Hydrochlorothiazide: Difference between revisions
No edit summary |
No edit summary |
||
| (40 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
<!--Overview--> | <!--Overview--> | ||
|genericName= | |genericName= | ||
Hydrochlorothiazide | Hydrochlorothiazide | ||
|aOrAn= | |aOrAn= | ||
a | a | ||
|drugClass= | |drugClass= | ||
[[thiazide]] [[diuretic]] | |||
|indication= | |indication= | ||
[[hypertension]] either as the sole therapeutic agent, or in combination with other [[antihypertensive]]s | |||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
|adverseReactions= | |adverseReactions= | ||
| Line 31: | Line 23: | ||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle= | ||
Title | Title | ||
|blackBoxWarningBody= | |blackBoxWarningBody= | ||
<i><span style="color:#FF0000;">ConditionName: </span></i> | <i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 43: | Line 33: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Hypertension===== | ||
* Dosing Information | * Dosing Information | ||
:* | :* '''12.5 mg once daily''' whether given alone or in combination with other [[antihypertensive]]s. | ||
:* Total daily doses greater than 50 mg are not recommended. | |||
:* | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
===== | =====Hypercalciuria===== | ||
* Dosing Information | * Dosing Information | ||
:* | :* '''25–50 mg/day'''<ref>{{Cite journal | issn = 0022-5347 | volume = 128 | issue = 5 | pages = 903–907 | last = Scholz | first = D. | coauthors = P. O. Schwille, A. Sigel | title = Double-blind study with thiazide in recurrent calcium lithiasis | journal = The Journal of Urology | date = 1982-11 | pmid = 7176047 }}</ref><ref>{{Cite journal | issn = 0036-5599 | volume = 18 | issue = 2 | pages = 143–149 | last = Laerum | first = E. | title = Metabolic effects of thiazide versus placebo in patients under long-term treatment for recurrent urolithiasis | journal = Scandinavian Journal of Urology and Nephrology | date = 1984 | pmid = 6463598 }}</ref><ref>{{Cite journal | issn = 0001-6101 | volume = 215 | issue = 4 | pages = 383–389 | last = Laerum | first = E. | coauthors = S. Larsen | title = Thiazide prophylaxis of urolithiasis. A double-blind study in general practice | journal = Acta Medica Scandinavica | date = 1984 | pmid = 6375276 }}</ref><ref>{{Cite journal | issn = 0391-6510 | volume = 8 | issue = 1 | pages = 29–34 | last = Bentur | first = L. | coauthors = U. Alon, M. Berant | title = Hypercalciuria in chronically institutionalized bedridden children: frequency, predictive factors and response to treatment with thiazides | journal = The International Journal of Pediatric Nephrology | date = 1987-03 | pmid = 3583554 }}</ref> | ||
=====Osteoporosis===== | |||
* Dosing Information | |||
:* '''12.5–25 mg/day'''<ref>{{Cite journal | issn = 0029-7844 | volume = 67 | issue = 4 | pages = 457–462 | last = Wasnich | first = R. D. | coauthors = P. D. Ross, L. K. Heilbrun, J. M. Vogel, K. Yano, R. J. Benfante | title = Differential effects of thiazide and estrogen upon bone mineral content and fracture prevalence | journal = Obstetrics and Gynecology | date = 1986-04 | pmid = 3960416 }}</ref><ref>{{Cite journal | issn = 0140-6736 | volume = 1 | issue = 8640 | pages = 687–690 | last = Ray | first = W. A. | coauthors = M. R. Griffin, W. Downey, L. J. Melton | title = Long-term use of thiazide diuretics and risk of hip fracture | journal = Lancet | date = 1989-04-01 | pmid = 2564506 }}</ref><ref>{{Cite journal | issn = 0003-4819 | volume = 133 | issue = 7 | pages = 516–526 | last = LaCroix | first = A. Z. | coauthors = S. M. Ott, L. Ichikawa, D. Scholes, W. E. Barlow | title = Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial | journal = Annals of Internal Medicine | date = 2000-10-03 | pmid = 11015164 }}</ref> | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed= | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 125: | Line 74: | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport= | ||
===== | =====Hypercalciuria===== | ||
* Dosing Information | * Dosing Information | ||
:* | :* '''0.5–2 mg/kg/day'''<ref>{{Cite journal | issn = 0022-5347 | volume = 128 | issue = 5 | pages = 903–907 | last = Scholz | first = D. | coauthors = P. O. Schwille, A. Sigel | title = Double-blind study with thiazide in recurrent calcium lithiasis | journal = The Journal of Urology | date = 1982-11 | pmid = 7176047 }}</ref> | ||
===== | =====Bronchopulmonary Dysplasia of Newborn===== | ||
* Dosing Information | |||
:* '''1 mg/kd/day'''<ref>{{Cite journal | issn = 0022-3476 | volume = 115 | issue = 4 | pages = 615–620 | last = Albersheim | first = S. G. | coauthors = A. J. Solimano, A. K. Sharma, J. A. Smyth, A. Rotschild, B. J. Wood, S. B. Sheps | title = Randomized, double-blind, controlled trial of long-term diuretic therapy for bronchopulmonary dysplasia | journal = The Journal of Pediatrics | date = 1989-10 | pmid = 2677293 }}</ref> | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications= | ||
