Perinatal infection
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1], Associate Editor(s)-in-Chief: Hanan E. Elkalawy, MD[2]
Overview
perinatal infections represent the most important causes of permanent disability among children worldwide. Referred to it by the acronym TORCH, denoting Toxoplasma gondii, rubella virus, cytomegalovirus, and herpes virus,etc, it can result in congenital infections from only a modest number of human pathogens which cross the placenta and infect the fetus. Although congenital rubella syndrome has been eliminated in the Americas by immunization, several pathogens discussed in this chapter cannot currently be prevented by vaccines or effectively treated with the available antimicrobial drugs. Due to the immaturity of the immune system, newborn infants are at risk for postnatally acquired infections with certain viruses and several bacteria. This chapter summarizes the epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, and prevention of selected pathogens that can damage the developing nervous system. As emphasized by the persisting challenges of preventing congenital cytomegalovirus infection and the emergence of severe brain damage associated with congenital Zika syndrome, these pathogens remain important causes of cerebral palsy, epilepsy, and intellectual disability.
Historical Perspective
Discovery
- over 100 years, The concept that there is certain human pathogens are able to damage the developing nervous system in utero or perinatally. Although female can get numerous infectious agents during their pregnancies, relatively few pathogens cross the placenta and cause intrauterine fetal infections.
- In the 1970s, There is investigators at Emory University and the Centers for Disease Control and Prevention (CDC) coined the term TORCH, an acronym underscoring Toxoplasma gondii, rubella virus, cytomegalovirus, and herpesvirusesas important, potential causes of congenital infection.
- The TORCH concept highlighted that these agents can produce the same clinical manifestations in infected infants.
- Although congenital rubella virus syndrome has disappeared in countries with compulsory immunization against this virus , the TORCH agents, as well as more recently recognized pathogens, such as lymphocytic choriomeningitis virus and Zika virus, remain major causes of long-term neurodevelopmental disabilities among children throughout the world.[1]
Classification
Perinatal infection is vertically transmitted infection ,which starts at gestational ages between 22[2] and 28 weeks[3] (with regional variations in the definition) and ending seven completed days after birth[2]
Pathophysiology
- In the scale of optimal virulence, vertical transmission tends to progress benign symbiosis, so is a critical idea for evolutionary medicine. Because the ability of reproducibility of pathogen in the host is the leading cause of pathogen to pass from mother to child, Its transmissibility tends to be inversely related to their virulence.[4]
- Although HIV is transmitted through perinatal transmission, it is vertical transmission is not the primary mode of transmission. in addition to the new medicine decreased the frequency of vertical transmission of HIV. The incidence of perinatal HIV cases in the United States has decreased as a result of the implementation of recommendations on HIV counselling and voluntary testing practices and the use of zidovudine therapy to reduce perinatal HIV transmission.[5]
- In dual inheritance theory, vertical transmission refers to the passing of cultural traits from parents to children.[6]
| maternal infection | |||||||||||||||||||
| placental infection and inflammation | |||||||||||||||||||
| intrauterine growth retardation | fetal infection | ||||||||||||||||||
Causes
the following organism responsible for perinatal infection are transmitted either by one of the following across the placenta (transplacental) and across the female reproductive tract during (childbirth).
Transplacental
The embryo and fetus have little or no immunity and depend on the immune function of their mother. Several pathogens can cross the placenta and cause perinatal infection. Often, microorganisms that produce minor illness in the mother are very dangerous for the developing embryo or fetus.
During childbirth
Babies can also become infected by their mothers during child birth. Some infectious agents may be transmitted to the embryo or fetus in the uterus, while passing through the birth canal, or even shortly after birth.
Types of infections
Bacteria, viruses, and other organisms are able to be passed from mother to child. Several vertically transmitted infections are included in the TORCH complex:[7]
- T – toxoplasmosis from Toxoplasma gondii
- O – other infections (see below)
- R – rubella
- C – cytomegalovirus
- H – herpes simplex virus-2 or neonatal herpes simplex
Other infections include:
- Parvovirus B19
- Coxsackievirus
- Chickenpox (caused by varicella zoster virus)
- Chlamydia infection
- HIV
- Human T-lymphotropic virus
- Syphilis
- Zika fever, caused by Zika virus, can cause microcephaly and other brain defects in the child.
