuals. The BCG vaccine is used in children susceptible to TB infections, such as children living in endemic areas or having close contact with a confirmed case of TB.
- Several preventive measures are used to avoid the transmission of the mycobacteria, such as respiratory isolation, use of respiratory masks among health-care professionals, and advising respiratory hygiene and cough etiquette.
Secondary Prevention
- Secondary prevention for tuberculosis includes methods for screening and early diagnosis, such as tuberculin skin test (TST) and IGRAs; and to guarantee the correct treatment regimen at the right time to prevent disease progression.
Cost effectiveness of therapy
- Treatment of tuberculosis must be analyzed for relative cost effectiveness of inpatient and outpatient models of care as it will benefit regions where tuberculosis is highly prevalent.
- Unless there is severe complications it is highly recommended to treat the TB patient in ambulatory care rather than inpatient services.
Future or investigational therapy
- Since new drug-resistant tuberculosis has been emerging, the role of future therapies is vital in curbing outbreaks.
- The new drugs should be more effective than the current regimen and a few drugs in clinical trials have been showing good results.
References
Template:WSHistorical Perspective
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [6]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[7]; João André Alves Silva, M.D. [8]
Overview
Tuberculosis has been detected for a long time. The earliest unambiguous detection of Mycobacterium tuberculosis was in the remains of bison, dated 18,000 BC.[1] However, whether tuberculosis originated in cattle and then transferred to humans, or diverged from a common ancestor, is unclear.[2] Through history tuberculosis has had many names including phthisis and Wasting disease, which were mostly derived from its symptoms. Robert Koch identified the Mycobacterium tuberculosis 1882. In the 19th and early 20th centuries, tuberculosis was considered an endemic disease of the urban poor and a public healthcare issue. In 1946, the development of the antibiotic streptomycin made the cure possible. In the 1980s, the drug-resistant strains appeared increasingly eliminating the hope of cure.
Historical Perspective

TB was present in prehistoric humans and the evidence of infection was found in the skeletal remains 4000 BC, in addition to the spines of mummies showing tubercular decay from 3000-2400 BC.[4] Phthisis is a Greek term for tuberculosis; around 460 BC. In terms of Hippocrates point of view, phthisis was the most widespread disease of the time involving fever and coughing up blood, which was almost always fatal.[5] TB was present in South America for about 2,000 years evidenced by some genetic studies.[6] In South America, the earliest evidence of tuberculosis was linked to the Paracas-Caverna culture (circa 750 BC to circa 100 AD).[7]
Egyptian mummies, that dates back to 2400 BC, showed skeletal deformities characteristic of tuberculosis of spine (Pott's lesions) and those findings were documented clearly in early Egyptian art.[8]
In the Ancient Greece TB was identified and named Phtisis. Hippocrates recognized it as a fatal disease especially for young adults. Isocrates was the first to suggest that TB is considered an infectious disease, while Aristotle suggested its contagious in oxes and pigs.[9]
Along time ago, Since tuberculosis was similar to the idea of consumption of the infected patients with fever, hemoptysis, pallor, and severe wasting, tuberculosis was called consumption. Other names are:[10][11][10][12]
- Phthisis (Greek for consumption)
- Phthisis pulmonalis
- Scrofula (in adults) - TB of the lymphatic system, resulting in swollen cervical lymph nodes
- Tabes mesenterica - TB of the abdomen
- Lupus vulgaris - TB of the skin
- Wasting disease
- White plague - due to paleness of the sufferers
- King's evil - as people had a false belief that that scrofula would heal by the king’s touch
- Pott's disease - TB of the spine
- Miliary tuberculosis – also called disseminated TB, occurs when the infection spreads in the blood stream, resulting in lesions in multiple organs with the appearance of millet seeds on x-ray.
Folklore
tuberculosis was sometimes considered as as vampirism before the industrial revolution. When one family member died from it, the other members became infected and died slowly. People had a false belief that this occurs because the first victim drains the life from the other family members. Moreover, people who had TB had symptoms such as red, swollen eyes (which also causes photosensitivity), pale skin and hemoptysis that are similar to what people knew about vampires. This suggested the idea that the only way for the afflicted to replenish this loss of blood was by sucking blood.[13] people also attributed it to being forced, nightly, to attend fairy revels, so that the victim wasted away due to lack of rest.[14] Similarly, but rarely, it was attributed to the victims being 'hagridden' - being transformed into horses by witches (hags) to travel to their nightly meetings leading to lack of rest.[14]
In the 19th century. Many people believed TB caused a sensation of euphoria called ''Spes phthisica'' or ''hope of the consumptive''. It was believed that artists affected with TB had bursts of creativity with TB progression. It was also believed that TB sufferers were having a final burst of energy just before their death that made women more beautiful and men more creative.[15]
Study and Treatment

Although Dr Richard Morton established that the pulmonary form was associated with 'tubercles' in 1689,[19][20] due to the variation of its symptoms, TB was not identified as a single disease until the 1820s and was not named 'tuberculosis' until 1839 by J. L. Schönlein.[21] During the years 1838-1845, Dr. John Croghan, the owner of Mammoth Cave, brought some tuberculosis sufferers into the cave to treat them with the constant temperature and purity of the cave air; however, they died within a year.[22] The first TB sanatorium opened in 1859 in Görbersdorf, Germany (today Sokołowsko, Poland) by Hermann Brehmer.[23]
Hermann Lebert published his project Traite Pratique des Maladies Scrofuleuses et Tuberculeuses in 1849, reporting that the TB or "King's evil" was a childhood disease that can affect multiple body's sites such as skin, eyes, ears, joints, and bones, causing ulceration and suppuration.[24]
Robert Koch identified and deascribed the bacillus causing tuberculosis, Mycobacterium tuberculosis, on March 24, 1882 for which He received the Nobel Prize in physiology or medicine in 1905.[25] Koch did not identify that bovine (cattle) and human tuberculosis were the same infection, which delayed considering infected milk as a source of infection. Later on, this source was eliminated by the process of milk pasteurization. Koch identified a glycerine extract of the tubercle bacilli as in 1890, calling it 'tuberculin'. It was not accurate, but was later adapted as a test for pre-symptomatic tuberculosis.[26]
The first achievement in tuberculosis immunization was 'BCG' (Bacillus of Calmette and Guerin) that was developed from attenuated bovine-strain tuberculosis by Albert Calmette and Camille Guerin in 1906. The BCG vaccine was first used on humans in 1921 in France, and after World War II, BCG received approval in the USA, Great Britain, and Germany.