* | * [[Anuria]] | ||
* [[Hypersensitivity]] to this product or other [[sulfonamide]] derived drugs | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings= | ||
* | * Acute Myopia and Secondary Angle-Closure Glaucoma | ||
:* Hydrochlorothiazide, a [[sulfonamide]], can cause an idiosyncratic reaction, resulting in acute transient [[myopia]] and acute [[angle-closure glaucoma]]. Symptoms include acute onset of decreased visual acuity or [[ocular pain]] and typically occur within hours to weeks of drug initiation. Untreated acute [[angle-closure glaucoma]] can lead to permanent [[vision loss]]. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the [[intraocular pressure]] remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of [[sulfonamide]] or [[penicillin]] allergy. | |||
* Diabetes and Hypoglycemia | |||
:* Latent [[diabetes mellitus]] may become manifest and [[diabetic]] patients given thiazides may require adjustment of their [[insulin]] dose. | |||
* Renal Disease | |||
:* Cumulative effects of the [[thiazides]] may develop in patients with impaired [[renal function]]. In such patients, [[thiazides]] may precipitate [[azotemia]]. | |||
====Precautions==== | ====Precautions==== | ||
* | * Electrolyte and Fluid Balance Status | ||
:* In published studies, clinically significant [[hypokalemia]] has been consistently less common in patients who received 12.5 mg of hydrochlorothiazide than in patients who received higher doses. Nevertheless, periodic determination of serum [[electrolyte]]s should be performed in patients who may be at risk for the development of [[hypokalemia]]. Patients should be observed for signs of fluid or [[electrolyte disturbance]]s, i.e., [[hyponatremia]], [[hypochloremic alkalosis]], and [[hypokalemia]] and [[hypomagnesemia]]. | |||
:* Warning signs or symptoms of fluid and [[electrolyte imbalance]] include dryness of mouth, [[thirst]], [[weakness]], [[lethargy]], [[drowsiness]], [[restlessness]], muscle pains or cramps, muscular fatigue, [[hypotension]], [[oliguria]], [[tachycardia]], and gastrointestinal disturbances such as [[nausea]] and [[vomiting]]. | |||
:* [[Hypokalemia]] may develop, especially with brisk [[diuresis]] when severe cirrhosis is present, during concomitant use of [[corticosteroid]] or [[ACTH|adrenocorticotropic hormone (ACTH)]] or after prolonged therapy. Interference with adequate oral electrolyte intake will also contribute to [[hypokalemia]]. [[Hypokalemia]] and [[hypomagnesemia]] can provoke ventricular [[arrhythmia]]s or sensitize or exaggerate the response of the heart to the toxic effects of [[digitalis]]. [[Hypokalemia]] may be avoided or treated by [[potassium]] supplementation or increased intake of [[potassium]] rich foods. | |||
:* Dilutional [[hyponatremia]] is life-threatening and may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than salt administration, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice. | |||
* Hyperuricemia | |||
:* [[Hyperuricemia]] or acute [[gout]] may be precipitated in certain patients receiving [[thiazide]] [[diuretics]]. | |||
* Impaired Hepatic Function | |||
:* [[Thiazides]] should be used with caution in patients with impaired hepatic function. They can precipitate hepatic coma in patients with severe [[liver disease]]. | |||
* Parathyroid Disease | |||
:* [[Calcium]] excretion is decreased by [[thiazides]], and pathologic changes in the parathyroid glands, with [[hypercalcemia]] and [[hypophosphatemia]], have been observed in a few patients on prolonged [[thiazide]] therapy. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |clinicalTrials= | ||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
* Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn. | |||
* The adverse reactions associated with hydrochlorothiazide have been shown to be dose related. In controlled clinical trials, the adverse events reported with doses of 12.5 mg hydrochlorothiazide once daily were comparable to [[placebo]]. The following [[adverse reactions]] have been reported for doses of hydrochlorothiazide 25 mg and greater and, within each category, are listed in the order of decreasing severity. | |||
======Body as a Whole====== | |||
[[Weakness]]. | |||
======Cardiovascular====== | ======Cardiovascular====== | ||
[[Hypotension]] including [[orthostatic hypotension]] (may be aggravated by [[alcohol]], [[barbiturate]]s, [[narcotic]]s or [[antihypertensive]] drugs). | |||
======Neurologic====== | |||
[[Vertigo]], [[paresthesia]], [[dizziness]], [[headache]], [[restlessness]]. | |||
======Hematologic====== | |||
[[Aplastic anemia]], [[agranulocytosis]], [[leukopenia]], [[hemolytic anemia]], [[thrombocytopenia]]. | |||