- Hepatitis B
The TORCH complex was originally considered to consist of the four conditions mentioned above, with the "TO" referring to Toxoplasma. The four-term form is still used in many modern references and the capitalization "TORCH" is sometimes used in these contexts.[8]
- A further expansion of this acronym, CHEAPTORCHES, was proposed by Ford-Jones and Kellner in 1995:[9]
- C – chickenpox and shingles
- H – hepatitis, C , (D), E
- E – enteroviruses
- A – AIDS (HIV infection)
- P – parvovirus B19 (produces hydrops fetalis secondary to aplastic anemia)
- T – toxoplasmosis
- O – other (group B streptococci, Listeria, Candida, and Lyme disease)
- R – rubella
- C – cytomegalovirus
- H – herpes simplex
- E – everything else sexually transmitted (gonorrhea, Chlamydia infection, Ureaplasma urealyticum, and human papillomavirus)
- S – Syphilis
Differentiating types of perinatal infection
| Disease | characteristics symptoms and signs | Lab finding & Other evaluation |
|---|---|---|
| Toxoplasmosis[1] | Classic triad Chorioretinitis: Hydrocephalus, Intracranial calcifications (ring-enhancing lesions), Petechiae and purpura (blueberry muffin rash) |
CT/MRI: intracranial calcifications, hydrocephalus, ring-enhancing lesions T. gondii-specific IgM antibodies (CSF, serum) PCR for T. gondii DNA (CSF, serum) Ophthalmological evaluation: chorioretinitis |
| Syphilis[1] |
|
Initial test: RPR or VDRL (serum) Confirmatory test: dark-field microscopy or PCR of lesions or bodily fluids
|
| listeriosis[1] | Spontaneous abortion and premature birth,Meningitis, sepsis,Vesicular and pustular skin lesions (granulomatosis infantiseptica) | Culture from blood or CSF samples (pleocytosis) |
| Varicella zoster virus (VZV)[1] | IUGR, premature birth,Chorioretinitis, cataract,Encephalitis,Pneumonia,CNS abnormalities,Hypoplastic limbs |
Usually clinical diagnosis is confirmed by appearance of skin lesions ( chickenpox and shingles.) DFA or PCR of fluid collected from blisters or cerebrospinal fluid (CSF) Serology
|
| Parvovirus B19[1] | Aplastic anemia,Fetal hydrops |
Positive IgM and negative IgG: very recent infection → refer to specialist Positive IgM and IgG: acute infection → refer to specialist Positive IgG and negative IgM: maternal immunity → reassurance Negative IgG and negative IgM: no maternal immunity → counseling
PCR for parvovirus B19 DNA (amniotic fluid or blood) Doppler ultrasound of fetal vessels in suspected hydrops fetalis |
| Rubella[1] |
|
PCR for rubella RNA (throat swab, CSF) Serology (abnormally high or persistent concentrations of IgM and/or IgG antibodies) Viral culture (nasopharynx, blood)
IgM antibody serology (chorionic villi, amniotic fluid) PCR for rubella RNA (chorionic villi, amniotic fluid) Newborn and mother |
| Cytomegaly virus (CMV)[1] | Jaundice, hepatosplenomegaly,IUGR,Chorioretinitis,Sensorineural deafness,Periventricular calcifications, Petechiae and purpura (blueberry muffin rash),Microcephaly,Seizures |
|
| Herpes simplex virus (HSV)[1] | Premature birth, IUGR, Skin, eyes, and mouth involvement: vesicular lesions, keratoconjunctivitis, Localized CNS involvement: meningitis ,Disseminated disease: multiple organ involvement, sepsis |
-Standard: viral culture of HSV from skin lesions, conjunctiva, oro/nasopharynx, or rectum -Alternative: PCR for HSV DNA (CSF, blood) |
Epidemiology and Demographics
| disease | prevalence/ epidemiology |
|---|---|
| congenital toxoplasmosis | reported to be anywhere from 1 in 1000 to 1 in 10,000 births.[10]The rate of congenital infection is about 15% in the first trimester, 25% in the second trimester, and 60% in the third trimester.[11] |
| congenital rubella syndrome | The prototype of the perinatal infections was first recognized by the Australian ophthalmologist Gregg in 1941 during a rubella epidemic.[12] Eighty to 90% of the adult population is immune, and with the use of rubella vaccine. Although the incidence of rubella reached an all-time reported low in 1988, there has been a distinct increase in the incidence since then, reaching the highest level since 1982 during 1990. Distinct outbreaks seemed to occur in two settings: (1) in locations in which unvaccinated adults congregate, such as workplaces, colleges, and prisons, and (2) among children in religious communities with low levels of vaccination.[13] |