Tuberculosis, or 'consumption' was considered an endemic disease of the urban poor and a public healthcare issue. In 1815, one in four deaths in England was of consumption; by 1918 one in six deaths in France were still caused by TB. In the 20th century, the number of deaths due to TB was 100 million people.[27] After the establishment in the 1880s that the disease was contagious, TB was reported as a notifiable disease in Britain.[23]

In the United States, concern about the spread of tuberculosis played a role in the movement to prohibit public spitting except into spittoons.
In Europe, deaths from TB decreased from 500 out of 100,000 in 1850 to 50 out of 100,000 by 1950. public health improvements had an impact regarding decreasing tuberculosis cases even before the development of antibiotics, but the disease was still representing a significant harm to public health.[28]
In 1946, the development of the antibiotic streptomycin made the cure possible. Before the discovery of this drug, the only treatment besides sanatoria were surgical interventions, such as the pneumothorax technique - in the form of collapsing an infected lung to rest it and allow lesions to heal - which was of little benefit and was largely stopped by the 1950s.[29] The emergence of multidrug-resistant TB has again introduced surgery as part of the treatment for these infections. Here, surgical removal of lung cavities will reduce the number of bacteria in the lungs, as well as increase the exposure of the remaining bacteria to drugs in the bloodstream, which is thought to increase the effectiveness of the chemotherapy.[30]
In the 1980s, the drug-resistant strains appeared increasingly eliminating the hope of cure. For example, tuberculosis cases in Britain, numbering around 117,000 in 1913, had fallen to around 5,000 in 1987, but cases increased again, reaching 6,300 in 2000 and 7,600 cases in 2005.[31] As a result of the elimination of public health facilities in New York and the emergence of HIV, there was another TB resurgence in the late 1980s.[32]
References
- ↑ Rothschild B, Martin L, Lev G, Bercovier H, Bar-Gal G, Greenblatt C, Donoghue H, Spigelman M, Brittain D (2001). "Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present". Clin Infect Dis. 33 (3): 305–11. PMID 11438894.
- ↑ Pearce-Duvet J (2006). "The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease". Biol Rev Camb Philos Soc. 81 (3): 369–82. PMID 16672105.
- ↑ 3.0 3.1 3.2 "Wikimedia Commons".
- ↑ Zink A, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, Nerlich A (2003). "Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping". J Clin Microbiol. 41 (1): 359–67. PMID 12517873.
- ↑ Hippocrates. Aphorisms. Accessed 07 October 2006.
- ↑ Konomi N, Lebwohl E, Mowbray K, Tattersall I, Zhang D (2002). "Detection of mycobacterial DNA in Andean mummies". J Clin Microbiol. 40 (12): 4738–40. PMID 12454182.
- ↑ "South America: Prehistoric Findings". Memorias do Instituto Oswaldo Cruz, Vol. 98 (Suppl.I) January 2003. Retrieved on 2007-02-08.
- ↑ MORSE D, BROTHWELL DR, UCKO PJ (1964). "TUBERCULOSIS IN ANCIENT EGYPT". Am Rev Respir Dis. 90: 524–41. doi:10.1164/arrd.1964.90.4.524. PMID 14221665.
- ↑ Barberis I, Bragazzi NL, Galluzzo L, Martini M (2017). "The history of tuberculosis: from the first historical records to the isolation of Koch's bacillus". J Prev Med Hyg. 58 (1): E9–E12. PMC 5432783. PMID 28515626.
- ↑ 10.0 10.1 Tuberculosis Encyclopedia Britannica, 11th ed.
- ↑ Rudy's List of Archaic Medical Terms English Glossary of Archaic Medical Terms, Diseases and Causes of Death. Accessed 09 Oct 06
- ↑ Disseminated tuberculosis NIH Medical Encyclopedia. Accessed 09 Oct 06
- ↑ Sledzik P, Bellantoni N (1994). "Brief communication: bioarcheological and biocultural evidence for the New England vampire folk belief". Am J Phys Anthropol. 94 (2): 269–74. PMID 8085617.
- ↑ 14.0 14.1 Katharine Briggs, An Encyclopedia of Fairies "Consumption" (Pantheon Books, 1976) p. 80. ISBN 0-394-73467-X
- ↑ Lawlor, Clark. "Transatlantic Consumptions: Disease, Fame and Literary Nationalism in the Davidson Sisters, Southey, and Poe". Studies in the Literary Imagination, Fall 2003. Available at findarticles.com. Retrieved on 2007-06-08.
- ↑ Y. A. Al-Sharrah (2003), "The Arab Tradition of Medical Education and its Relationship with the European Tradition", Prospects 33 (4), Springer.
- ↑ George Sarton, Introduction to the History of Science.
(cf. Dr. A. Zahoor and Dr. Z. Haq (1997). Quotations From Famous Historians of Science, Cyberistan.) - ↑ David W. Tschanz, MSPH, PhD (August 2003). "Arab Roots of European Medicine", Heart Views 4 (2).
- ↑ Who Named It? Léon Charles Albert Calmette. Retrieved on 6 October 2006.
- ↑ Trail R (1970). "Richard Morton (1637–1698)". Med Hist. 14 (2): 166–74. PMID 4914685.
- ↑ Zur Pathogenie der Impetigines. Auszug aus einer brieflichen Mitteilung an den Herausgeber. [Müller’s] Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. 1839, page 82.
- ↑ Kentucky: Mammoth Cave long on history. CNN. 27 February 2004. Accessed 08 October 2006.
- ↑ 23.0 23.1 McCarthy OR (2001). "The key to the sanatoria". J R Soc Med. 94 (8): 413–7. PMID 11461990.
- ↑ Barberis I, Bragazzi NL, Galluzzo L, Martini M (2017). "The history of tuberculosis: from the first historical records to the isolation of Koch's bacillus". J Prev Med Hyg. 58 (1): E9–E12. PMC 5432783. PMID 28515626.
- ↑ Nobel Foundation. The Nobel Prize in Physiology or Medicine 1905. Accessed 07 October 2006.