======Gastrointestinal====== | ======Gastrointestinal====== | ||
[[Pancreatitis]], [[jaundice]] (intrahepatic cholestatic jaundice), [[diarrhea]], [[vomiting]], [[sialadenitis]], [[cramping]], [[constipation]], gastric irritation, [[nausea]], [[anorexia]]. | |||
======Hypersensitivity====== | ======Hypersensitivity====== | ||
[[Anaphylactic reaction]]s, [[necrotizing angiitis]] ([[vasculitis]] and [[cutaneous vasculitis]]), [[respiratory distress]] including [[pneumonitis]] and [[pulmonary edema]], [[photosensitivity]], [[fever]], [[urticaria]], [[rash]], [[purpura]]. | |||
======Metabolic====== | |||
[[Electrolyte imbalance]], [[hyperglycemia]], [[glycosuria]], [[hyperuricemia]]. | |||
======Musculoskeletal====== | |||
[[Muscle spasm]]. | |||
======Renal====== | |||
[[Renal failure]], [[renal dysfunction]], [[interstitial nephritis]]. | |||
====== | ======Skin====== | ||
[[Erythema multiforme]] including [[Stevens-Johnson syndrome]], [[exfoliative dermatitis]] including [[toxic epidermal necrolysis]], [[alopecia]]. | |||
======Special Senses====== | |||
Transient [[blurred vision]], [[xanthopsia]]. | |||
======Urogenital====== | |||
[[Impotence]]. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions= | ||
* | * [[Alcohol]], [[barbiturate]]s, or [[narcotic]]s - potentiation of [[orthostatic hypotension]] may occur. | ||
* Antidiabetic drugs - (oral agents and [[insulin]]) dosage adjustment of the antidiabetic drug may be required. | |||
* Other [[antihypertensive]] drugs - additive effect or potentiation. | |||
* [[Cholestyramine]] and [[colestipol]] resins - [[Cholestyramine]] and colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85 and 43 percent, respectively. | |||
* [[Corticosteroid]], [[ACTH]] – intensified [[electrolyte]] depletion, particularly [[hypokalemia]]. | |||
* Pressor amines (e.g., [[norepinephrine]]) - possible decreased response to pressor amines but not sufficient to preclude their use. | |||
* Skeletal [[muscle relaxant]]s, nondepolarizing (e.g., tubocurarine) - possible increased responsiveness to the muscle relaxant. | |||
* [[Lithium]] - generally should not be given with [[diuretics]]. [[Diuretic]] agents reduce the renal clearance of [[lithium]] and greatly increase the risk of [[lithium]] toxicity. Refer to the package insert for lithium preparations before use of such preparations with Microzide. | |||
* [[NSAID|Non-steroidal anti-inflammatory drugs]] - In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the [[diuretic]], [[natriuretic]], and [[antihypertensive]] effects of loop, [[potassium]]-sparing and [[thiazide]] [[diuretics]]. When Microzide and [[NSAID|non-steroidal anti-inflammatory agents]] are used concomitantly, the patients should be observed closely to determine if the desired effect of the [[diuretic]] is obtained. | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category B''' | ||
:* Teratogenic Effects | |||
::* Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 and 1000 mg hydrochlorothiazide/kg, respectively, provided no evidence of harm to the fetus. | |||
::* There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during [[pregnancy]] only if clearly needed. | |||
:* Nonteratogenic Effects | |||
::* Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal [[jaundice]], [[thrombocytopenia]], and possibly other [[adverse reactions]] that have occurred in adults. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | * '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery= | |useInLaborDelivery= | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing= | ||
* Thiazides are excreted in breast milk. Because of the potential for serious [[adverse reactions]] in nursing infants, a decision should be made whether to discontinue nursing or to discontinue hydrochlorothiazide, taking into account the importance of the drug to the mother. | |||
|useInPed= | |useInPed= | ||
* Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri= | |useInGeri= | ||
* A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., > 65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of hydrochlorothiazide (12.5 mg) is therefore recommended. If further titration is required, 12.5 mg increments should be utilized. | |||
|useInGender= | |useInGender= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace= | |useInRace= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair= | |useInRenalImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair= | |useInHepaticImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential= | |useInReproPotential= | ||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp= | |useInImmunocomp= | ||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration= | |administration= | ||
* Oral | * Oral | ||
|monitoring= | |monitoring= | ||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat= | |IVCompat= | ||