| cytomegalovirus | CMV is acquired by 1–2% of all newborns, and approximately 10% of these newborns show some evidence of damage if they are carefully followed. This makes the incidence of significant neonatal infection 1 in 500 to 1000 births. [14] |
| Herpesvirus | Most (85%) genital infections are caused by Herpesvirus hominis (HSV) type II, with the major perinatal concern being infection acquired by the infant during the birth process. Such infections are infrequent (1 in 5000–20,000), but the morbidity and mortality are high.[15] |
| Parvovirus | The annual incidence of acute parvovirus infection during pregnancy is 1 in 400 pregnancies. The seroconversion rate is approximately 16%.The risk of vertical transmission to the fetus is 33%. [16] |
| human immunodeficiency virus | The human immunodeficiency virus (HIV) epidemic is now over 30 years old. The number of cases of people living with HIV/AIDS globally rose from 29 million in 2001 to 33.2 million in 2007.[17] |
| Varicella zoster virus | The varicella zoster virus (VZV) is a member of the herpesvirus group. This is in part due to the fact that infection is rare in pregnancy (90% of adults are immune). [18] |
| Hepatitis | It is not known to cause any fetal or neonatal disease but is, nonetheless, a serious illness. Anyone, pregnant or not, who is exposed by contact or travel in endemic areas should receive immune serum globulin (0.02–0.05 mL/kg). If exposure is prolonged and close, the higher dose should be used and repeated every 4–6 months.[19] |
| Influenza | Influenza is one of the more common viral infections to which pregnant women are exposed. When epidemics occur, the problem is magnified because of the patient's susceptibility to a new strain. In addition to the risks of seasonal influenza, pregnant women have experienced excess mortality during the influenza pandemics of 1918–19, 1957–58, and, most recently, the 2009 H1N1 pandemic.[20] |
| Mumps | Mumps is a rare complication of pregnancy, with estimates of incidence varying from 0.8 to 10 cases per 10,000.[21] |
| Genital condylomata | Although the exact prevalence is not known, new techniques, especially DNA sequencing, provide evidence of the ubiquity of this infection. Although the major concern about HPV in women is its role in genital dysplasia and neoplasia, the other concern in pregnancy is fetal/neonatal infection. [22] |
| group B streptococcal infection | Despite the high colonization rate of GBS, the attack rate is quite low. Early-onset infection occurs at a rate of 3–4 per 1000 live births and is manifest within the first 5–7 days of life, usually within 48 hours.[23] |
| Listeriosis | here is very little known about its ecology, colonization rates, or attack rates. There are several reasons for this lack of information, including culture difficulties.[24] |
| Tuberculosis | During the 19th and early 20th centuries, it was the subject of many novels and dramatic operas. The advent of chemotherapeutic agents radically changed the attitudes toward and management of this dreaded disease.[25] The development of effective treatment has essentially reduced the possibility of this disease having any substantial effects on pregnancy. There may be an increase in disease activity in the postpartum period, but since the advent of effective therapy this has little clinical significance.[26] |
| Syphilis | The incidence of syphilis especially congenital syphilis in adults has risen dramatically in the past few years, particularly in endemic urban areas.[27] |
| Gonorrhea | Maternal infection most often is asymptomatic, and in some populations the rate of endocervical colonization exceeds 5%. Salpingitis rarely occurs in the first trimester, and with PROM, cervical colonization can lead to chorioamnionitis in late pregnancy.[28] |
| Mycoplasmas | These associations have not been conclusively established, and, treatment should be used only if there is reasonable evidence for causality in a given situation. [29] |