- ↑ Waddington K (2004). "To stamp out "so terrible a malady": bovine tuberculosis and tuberculin testing in Britain, 1890–1939". Med Hist. 48 (1): 29–48. PMID 14968644.
- ↑ Torrey EF and Yolken RH. 2005. Their bugs are worse than their bite. Washington Post, April 3, p. B01.
- ↑ [[Medical Research Council (UK)|]]. MRC's contribution to Tuberculosis research. Accessed 02 July 2007.
- ↑ Wolfart W (1990). "[Surgical treatment of tuberculosis and its modifications—collapse therapy and resection treatment and their present-day sequelae]". Offentl Gesundheitswes. 52 (8–9): 506–11. PMID 2146567.
- ↑ Lalloo U, Naidoo R, Ambaram A (2006). "Recent advances in the medical and surgical treatment of multi-drug resistant tuberculosis". Curr Opin Pulm Med. 12 (3): 179–85. PMID 16582672.
- ↑ "Tuberculosis – Respiratory and Non-respiratory Notifications, England and Wales, 1913-2005". Health Protection Agency Centre for Infections. 21 March 2007. Retrieved 2007-08-01.
- ↑ Paolo W, Nosanchuk J (2004). "Tuberculosis in New York city: recent lessons and a look ahead". Lancet Infect Dis. 4 (5): 287–93. PMID 15120345.
Pathophysiology
| https://https://www.youtube.com/watch?v=yR51KVF4OX0%7C350}} |
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[10], João André Alves Silva, M.D. [11]
Overview
Transmission of M. tuberculosis occurs when individuals with active pulmonary disease cough, speak, sneeze or sing expelling the infectious droplets. The mycobacterium tuberculosis favors the upper lung lobes due to the high oxygen level. Tuberculosis is a prototypical granulomatous infection. The granuloma surrounds the mycobacteria and prevents their dissemination and facilitates the immune cell interaction. Within the granuloma, CD4 T lymphocytes release chemokines that activate local macrophages and recruit other immune cells..
Pathogenesis
Transmission of M. tuberculosis occurs when individuals with active pulmonary disease cough, speak, sneeze or sing expelling the infectious droplets that can pass to the terminal bronchioles and alveoli then phagocytosed by alveolar macrophages where they can replicate in the endosomes of alveolar macrophages. As a part of the immune response by these macrophages, the alveolar macrophages release cytokines that recruits further macrophages, neutrophils, and monocytes, surrounding the bacilli. Despite having a very low infectious dose (ID<200 bacteria), 90% of the infected immunocompetent individuals are asymptomatic. In most cases, the bacteria may either be eliminated or enclosed within a granuloma. The granuloma is a structured, radial aggregation of macrophages, epithelioid cells, T lymphocytes, B lymphocytes, and fibroblasts that prevents the spreading of mycobacteria and enhances interaction of the immune cells.[1] The primary site of infection in the lung is called the Ghon focus that is mainly located in either the upper part of the lower lobe, or the lower part of the upper lobe.[1][2]
Primary Infection
The infected macrophages are transported through the lymphatics to the regional lymph nodes in the immunocompetent individuals. However, with impaired immune response, these macrophages can pass through the bloodstream to enter any part of the body. Those foci of primary infection usually resolve without any consequences, but they can act as a foci of M. tuberculosis dissemination. There are particular organs that are more susceptible to bacterial replication as well as being potential metastatic foci which include:[1][2]
- Apical-posterior regions of the lungs
- Lymph nodes
- Kidneys
- Vertebral bodies
- Extremities of long bones
- Juxta ependymal meningeal regions
Although TB is a systemic disease and all organs can be affected, the heart, pancreas, skeletal muscles and thyroid are rarely involved.[3] In a few cases, when the infectious dose is high and antigens concentration in the primary focus is high, the immune response and hypersensitivity can lead to necrosis and calcification of this lesion, and these primary calcified foci are then called Ranke complex.[1][4]
Progression of the Primary Infection
Primary foci of infection can enter the large pulmonary lymph nodes. These may lead to:[1]
- Bronchial collapse
- atelectasis
- Bronchial erosion, with more dissemination of infection
- In non-caucasian children, elderly patients and HIV/AIDS, the immune response is impaired, consequently the primary focus of infection can deteriorate into progressive primary disease, with advancing pneumonia.
- In addition,the infection may result in cavity formation with transmission of the infection through the bronchi.[1][5][6]
- In young children, the onset of immune response may be delayed after the bacterial dissemination resulting in military tuberculosis. Bacteria can spread directly from the primary focus, or from the Weigart focus (metastatic focus adjacent to a pulmonary vein) through the blood.[1][7]
- In younger patients, rupture of subpleural foci into the pleural space may occur leading to serofibrinous pleurisy.[1] The most serious site of the M. tuberculosis dissemination is the postero-apical regions of the lung where it can replicate hidden from the immune system.[1]
Immunopathogenesis
There are two types of immune response against tuberculosis that include the innate and acquired immune responses. However, the cell-mediated immune response predominates over the humoral type.
Innate Immune Response
Initially, The immune response generated against M. tuberculosis is minimal, enabling it to replicate inside the alveolar macrophages forming the Ghon focus, or metastatic foci. Recognition and phagocytosis of the M. tuberculosis bacilli by the alveolar macrophages occurs through interaction with certain receptors that are located on the surface of macrophages:[8]
- Toll-like Receptor 2 (TLR2)
- TLR4
- TLR9
- Dectin-1
- DC-SIGN
- Mannose receptor
- Complement receptors
- NOD2
Acquired Immunity and Granuloma Formation
- The granuloma control the infection; however, it enables the mycobacterium to survive inside for a long time.
- It is important to maintain a balance between the pro-inflammatory and anti-inflammatory cytokines released to decrease or control the mycobacterial proliferation.
- TNF-α and IFN-γ stimulate granuloma formation. On the other hand, IL-10 is one of the major negative regulators and inhibitors of granuloma formation.
- The granuloma is structured by blood-derived macrophages (derived from monocytes), epithelioid cells (differentiated macrophages), and multinucleated giant cells (also known as Langhans giant cells), surrounded by T lymphocytes.