| Line 306: | Line 245: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose= | ||
| Line 313: | Line 251: | ||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
* | * The most common signs and symptoms observed are those caused by electrolyte depletion ([[hypokalemia]], [[hypochloremia]], [[hyponatremia]]) and [[dehydration]] resulting from excessive [[diuresis]]. If [[digitalis]] has also been administered, [[hypokalemia]] may accentuate cardiac [[arrhythmia]]s. | ||
* The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat. | |||
====Management==== | ====Management==== | ||
* | * In the event of overdosage, symptomatic and supportive measures should be employed. [[Emesis]] should be induced or [[gastric lavage]] performed. Correct [[dehydration]], [[electrolyte imbalance]], [[hepatic coma]] and [[hypotension]] by established procedures. If required, give oxygen or artificial respiration for respiratory impairment. The degree to which hydrochlorothiazide is removed by [[hemodialysis]] has not been established. | ||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 326: | Line 265: | ||
<!--Drugbox2--> | <!--Drugbox2--> | ||
|drugBox= | |||
{{Drugbox2 | |||
| verifiedrevid = 443860485 | |||
| IUPAC_name = 6-chloro-1,1-dioxo-3,4-dihydro-2''H''-1,2,4-benzothiadiazine-7-sulfonamide | |||
| image = Hydrochlorothiazide-2D-skeletal.png | |||
| width = 200px | |||
| image2 = Hydrochlorothiazide-from-xtal-3D-balls.png | |||
| | <!--Clinical data--> | ||
| tradename = Apo-hydro, Aquazide h, Dichlotride among others, Oretic | |||
| Drugs.com = {{drugs.com|monograph|hydrochlorothiazide}} | |||
| MedlinePlus = a682571 | |||
| pregnancy_category = B (D if used to treat pregnancy-induced hypertension) | |||
| legal_status = Rx-only | |||
| routes_of_administration = Oral (capsules, tablets, oral solution) | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = Variably absorbed from GI tract. Bioavailability ~ 70% | |||
| metabolism = does not undergo significant metabolism (>95% excreted unchanged in urine)<ref>{{cite journal | author = Beermann B, Groschinsky-Grind M, Rosén A. | title = Absorption, metabolism, and excretion of hydrochlorothiazide | journal = Clin Pharmacol Ther | volume = 19 | issue = 5 (Pt 1) | pages = 531–7 | year = 1976}}</ref> | |||
| elimination_half-life = 5.6–14.8 h | |||
| excretion = Primarily excreted unchanged in urine | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 58-93-5 | |||
| ATC_prefix = C03 | |||
| ATC_suffix = AA03 | |||
| PubChem = 3639 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00999 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3513 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 0J48LPH2TH | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00340 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 435 | |||
<!--Chemical data--> | |||
| C=7 | H=8 | Cl=1 | N=3 | O=4 | S=2 | |||
| molecular_weight = 297.74 | |||
| smiles = O=S(=O)(c1c(Cl)cc2c(c1)S(=O)(=O)NCN2)N | |||
| InChI = 1/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) | |||
| InChIKey = JZUFKLXOESDKRF-UHFFFAOYAN | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = JZUFKLXOESDKRF-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction= | |mechAction= | ||
* | * Hydrochlorothiazide blocks the reabsorption of sodium and chloride ions, and it thereby increases the quantity of sodium traversing the distal tubule and the volume of water excreted. A portion of the additional sodium presented to the distal tubule is exchanged there for [[potassium]] and [[hydrogen ion]]s. With continued use of hydrochlorothiazide and depletion of [[sodium]], compensatory mechanisms tend to increase this exchange and may produce excessive loss of [[potassium]], [[hydrogen]] and [[chloride]] ions. Hydrochlorothiazide also decreases the excretion of [[calcium]] and [[uric acid]], may increase the excretion of [[iodide]] and may reduce glomerular filtration rate. Metabolic toxicities associated with excessive electrolyte changes caused by hydrochlorothiazide have been shown to be dose-related. | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure= | ||
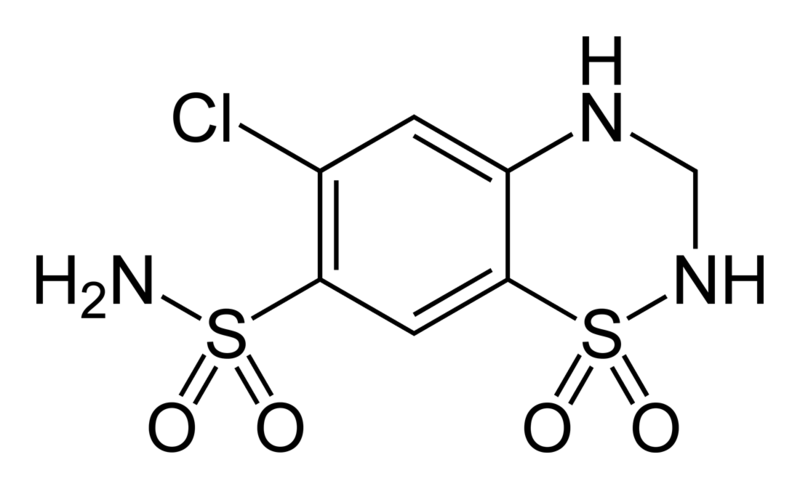
* | * Microzide® (hydrochlorothiazide, USP) Capsules 12.5 mg is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2; its molecular weight is 297.74; and its structural formula is: | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* It is a white, or practically white, crystalline powder which is slightly soluble in water, but freely soluble in sodium hydroxide solution. | |||
* Microzide is supplied as 12.5 mg capsules for oral use. | |||
* Inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate. Gelatin capsules contain D&C Red No. 28, D&C Yellow No. 10, FD&C Blue No. 1, gelatin, titanium dioxide. The capsules are printed with edible ink containing black iron oxide, D&C Yellow No. 10, FD&C Blue No. 1, FD&C Blue No. 2, FD&C Red No. 40. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD= | |PD= | ||