| Chlamydia | The rate of asymptomatic cervical infection in obstetric populations is high (5–10%), as is urethral infection in the male (sexual transmission occurs). Newborns acquire the organism at birth in significant numbers, and conjunctivitis is common.[30] |
| Salmonella | Typhoid fever is currently a rare disease in the United States. When the disease occur in pregnancy, it likes any serious febrile illness, result in spontaneous abortion or premature labor. In those cases in which the exposure of the fetus to maternal disease has been less than 2–3 weeks, the organism has not been recovered from aborted fetuses.[31] |
| Trichomonas vaginalis | Trichomonas vaginalis is likely the most common parasite to infect women. Newborns can be infected at birth; however, the manifestations are benign.[11] |
| Malaria | Malaria is not a common problem for obstetric practice in the United States, but in endemic areas it is a serious concern and a leading cause of anemia in pregnancy.[32] |
| Candidiasis | Vaginitis caused by Candida albicans is very common during pregnancy.[33] |
| Coccidioidomycosis | Coccidioides immitis most often produces a rather benign and self-limited respiratory infection. It is endemic in the Southwestern United States and in 10% of cases progresses to disseminated infection. If the latter occurs in pregnancy, the placenta may be involved; however, there are no documented cases of congenital infection.[34] |
Age
- Patients of all age groups may develop perinatal infection.
Gender
- perinatal infection affects boy and girls children equally.
Race
- There is no racial predilection for perinatal infection.
Risk Factors
- Common risk factors in the development of Perinatal infection.[35] are
| Fetal causes | maternal causes |
|---|---|
| Birth weight | chorioamnionitis |
| Ceseran delivary | Hypertension (pregestational and gestational including preeclampsia) |
| Multiple delivary | Diabetes (pregestational and gestational) |
| Fetal distress | |
| Meconium aspiration | |
| Patent ductus arteriosus |
Infant outcomes
| Infant outcome |
|---|
| Mechanical ventilation |
| Pneumothorax |
| Respiratory distress syndrome |
| Chronic lung disease |
| Necrotizing enterocolitis |
| Interventricular hemorrhage |
| Hypoxic - ischemic encephalopathy |
| Retinopathy of prematurity |
| Extracorporeal life support |
| In hospital death |
In addition, we evaluated combined grade 3 and grade 4 intraventricular hemorrhage and combined stages 3 through 5 ROP to align with common categorization of these more clinically important outcomes.
Natural History, Complications and Prognosis
Early clinical features include
| Feature | CMV | LCM VIRUS | Rubella virus | Toxoplasma gondii | Treponema pallidum | Zika virus |
|---|---|---|---|---|---|---|
| Systemic | ||||||
| Jaundice | +++ | - | ++ | +++ | +++ | - |
| Hepatosplenomegaly | +++ | - | ++ | +++ | ++ | - |
| Rash | Petechial | Bullous (rare) | Petechial “blue-berry” | Petechial | Petechial | - |
| Anemia | ++ | - | + | - | + | - |
| Neurologic/eye | ||||||
| Microcephaly | ++ | + | ++ | +/- | - | +++ |
| Macrocephaly | +/- | ++ | - | +++ | - | + |
| Chorioretinitis | + | +++ | + | +++ | + | ++ |
| Cataract | - | - | ++ | - | - | + |
-: not seen; +/: rare; +: occasional; ++: common; +++: very common. [1]
Diagnosis
Diagnostic Criteria
- The diagnosis of [perinatal diagnosis][36] is made when
Chlamydia can be diagnosed by taking a cotton swab sample of the cervix and vagina during the third trimester of the pregnancy. Chlamydial cell cultures take three to seven days to grow. DNA probes are available for more rapid diagnosis.
- Past or recent infection with cytomegalovirus (CMV) can be identified by documentation of seroconversion of a previously seronegative patient (the development of IgG antibodies to CMV in a patient who was previously negative for these antibodies) and CMV can be grown from body fluids. [37]
- Genital herpes is suspected with the outbreak of a particular kind of genital sore. The sore can be cultured and tested to confirm that HSV-2 is present.