- Caseous granulomas are the main characteristic of tuberculosis. The caseous granulomas include epithelioid macrophages and some lymphocytes with a necrotic center. Other types of granuloma include non-necrotizing granulomas, that are mainly formed of macrophages and a few lymphocytes, necrotic neutrophilic granulomas, and completely fibrotic granulomas.[9]
- Several chemokines are involved in granuloma formation released either from the respiratory tract epithelium or the immune cells themselves.
- Interaction with CCR2 receptor with (CCL2/MCP-1, CCL12, and CCL13) is necessary for the initial recruitment of macrophages.
- Macrophages and lymphocytes release a chemokine called osteopontin that enhances the adhesion and recruitment of the immune cells.
- CCL19 and CCL21 are important for recruitment of IFN--producing T cells.
- In TNF-deficient mice, absence of these chemokines as a result of inhibition of the expression of the CC and CXC chemokines prevents the recruitment of other macrophages and T lymphocytes. This finding sheds the light on the role of TNF in granuloma formation.[9]
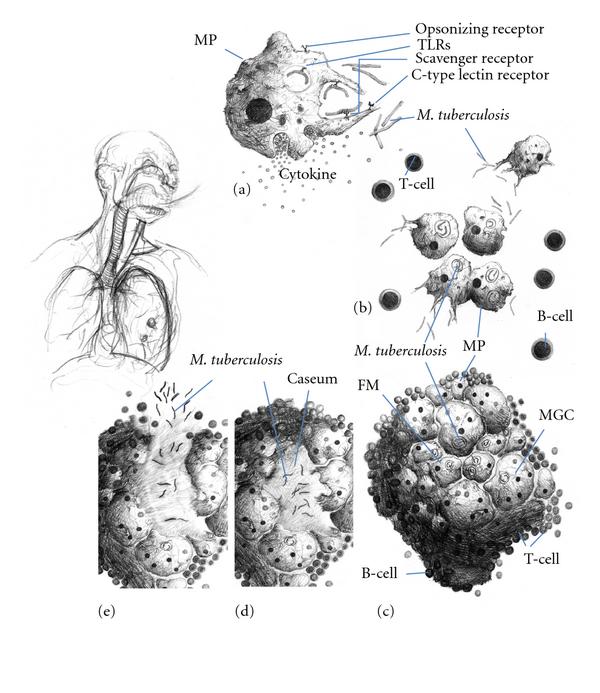
Molecular Pathogenesis
- The mycobacterial antigens are presented on thee surfaces of alveolar macrophages and dendritic cells through class II major histocompatibility complex. These antigens are recognized by CD4 lymphocytes through αβ T-cell receptors. Following that, CD4 lymphocytes release chemokines that recruit more macrophages to the foci of infection.
- Interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) signaling activates additional macrophages. [10]
- Metalloproteinase converts the transmembrane protein to soluble TNF-α which interacts with the TNFR1 and TNFR2 receptors inducing apoptosis through caspase-dependent pathways
- TNF along side the synergistic action of interferon-gamma enhances the phagocytic activity of the macrophages and facilitates the intracellular killing of mycobacteria by reactive nitrogen and oxygen intermediates.
- Neutralization of the TNF-α activity leads to the mycobacteria survival within the granuloma in latent infection.
- TNF activates release of CCL2, CCL3, CCL4, CCL5, CCL8 chemokines and increases CD54 leading to accumulation of immune cells and it is the main element in the process of granuloma formation and maintenance. [10]
- The immune cells release large amounts of lytic enzymes leading to tissue necrosis.

Once within alveolar macrophages, M. tuberculosis uses multiple mechanisms in order to survive:[1]
- Urease - prevents acidification of macrophageal lysosomes, limiting action of cellular enzymes
- Secretion of antioxidants, for suppression of reactive oxygen species, such as:
Transmission
After contact with a patient having the active TB, and inhalation of the M. tuberculosis, the risk of developing active tuberculosis is low with a life-time risk of about 10%.[12] The probability of transmission between individuals depends on the number of expelled infectious droplets the ventilation, the duration of the exposure, immunity, and the virulence of the M. tuberculosis strain.[13] The probability of transmitting the infection is highest during the first years of getting the infection. After that, it decreases.[14]
In rare occasions, the mycobacteria can be transmitted by other ways apart from the respiratory route in which, the formation of foci in the regional lymph nodes frequently occurs. Those routes include:[1]
Associated Conditions
AIDS
- Tuberculosis influence the progression of HIV replication leading to an increase in in the mortality rate.[15]
- HIV infected patients, particularly those having low CD4 lymphocytes counts, are more likely to develop reactivation of latent tuberculosis. Moreover, when an individual has been recently infected with M. tuberculosis, they progress rapidly into active disease.[1][16][17] The correlation between AIDS and the risk of TB infection is still not fully understood.[1]
Patients with AIDS are more prone to get pulmonary and extrapulmonary tuberculosis. Extrapulmonary disease in AIDS patients has characteristic manifestations, such as:[1]
- Higher risk of progression into disseminated disease[18]
- DIC and acute respiratory failure
- Tuberculous pleuritis occurs bilaterally
- Abdominal and mediastinal lymphadenopathy frequently occurs
- Higher risk of Immune reconstitution inflammatory syndrome (IRIS)[19]
- Common abscesses of:[1]
Gallery
-
Left lateral margin of a tongue of a tuberculosis patient, which had been retracted in order to reveal the lesion that had been caused by the Gram-positive bacterium Mycobacterium tuberculosisAdapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection.[ http://phil.cdc.gov/phil/ Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.][20]
-
Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Photomicrograph describing tuberculosis of the placenta.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Histopathology of tuberculosis, endometrium. Ziehl-Neelsen stain.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Histopathology of tuberculosis, placenta.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]
-
Miliar Tuberculosis
-
Renal Tuberculosis lesion
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Mandell, Gerald (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone/Elsevier. ISBN 0443068399.
- ↑ 2.0 2.1 Herrmann J, Lagrange P (2005). "Dendritic cells and Mycobacterium tuberculosis: which is the Trojan horse?". Pathol Biol (Paris). 53 (1): 35–40. PMID 15620608.
- ↑ Agarwal R, Malhotra P, Awasthi A, Kakkar N, Gupta D (2005). "Tuberculous dilated cardiomyopathy: an under-recognized entity?". BMC Infect Dis. 5 (1): 29. PMID 15857515.