* Acute [[antihypertensive]] effects of [[thiazides]] are thought to result from a reduction in blood volume and [[cardiac output]], secondary to a [[natriuretic]] effect, although a direct vasodilatory mechanism has also been proposed. With chronic administration, plasma volume returns toward normal, but peripheral vascular resistance is decreased. The exact mechanism of the [[antihypertensive]] effect of hydrochlorothiazide is not known. | |||
* [[Thiazides]] do not affect normal [[blood pressure]]. Onset of action occurs within 2 hours of dosing, peak effect is observed at about 4 hours, and activity persists for up to 24 hours. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK= | ||
* Hydrochlorothiazide is well absorbed (65% to 75%) following oral administration. Absorption of hydrochlorothiazide is reduced in patients with [[congestive heart failure]]. | |||
* Peak plasma concentrations are observed within 1 to 5 hours of dosing, and range from 70 to 490 ng/mL following oral doses of 12.5 to 100 mg. Plasma concentrations are linearly related to the administered dose. Concentrations of hydrochlorothiazide are 1.6 to 1.8 times higher in whole blood than in plasma. Binding to serum proteins has been reported to be approximately 40% to 68%. The plasma elimination half-life has been reported to be 6 to 15 hours. Hydrochlorothiazide is eliminated primarily by renal pathways. Following oral doses of 12.5 to 100 mg, 55% to 77% of the administered dose appears in urine and greater than 95% of the absorbed dose is excreted in urine as unchanged drug. In patients with renal disease, plasma concentrations of hydrochlorothiazide are increased and the elimination half-life is prolonged. | |||
* When Microzide is administered with food, its bioavailability is reduced by 10%, the maximum plasma concentration is reduced by 20%, and the time to maximum concentration increases from 1.6 to 2.9 hours. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |nonClinToxic= | ||
| Line 364: | Line 353: | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies= | ||
* In an 87 patient 4-week double-blind, placebo controlled, parallel group trial, patients who received Microzide had reductions in seated [[SBP|systolic]] and [[DBP|diastolic blood pressure]] that were significantly greater than those seen in patients who received [[placebo]]. In published placebo-controlled trials comparing 12.5 mg of hydrochlorothiazide to 25 mg, the 12.5 mg dose preserved most of the placebo-corrected [[blood pressure]] reduction seen with 25 mg. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
* | * Microzide® (hydrochlorothiazide, USP) Capsules 12.5 mg are #4 Teal Opaque/Teal Opaque two piece hard gelatin capsules imprinted with Microzide and 12.5 mg in black ink. They are supplied in bottles of 100 with child resistant closures (NDC 52544-622-01). | ||
* Dispense in a tight, light-resistant container as defined in the USP. | |||
* Keep out of reach of children. | |||
* Store at 20°-25°C (68°-77°F). [See USP controlled room temperature.] Protect from light, moisture, freezing, -20°C (-4°F). Keep container tightly closed. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
| Line 386: | Line 371: | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol= | |alcohol= | ||
* | * [[Hypotension]] including [[orthostatic hypotension]] may be aggravated by [[alcohol]]. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames= | |brandNames= | ||
* Microzide®<ref>{{Cite web | title = | * Microzide®<ref>{{Cite web | title = Microzide (hydrochlorothiazide) capsule, gelatin coated | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=afbefaff-97c4-4f84-b989-1595f882a885 }}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |||
| | * Microzide® — Maxzide®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
* | * Microzide® — Micronase®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
| Line 428: | Line 411: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}02.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
<!--Category--> | <!--Category--> | ||
[[Category:Diuretics]] | |||
[[Category:Thiazides]] | |||
[[Category:Sulfonamides]] | |||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 00:54, 25 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hydrochlorothiazide is a thiazide diuretic that is FDA approved for the {{{indicationType}}} of hypertension either as the sole therapeutic agent, or in combination with other antihypertensives. Common adverse reactions include hypotension, phototoxicity, and vertigo.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- 12.5 mg once daily whether given alone or in combination with other antihypertensives.
- Total daily doses greater than 50 mg are not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydrochlorothiazide in adult patients.
Non–Guideline-Supported Use
Hypercalciuria
- Dosing Information
Osteoporosis
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Hydrochlorothiazide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydrochlorothiazide in pediatric patients.
Non–Guideline-Supported Use
Hypercalciuria
- Dosing Information
- 0.5–2 mg/kg/day[8]
Bronchopulmonary Dysplasia of Newborn
- Dosing Information
- 1 mg/kd/day[9]
Contraindications
- Anuria
- Hypersensitivity to this product or other sulfonamide derived drugs
Warnings
- Acute Myopia and Secondary Angle-Closure Glaucoma
- Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
- Diabetes and Hypoglycemia
- Latent diabetes mellitus may become manifest and diabetic patients given thiazides may require adjustment of their insulin dose.
- Renal Disease
- Cumulative effects of the thiazides may develop in patients with impaired renal function. In such patients, thiazides may precipitate azotemia.
Precautions
- Electrolyte and Fluid Balance Status
- In published studies, clinically significant hypokalemia has been consistently less common in patients who received 12.5 mg of hydrochlorothiazide than in patients who received higher doses. Nevertheless, periodic determination of serum electrolytes should be performed in patients who may be at risk for the development of hypokalemia. Patients should be observed for signs of fluid or electrolyte disturbances, i.e., hyponatremia, hypochloremic alkalosis, and hypokalemia and hypomagnesemia.
- Warning signs or symptoms of fluid and electrolyte imbalance include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
- Hypokalemia may develop, especially with brisk diuresis when severe cirrhosis is present, during concomitant use of corticosteroid or adrenocorticotropic hormone (ACTH) or after prolonged therapy. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia and hypomagnesemia can provoke ventricular arrhythmias or sensitize or exaggerate the response of the heart to the toxic effects of digitalis. Hypokalemia may be avoided or treated by potassium supplementation or increased intake of potassium rich foods.
- Dilutional hyponatremia is life-threatening and may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than salt administration, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
- Hyperuricemia
- Hyperuricemia or acute gout may be precipitated in certain patients receiving thiazide diuretics.
- Impaired Hepatic Function
- Thiazides should be used with caution in patients with impaired hepatic function. They can precipitate hepatic coma in patients with severe liver disease.
- Parathyroid Disease
- Calcium excretion is decreased by thiazides, and pathologic changes in the parathyroid glands, with hypercalcemia and hypophosphatemia, have been observed in a few patients on prolonged thiazide therapy.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Hydrochlorothiazide in the drug label.
Postmarketing Experience
- Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
- The adverse reactions associated with hydrochlorothiazide have been shown to be dose related. In controlled clinical trials, the adverse events reported with doses of 12.5 mg hydrochlorothiazide once daily were comparable to placebo. The following adverse reactions have been reported for doses of hydrochlorothiazide 25 mg and greater and, within each category, are listed in the order of decreasing severity.
Body as a Whole
Cardiovascular
Hypotension including orthostatic hypotension (may be aggravated by alcohol, barbiturates, narcotics or antihypertensive drugs).
Neurologic
Vertigo, paresthesia, dizziness, headache, restlessness.
Hematologic
Aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia.
Gastrointestinal
Pancreatitis, jaundice (intrahepatic cholestatic jaundice), diarrhea, vomiting, sialadenitis, cramping, constipation, gastric irritation, nausea, anorexia.
Hypersensitivity
Anaphylactic reactions, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, photosensitivity, fever, urticaria, rash, purpura.
Metabolic
Electrolyte imbalance, hyperglycemia, glycosuria, hyperuricemia.
Musculoskeletal
Renal
Renal failure, renal dysfunction, interstitial nephritis.
Skin
Erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis, alopecia.
Special Senses
Transient blurred vision, xanthopsia.