- ↑ Grosset J (2003). "Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary". Antimicrob Agents Chemother. 47 (3): 833–6. PMID 12604509.
- ↑ Stead WW, Lofgren JP, Warren E, Thomas C (1985). "Tuberculosis as an endemic and nosocomial infection among the elderly in nursing homes". N Engl J Med. 312 (23): 1483–7. doi:10.1056/NEJM198506063122304. PMID 3990748.
- ↑ Murray JF (1990). "Cursed duet: HIV infection and tuberculosis". Respiration. 57 (3): 210–20. PMID 2274719.
- ↑ Kim J, Park Y, Kim Y, Kang S, Shin J, Park I, Choi B (2003). "Miliary tuberculosis and acute respiratory distress syndrome". Int J Tuberc Lung Dis. 7 (4): 359–64. PMID 12733492.
- ↑ Aderem A, Underhill DM (1999). "Mechanisms of phagocytosis in macrophages". Annu Rev Immunol. 17: 593–623. doi:10.1146/annurev.immunol.17.1.593. PMID 10358769.
- ↑ 9.0 9.1 9.2 Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F (2012). "The tuberculous granuloma: an unsuccessful host defense mechanism providing a safe shelter for the bacteria?". Clin Dev Immunol. 2012: 139127. doi:10.1155/2012/139127. PMC 3395138. PMID 22811737.
- ↑ 10.0 10.1 "Tumor Necrosis Factor alpha".
- ↑ "TNF Alpha". Missing or empty
|url=(help) - ↑ Glaziou P, Falzon D, Floyd K, Raviglione M (2013). "Global epidemiology of tuberculosis". Semin Respir Crit Care Med. 34 (1): 3–16. doi:10.1055/s-0032-1333467. PMID 23460002.
- ↑ "Causes of Tuberculosis". Mayo Clinic. 2006-12-21. Retrieved 2007-10-19.
- ↑ Lawn SD, Zumla AI (2011). "Tuberculosis". Lancet. 378 (9785): 57–72. doi:10.1016/S0140-6736(10)62173-3. PMID 21420161.
- ↑ Zumla A, Raviglione M, Hafner R, von Reyn CF (2013). "Tuberculosis". N Engl J Med. 368 (8): 745–55. doi:10.1056/NEJMra1200894. PMID 23425167.
- ↑ Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR; et al. (1992). "An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms". N Engl J Med. 326 (4): 231–5. doi:10.1056/NEJM199201233260404. PMID 1345800.
- ↑ Bouvet E, Casalino E, Mendoza-Sassi G, Lariven S, Vallée E, Pernet M; et al. (1993). "A nosocomial outbreak of multidrug-resistant Mycobacterium bovis among HIV-infected patients. A case-control study". AIDS. 7 (11): 1453–60. PMID 8280411.
- ↑ Shafer RW, Kim DS, Weiss JP, Quale JM (1991). "Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection". Medicine (Baltimore). 70 (6): 384–97. PMID 1956280.
- ↑ Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W; et al. (2008). "Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings". Lancet Infect Dis. 8 (8): 516–23. doi:10.1016/S1473-3099(08)70184-1. PMC 2804035. PMID 18652998.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 "Public Health Image Library (PHIL), Centers for Disease Control and Prevention".
Epidemiology and Demographics
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [12]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[13]; João André Alves Silva, M.D. [14], Dima Nimri, M.D. [15], Tarek Nafee, M.D. [16]
Overview
In 2019, about 8,920 TB cases were documented in the US with an incidence of 2.7 cases/100,000 persons. This represented a decrease by 1.1% from 2018. In 2015, about 10.4 million people developed symptomatic TB and 1.8 million died from the disease. In 2014, approximately 9,421 cases were documented in the United States, with an incidence of 3.0 cases/100 000 persons. Since 1990, there has been a decrease in the mortality rate . TB is more prevalent in older men. Racial and ethnic minorities have a higher prevalence of TB than non-Hispanic whites. Coinfection with HIV is an important leading cause of death in TB. In 2015, 60% of the worldwide TB cases were in 6 countries: South Africa, Indonesia, China, Pakistan, India, and Nigeria. The WHO reported 24 other high-burden TB countries including Bangladesh, Korea, Columbia, Cambodia, Congo, Brazil, Ethiopia, Myanmar, Philippines, Thailand, Liberia, Vietnam, Kenya, Central Africa, Russia, Angola, Zimbabwe, Namibia, Mozambique, Tanzania, Sierra Leone, Zambia, Papua New Guinea, and Lesotho.[1]
Epidemiology
- Worldwide, in 2018, there were approximately 10 million individuals with incident TB and about 1.5 million TB-related deaths.
- Approximately 862,000 of these cases were in patients coinfected with HIV [12]
- Worldwide, in 2015, approximately 10.4 million people had symptomatic TB.[2][3]
- 1.17 million of these cases occurred were in patients coinfected with HIV.[2][3]
- 400,000 of these cases were in patients coinfected with HIV.[2][3]
- In the United States, in 2014, approximately 9,421 cases were reported with an incidence of 3.0 cases/100 000 persons.[2][3]
Incidence and Mortality
Worldwide Tuberculosis
- Over 95% of TB deaths occur in low- and middle-income countries, and it is among the top three causes of death for women aged 15 to 44.
- The TB death rate dropped 45% between 1990 and 2012.
- In 2015, 3 million lives were saved by the global TB response.
-
Incidence of All Forms of TB in 2015. - WHO 2016 TB Report)[1]
-
Trend in TB incidence from 2000 to 2015. - WHO 2016 TB Report)[1]
-
TB mortality trends (2000-2015) - WHO 2016 TB Report)[1]
-
TB Global Mortality in 2015. - WHO 2016 TB Report)[1]
-
Image 5 - TB is in the top 10 causes of death in 2015. - WHO 2016 TB Report)[1]
-
Trends in Estimated Incident Tuberculosis 2000-2018 (TB)[1]
Global Regional Incidence & Mortality
The following global regional trends in TB incidence are observed from 2000 to 2015:
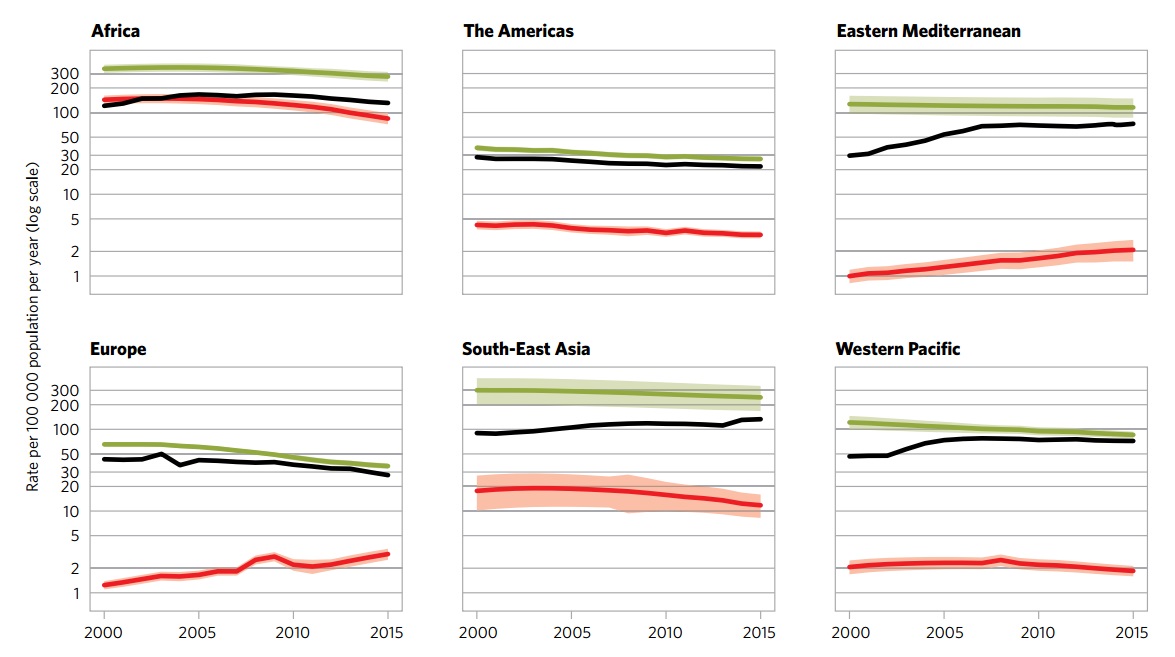
| African Region | American Region | Eastern Mediterranean Region | European Region | South-East Asian Region | Western-Pacific Region | |
|---|---|---|---|---|---|---|
| Incidence (per 100 000) |
275 | 27 | 116 | 36 | 246 | 86 |
| Incidence of Multi-Drug Resistant TB (per 100 000) |
11 | 1.1 | 6 | 14 | 10 | 5.5 |
| Mortality (excluding HIV-TB coinfection) (per 100 000) |
45 | 1.9 | 12 | 3.5 | 37 | 4.8 |
| Mortality (only HIV-TB coinfection) (per 100 000) |
30 | 0.59 | 0.46 | 0.54 | 3.9 | 0.31 |
| Total new cases in 2016 | 1 333 504 | 230 519 | 484 733 | 297 448 | 2 656 560 | 1 361 430 |
| Table adapted from WHO Global Report 2016 [1] | ||||||
TB and HIV
Immunosuppression secondary to HIV is strongly associated with incidence of TB and its subsequent complications. Tuberculosis contributes to a considerable proportion of HIV/AIDS related deaths.
-
Incidence of TB and HIV in 2015 - WHO 2016 TB Report)[1]
-
Trend in TB and HIV mortality (2000-2015) - WHO 2016 TB Report)[1]
-
Estimated TB and HIV deaths in 2015 - WHO 2016 TB Report)[1]
Tuberculosis in Endemic Countries
In 2015, 60% of the worldwide TB cases were reported in six countries:
South Africa
- The incidence of tuberculosis was reported as 834 per 100,000 of the general population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 46 per 100,000 of the general population.[1]
Indonesia
- The incidence of tuberculosis was reported as 395 per 100,000 of the overall population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 40 per 100,000 of the overall population.[1]
Nigeria
- The incidence of tuberculosis was reported as 322 per 100,000 of the general population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 99 per 100,000 of the general population.[1]
Pakistan
- The incidence of tuberculosis was reported as 270 per 100,000 of the general population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 23 per 100,000 of the general population.[1]
India
- The incidence of tuberculosis was reported as 217 per 100,000 of the general population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 36 per 100,000 of the general population.[1]
China
- The incidence of tuberculosis was reported as 67 per 100,000 of the general population.[1]
- The mortality rate of tuberculosis (excluding HIV-TB coinfection) was reported as 2.6 per 100,000 of the general population.[1]
Tuberculosis in the United States
TB resurge occurred in the mid-1980s since when the number of cases steadily increased with a peak occurred in 1992. Following this peak, the number of reported TB cases has decreased annually. The year of 2014 represented the twenty-second year of decline in the reported TB cases in the United States since that peak. In 2014, approximately a total number of 9,421 cases were reported in the 50 states and the District of Columbia (DC). This was considered a decline of 1.5% from 2013. The number of TB cases per 100,000 in 2013 and 2014 was at a stable rate of 3.0.[1]
 |
 |
Demographics
Age
In 2014, TB cases in most age groups decreased by approximately 70% from the 1993 values. Below is the comparison between the case rates (per 100 000 persons) of these two years, according to different ages:[4]
| Age | Case rate in 1993 | Case rate in 2014 |
|---|---|---|
| >65 years | 17.7 | 4.8 |
| 45 - 64 years | 12.4 | 3.5 |
| 25 - 44 years | 11.5 | 3.4 |
| 15 - 24 years | 5.0 | 2.2 |
| < 15 years | 2.9 | 0.8 |
| Data provided by the CDC[2][3][4] | ||
 |
 |
Depending on the age of the patient, tuberculosis may have different clinical manifestations, progression, and prognosis:[6][7][8][9][7][10]
| Factor | Influence |
|---|---|
| Infants and Children |
|
| Adolescents |
|
| Midadulthood |
|
| Elderly |
|
Gender
In 2012, there were 410,000 total deaths in women as a result of TB in the United States, 160,000 of them were HIV-positive. Out of the total TB deaths among HIV-positive people, 50% were women.[2][3] TB rates tend to increase with age, ranging from a low rate of less than 1 per 100,000 in children aged 5 - 14 to a high rate of 6.9 per 100,000 in men aged 65 years and older. With age increasing, the case rate increases faster in men than in women; the rates in men aged 45 years and older were approximately more than double the case rate in women of the same age.[2][3]

Race
The highest TB rates was reported in Asians, a decline from 29.9 per 100,000 persons in 2003 to 17.8 in 2014.[2][11]
| Racial/ethnic groups | Case rate in 2003 | Case rate in 2014 |
|---|---|---|
| Non-Hispanic blacks or African-Americans | 11.7 | 5.1 |
| Hispanics | 10.3 | 5.0 |
| American Indians and Alaska Natives | 8.2 | 5.0 |
| Non-Hispanic whites | 1.4 | 0.6 |
| Native Hawaiian or Other Pacific Islanders | 16.2 | 16.9 |
| Data provided by the CDC[2][3][11] | ||