Urogenital
Drug Interactions
- Alcohol, barbiturates, or narcotics - potentiation of orthostatic hypotension may occur.
- Antidiabetic drugs - (oral agents and insulin) dosage adjustment of the antidiabetic drug may be required.
- Other antihypertensive drugs - additive effect or potentiation.
- Cholestyramine and colestipol resins - Cholestyramine and colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85 and 43 percent, respectively.
- Corticosteroid, ACTH – intensified electrolyte depletion, particularly hypokalemia.
- Pressor amines (e.g., norepinephrine) - possible decreased response to pressor amines but not sufficient to preclude their use.
- Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) - possible increased responsiveness to the muscle relaxant.
- Lithium - generally should not be given with diuretics. Diuretic agents reduce the renal clearance of lithium and greatly increase the risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with Microzide.
- Non-steroidal anti-inflammatory drugs - In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. When Microzide and non-steroidal anti-inflammatory agents are used concomitantly, the patients should be observed closely to determine if the desired effect of the diuretic is obtained.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Teratogenic Effects
- Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 and 1000 mg hydrochlorothiazide/kg, respectively, provided no evidence of harm to the fetus.
- There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Nonteratogenic Effects
- Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydrochlorothiazide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hydrochlorothiazide during labor and delivery.
Nursing Mothers
- Thiazides are excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue hydrochlorothiazide, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., > 65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of hydrochlorothiazide (12.5 mg) is therefore recommended. If further titration is required, 12.5 mg increments should be utilized.
Gender
There is no FDA guidance on the use of Hydrochlorothiazide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydrochlorothiazide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Hydrochlorothiazide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Hydrochlorothiazide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydrochlorothiazide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydrochlorothiazide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Hydrochlorothiazide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Hydrochlorothiazide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
- The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat.
Management
- In the event of overdosage, symptomatic and supportive measures should be employed. Emesis should be induced or gastric lavage performed. Correct dehydration, electrolyte imbalance, hepatic coma and hypotension by established procedures. If required, give oxygen or artificial respiration for respiratory impairment. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established.
Chronic Overdose
There is limited information regarding Chronic Overdose of Hydrochlorothiazide in the drug label.
Pharmacology
Mechanism of Action
- Hydrochlorothiazide blocks the reabsorption of sodium and chloride ions, and it thereby increases the quantity of sodium traversing the distal tubule and the volume of water excreted. A portion of the additional sodium presented to the distal tubule is exchanged there for potassium and hydrogen ions. With continued use of hydrochlorothiazide and depletion of sodium, compensatory mechanisms tend to increase this exchange and may produce excessive loss of potassium, hydrogen and chloride ions. Hydrochlorothiazide also decreases the excretion of calcium and uric acid, may increase the excretion of iodide and may reduce glomerular filtration rate. Metabolic toxicities associated with excessive electrolyte changes caused by hydrochlorothiazide have been shown to be dose-related.
Structure
- Microzide® (hydrochlorothiazide, USP) Capsules 12.5 mg is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2; its molecular weight is 297.74; and its structural formula is:
- It is a white, or practically white, crystalline powder which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
- Microzide is supplied as 12.5 mg capsules for oral use.
- Inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate. Gelatin capsules contain D&C Red No. 28, D&C Yellow No. 10, FD&C Blue No. 1, gelatin, titanium dioxide. The capsules are printed with edible ink containing black iron oxide, D&C Yellow No. 10, FD&C Blue No. 1, FD&C Blue No. 2, FD&C Red No. 40.
Pharmacodynamics
- Acute antihypertensive effects of thiazides are thought to result from a reduction in blood volume and cardiac output, secondary to a natriuretic effect, although a direct vasodilatory mechanism has also been proposed. With chronic administration, plasma volume returns toward normal, but peripheral vascular resistance is decreased. The exact mechanism of the antihypertensive effect of hydrochlorothiazide is not known.
- Thiazides do not affect normal blood pressure. Onset of action occurs within 2 hours of dosing, peak effect is observed at about 4 hours, and activity persists for up to 24 hours.
Pharmacokinetics
- Hydrochlorothiazide is well absorbed (65% to 75%) following oral administration. Absorption of hydrochlorothiazide is reduced in patients with congestive heart failure.
- Peak plasma concentrations are observed within 1 to 5 hours of dosing, and range from 70 to 490 ng/mL following oral doses of 12.5 to 100 mg. Plasma concentrations are linearly related to the administered dose. Concentrations of hydrochlorothiazide are 1.6 to 1.8 times higher in whole blood than in plasma. Binding to serum proteins has been reported to be approximately 40% to 68%. The plasma elimination half-life has been reported to be 6 to 15 hours. Hydrochlorothiazide is eliminated primarily by renal pathways. Following oral doses of 12.5 to 100 mg, 55% to 77% of the administered dose appears in urine and greater than 95% of the absorbed dose is excreted in urine as unchanged drug. In patients with renal disease, plasma concentrations of hydrochlorothiazide are increased and the elimination half-life is prolonged.