The disproportionate burden of TB in minorities is due to many factors. In persons who were born in countries where TB is endemic, the disease can be a result of an acquired infection in their country of origin. Unequal distribution of TB risk factors, such as HIV infection, leads to increased TB exposure or to an increased risk of developing TB after infection with with M. tuberculosis.[2]
- Image 8 shows that above the age of 5, there is an increased risk of TB with age across all racial and ethnic groups. The case rates were higher in minority racial and ethnic groups than in non-Hispanic whites and were highest in Asians, Native Hawaiians and Other Pacific Islanders, especially in the adult age groups.[5]
- Image 9 shows that in 2014, 85% of all reported TB cases occurred in racial and ethnic minorities, whereas 13% of cases occurred in non-Hispanic whites. Persons reporting two or more races accounted for 2% of all cases[5]
 |
 |
 |
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 "WHO 2016 TB Report" (PDF).
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "Tuberculosis (TB)".
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Tuberculosis".
- ↑ 4.0 4.1 Center for Disease Control and Prevention http://www.cdc.gov/tb/statistics/reports/2014/pdfs/2014-surveillance-report_table4.pdf
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Center for Disease Control and Prevention (CDC)" (PDF).
- ↑ Stead WW, Lofgren JP, Warren E, Thomas C (1985). "Tuberculosis as an endemic and nosocomial infection among the elderly in nursing homes". N Engl J Med. 312 (23): 1483–7. doi:10.1056/NEJM198506063122304. PMID 3990748.
- ↑ 7.0 7.1 DAHL RH (1952). "[The first appearance of a pulmonary cavity after primary infection with relation to time and age]". Acta Tuberc Scand. 27 (1–2): 140–9. PMID 13007533.
- ↑ Stead WW (1967). "Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection?". Am Rev Respir Dis. 95 (5): 729–45. PMID 4960690.
- ↑ "Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement". Am J Respir Crit Care Med. 161 (4 Pt 2): S221–47. 2000. doi:10.1164/ajrccm.161.supplement_3.ats600. PMID 10764341.
- ↑ Stead WW, Kerby GR, Schlueter DP, Jordahl CW (1968). "The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis". Ann Intern Med. 68 (4): 731–45. PMID 5642961.
- ↑ 11.0 11.1 Center for Disease Control and Prevention http://www.cdc.gov/tb/statistics/reports/2014/pdfs/2014-surveillance-report_table17.pdf
Risk Factors
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [17]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[18]; João André Alves Silva, M.D. [19]
Overview
The risk factors for developing tuberculosis include: living or traveling to endemic areas for TB, elderly people and infants, immunosuppression, history of frequent or prolonged contact with infected patients, IV drug users, smoking, bad hygiene, and poor nutrition. In addition, the risk factors for multidrug-resistant TB include: non-adherence to treatment regimen, inadequate treatment for that bacterial strain, and contact with patients with multidrug-resistant TB.
Risk Factors
Primary TB, which represents 1-5% cases, occurs after infection. However, most of the cases occur with latent infection which is asymptomatic. The dormant bacilli can cause tuberculosis in 2 to 23% of the latent cases, usually several years following the primary infection.[1] The risk of reactivation is much higher with immunosuppression, such as HIV. In patients with HIV coinfection, the risk of reactivation increases reaching up to 10% per year.
The following are risk factors for active TB:[2][3]
- Living or traveling to endemic areas (Sub-saharan African, Russia, India, Pakistan, China)
- Elderly
- Infants
- IV drug users
- Immunosuppression:
- AIDS
- Diabetes
- Silicosis
- Malignancy, such as head and neck cancers
- Hematologic and reticuloendothelial disease, such as leukemia and Hodgkin's disease
- Or those taking medications, such as:
- Chemotherapy
- Immunosuppressive medications, such as prolonged corticosteroid therapy, tumor necrosis factor-alpha blockers[4]
- Tocilizumab
The risk of contracting TB increases in cases where there is:[2]
The following factors may increase the rate of TB infection in a population:[2]
- Chest X-ray with evidence of previous TB disease (fibrotic lesions and nodules)
- Increased number of HIV infections
- Increased number of homeless people
- The appearance of drug-resistant strains of TB
Drugs With Increased Risk of Tuberculosis Reactivation
- Treatment with the following drugs have been reported with increased risk of reactivation of latent tuberculosis.
Multidrug-Resistant Tuberculosis
Drug resistance is more common in people who:[5]
- Do not take their TB medicine regularly
- Do not take all of their TB medicine as told by their doctor or nurse
- Develop TB disease again, after having taken TB medicine in the past
- Come from areas of the world where drug-resistant TB is common
- Have spent time with someone known to have drug-resistant TB disease
References
- ↑ Parrish N, Dick J, Bishai W (1998). "Mechanisms of latency in Mycobacterium tuberculosis". Trends Microbiol. 6 (3): 107–12. PMID 9582936.
- ↑ 2.0 2.1 2.2 "Tuberculosis Fact Sheet".
- ↑ Griffith D, Kerr C (1996). "Tuberculosis: disease of the past, disease of the present". J Perianesth Nurs. 11 (4): 240–5. PMID 8964016.
- ↑ Mutlu G, Mutlu E, Bellmeyer A, Rubinstein I (2006). "Pulmonary adverse events of anti-tumor necrosis factor-alpha antibody therapy". Am J Med. 119 (8): 639–46. PMID 16887405.
- ↑ "Multidrug-resistant tuberculosis".
Causes
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis On the Web |
|
American Roentgen Ray Society Images of Tuberculosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [20]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[21]; João André Alves Silva, M.D. [22]
Synonyms and keywords: M. Tuberculosis
Overview
Mycobacterium tuberculosis is the bacterium responsible for tuberculosis. M. tuberculosis is an obligate aerobe, non-encapsulated, non-motile, acid-fast bacillus. . M. tuberculosis is one of the Mycobacterium tuberculosis complex, which also includes bacteria, such as M. bovis and M. africanum. The bacterium has a very slow rate of replication, and its genetic variations account for the different strains and the growing drug resistance. M. tuberculosis has tropism for different kinds of human cells, with preference for cells of the lung. The main natural reservoir for M. tuberculosis are Human beings; however, the bacteria can also infect other species.
Taxonomy
 |
 |
Cellular organisms; bacteria; Actinobacteria; Actinobacteria; Actinobacteridae; Actinomycetales; Corynebacterineae; Mycobacteriaceae; Mycobacterium; Mycobacterium tuberculosis complex; M. tuberculosis[3]
Biology
- Mycobacterium tuberculosis belongs to the Mycobacterium tuberculosis complex. This complex includes M. tuberculosis, M. bovis, M. africanum, M. canetti, and M. microti.[4]
- M. tuberculosis is an obligate aerobe, non-encapsulated, non-motile, acid-fast bacillus.
- Its shape is slender, straight or slightly curved bacillus with rounded ends.
- It can be present singly, in pairs or in small groups or clumps.
- It cannot form spores.
- It favors tissues with high oxygen levels.
- Due to the high lipid and mycolic acid content of its cell wall, It stains weakly gram-positive or does not retain the dye.
- Microscopic examination of sputum samples cannot differentiate it from other acid-fast bacteria, such as Nocardia spp.[4]
- M. tuberculosis divides every 15-20 hours which is considered an extremely low rate of division. This feature, in addition to its ability to remain latent for long time, are responsible for the long treatment duration needed.[4]
- It can withstand dryness for weeks and also weak disinfectants.
- Genetic variations in the M. tuberculosis genome lead to important phenotypical changes. As a result, there are several variable strains of the bacteria, six of which have particular geographical distribution. Three strains, the Beijing family, strain W and the W-like strains, were reported to be associated with higher incidence of multi-drug resistance.[5][6]
Tropism
There is no particular tissue tropism for M. tuberculosis and it can infect almost all human tissues. However, M. tuberculosis prefers tissues with high levels of oxygen , hence, pulmonary tuberculosis has the highest rate. [4]
Natural Reservoir
The main natural reservoir for M. tuberculosis are Human beings; however, the bacteria can also infect other species.[4]
References
- ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ "Mycobacterium tuberculosis".
- ↑ 4.0 4.1 4.2 4.3 4.4 Lawn SD, Zumla AI (2011). "Tuberculosis". Lancet. 378 (9785): 57–72. doi:10.1016/S0140-6736(10)62173-3. PMID 21420161.
- ↑ Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV (2009). "Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis". Nat Rev Microbiol. 7 (7): 537–44. doi:10.1038/nrmicro2165. PMID 19483712.
- ↑ Gagneux S, Small PM (2007). "Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development". Lancet Infect Dis. 7 (5): 328–37. doi:10.1016/S1473-3099(07)70108-1. PMID 17448936.
Natural History, Complications & Prognosis
Diagnosis
{{#ask:Used To Diagnose::Tuberculosis |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTestQuery |sort=Sort Order }}
Treatment
{{#ask:Used To Treat::Tuberculosis |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery |sort=Sort Order }} {{#ask:Prevents::Tuberculosis |?Sort Order |intro= | |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery2 |sort=Sort Order }}
![Left lateral margin of a tongue of a tuberculosis patient, which had been retracted in order to reveal the lesion that had been caused by the Gram-positive bacterium Mycobacterium tuberculosisAdapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/0/09/TB1.jpg)
![Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection.[ http://phil.cdc.gov/phil/ Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.][20]](/images/e/ed/TB2.jpg)
![Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/7/79/Leprosy-35.jpg)
![Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/6/60/Leprosy-36.jpg)
![Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/9/93/Leprosy-37.jpg)
![Light photomicrograph revealing some of the histopathologic cytoarchitectural characteristics seen in a mycobacterial skin infection Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/b/b5/Leprosy-38.jpg)
![Photomicrograph describing tuberculosis of the placenta.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/b/be/TB3.jpg)
![Histopathology of tuberculosis, endometrium. Ziehl-Neelsen stain.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/d/dd/TB4.jpg)
![Histopathology of tuberculosis, placenta.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[20]](/images/9/97/TB5.jpg)
![Incidence of All Forms of TB in 2015. - WHO 2016 TB Report)[1]](/images/0/05/TB_incidence_all_forms.jpg)
![Trend in TB incidence from 2000 to 2015. - WHO 2016 TB Report)[1]](/images/d/dd/TB_incidence_trends.jpg)
![TB mortality trends (2000-2015) - WHO 2016 TB Report)[1]](/images/9/94/TB_mortality_trends.jpg)
![TB Global Mortality in 2015. - WHO 2016 TB Report)[1]](/images/3/3d/TBmortality2015.jpg)
![Image 5 - TB is in the top 10 causes of death in 2015. - WHO 2016 TB Report)[1]](/images/6/65/Topworldwidecauseofdeath.jpg)
![Trends in Estimated Incident Tuberculosis 2000-2018 (TB)[1]](/images/c/c2/Screen_Shot_2021-01-19_at_8.30.45_PM.png)
![Incidence of TB and HIV in 2015 - WHO 2016 TB Report)[1]](/images/b/b0/TB_HIV_incidence.jpg)
![Trend in TB and HIV mortality (2000-2015) - WHO 2016 TB Report)[1]](/images/c/c7/TrendinTBandHIVdeath.jpg)
![Estimated TB and HIV deaths in 2015 - WHO 2016 TB Report)[1]](/images/b/b6/HIVandTBdeath2015.jpg)