- When Microzide is administered with food, its bioavailability is reduced by 10%, the maximum plasma concentration is reduced by 20%, and the time to maximum concentration increases from 1.6 to 2.9 hours.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Hydrochlorothiazide in the drug label.
Clinical Studies
- In an 87 patient 4-week double-blind, placebo controlled, parallel group trial, patients who received Microzide had reductions in seated systolic and diastolic blood pressure that were significantly greater than those seen in patients who received placebo. In published placebo-controlled trials comparing 12.5 mg of hydrochlorothiazide to 25 mg, the 12.5 mg dose preserved most of the placebo-corrected blood pressure reduction seen with 25 mg.
How Supplied
- Microzide® (hydrochlorothiazide, USP) Capsules 12.5 mg are #4 Teal Opaque/Teal Opaque two piece hard gelatin capsules imprinted with Microzide and 12.5 mg in black ink. They are supplied in bottles of 100 with child resistant closures (NDC 52544-622-01).
- Dispense in a tight, light-resistant container as defined in the USP.
- Keep out of reach of children.
- Store at 20°-25°C (68°-77°F). [See USP controlled room temperature.] Protect from light, moisture, freezing, -20°C (-4°F). Keep container tightly closed.
Storage
There is limited information regarding Hydrochlorothiazide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Hydrochlorothiazide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Hydrochlorothiazide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Hydrochlorothiazide in the drug label.
Precautions with Alcohol
- Hypotension including orthostatic hypotension may be aggravated by alcohol.
Brand Names
- Microzide®[11]
Look-Alike Drug Names
- Microzide® — Maxzide®[12]
- Microzide® — Micronase®[12]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Scholz, D. (1982-11). "Double-blind study with thiazide in recurrent calcium lithiasis". The Journal of Urology. 128 (5): 903–907. ISSN 0022-5347. PMID 7176047. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Laerum, E. (1984). "Metabolic effects of thiazide versus placebo in patients under long-term treatment for recurrent urolithiasis". Scandinavian Journal of Urology and Nephrology. 18 (2): 143–149. ISSN 0036-5599. PMID 6463598.
- ↑ Laerum, E. (1984). "Thiazide prophylaxis of urolithiasis. A double-blind study in general practice". Acta Medica Scandinavica. 215 (4): 383–389. ISSN 0001-6101. PMID 6375276. Unknown parameter
|coauthors=ignored (help) - ↑ Bentur, L. (1987-03). "Hypercalciuria in chronically institutionalized bedridden children: frequency, predictive factors and response to treatment with thiazides". The International Journal of Pediatric Nephrology. 8 (1): 29–34. ISSN 0391-6510. PMID 3583554. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Wasnich, R. D. (1986-04). "Differential effects of thiazide and estrogen upon bone mineral content and fracture prevalence". Obstetrics and Gynecology. 67 (4): 457–462. ISSN 0029-7844. PMID 3960416. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Ray, W. A. (1989-04-01). "Long-term use of thiazide diuretics and risk of hip fracture". Lancet. 1 (8640): 687–690. ISSN 0140-6736. PMID 2564506. Unknown parameter
|coauthors=ignored (help) - ↑ LaCroix, A. Z. (2000-10-03). "Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial". Annals of Internal Medicine. 133 (7): 516–526. ISSN 0003-4819. PMID 11015164. Unknown parameter
|coauthors=ignored (help) - ↑ Scholz, D. (1982-11). "Double-blind study with thiazide in recurrent calcium lithiasis". The Journal of Urology. 128 (5): 903–907. ISSN 0022-5347. PMID 7176047. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Albersheim, S. G. (1989-10). "Randomized, double-blind, controlled trial of long-term diuretic therapy for bronchopulmonary dysplasia". The Journal of Pediatrics. 115 (4): 615–620. ISSN 0022-3476. PMID 2677293. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Beermann B, Groschinsky-Grind M, Rosén A. (1976). "Absorption, metabolism, and excretion of hydrochlorothiazide". Clin Pharmacol Ther. 19 (5 (Pt 1)): 531–7.
- ↑ "Microzide (hydrochlorothiazide) capsule, gelatin coated".
- ↑ 12.0 12.1 "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Hydrochlorothiazide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Hydrochlorothiazide |Label Name=Hydrochlorothiazide02.png
}}
{{#subobject:
|Label Page=Hydrochlorothiazide |Label Name=Hydrochlorothiazide03.png
}}