- Hepatitis B can be identified through a blood test for the hepatitis B surface antigen (HBsAg) in pregnant women. The test is part of prenatal health programs.
- Human immunodeficiency virus (HIV) can be detected using a blood test and is part of most prenatal screening programs.
- Human papillomavirus (HPV) causes the growth of warts in the genital area. The wart tissue can be removed with a scalpel and tested to determine what type of HPV virus caused the infection.
- Pregnant women are usually tested for antibodies to rubella, which would indicate that they have been previously exposed to the virus and, therefore, would not develop infection during pregnancy if exposed.
- Group beta streptococcus (GBS) can be detected by a vaginal or rectal swab culture and sometimes from a urine culture. Blood tests can be used to confirm GBS infection in infants who exhibit symptoms.
- Pregnant women are usually tested for syphilis as part of the prenatal screening, generally with a blood test.
- ZIKA virus Methods for testing include both serologic and molecular tests. Laboratory tests in include ZIKV IgM, ZIKV NAT, and plaque reduction neutralization testing.[38]
History and Symptoms
If a developing fetus is infected by a TORCH agent, the outcome of the pregnancy may be miscarriage, stillbirth, delayed fetal growth and maturation (intrauterine growth retardation), or early delivery. In addition, newborns infected by any one of the TORCH agents may develop a spectrum of similar symptoms and findings. These may include
- listlessness (lethargy),
- fever,
- difficulties feeding,
- hepatosplenomegaly,
- anemia.
In addition, affected infants may develop
- petechia or purpura;
- jaundice;
- chorioretinitis; and/or other symptoms and findings.
Each infectious agent may also cause additional abnormalities that may vary in degree and severity, depending upon the stage of fetal development at time of infection and/or other factors. Following is a more specific description of the TORCH agents. ❑ Classic triad of toxoplasmosis Chorioretinitis (a form of posterior uveitis), Diffuse intracranial calcifications, Hydrocephalus ❑ Rubella is a viral infection characterized by fever, upper respiratory infection, swelling of the lymph nodes, skin rash, and joint pain. Severely affected newborns and infants may have visual and/or hearing impairment, heart defects, calcium deposits in the brain, and/or other abnormalities. ❑ Cytomegalovirus (CMV) Infection is a viral infection associated finding: growth retardation, microcephaly, hepatosplenomegaly, hepatitis, hemolytic anemia, calcium deposits in the brain. ❑ Herpes simplex virus may lead to neonatal herpes. the disorder is transmitted to an infant from an infected mother with active genital lesions at the time of delivery. In the event that a mother has a severe primary genital outbreak, it is possible that a mother may transmit the infection to the fetus. Severely affected newborns may develop cutaneous vesicles in the mouth area, conjunctivitis, abnormally diminished muscle tone, hepatitis, difficulties breathing. ❑ Parvovirus B19 Infection can cause miscarriage, fetal anemia, hydrops fetalis, myocarditis, and/or intrauterine fetal death. ❑ Syphilis Early congenital syphilis: Hepatomegaly and jaundice, Rhinorrhea with white or bloody nasal discharge , Maculopapular rash on palms and soles , Skeletal abnormalities (e.g., metaphyseal dystrophy, periostitis) Generalized lymphadenopathy (nontender). ❑ listeriosis Increased risk of premature birth and spontaneous abortion ,Early-onset syndrome: granulomatosis infantiseptica ,Severe systemic infection ,Most common findings: respiratory distress and skin lesions, Signs of meningitis may already develop. ❑ enterovirus Wide spectrum of clinical presentations, from non-specific febrile illness to fatal multisystem disease, Fever, irritability, poor feeding, lethargy, Maculopapular rash in 50% ,Respiratory symptoms in 50% ,Gastrointestinal symptoms in 20% , Hepatitis in 50% ,may have myocarditis, meningoencephalitis
Physical Examination
| Finding(s) | Possible congenital infections |
|---|---|
| Intrauterine growth retardation | Rubella, cytomegalovirus (CMV), toxoplasmosis |
| Anemia with hydrops | Parvovirus B19, syphilis, CMV, toxoplasmosis |
| Bone lesions | Syphilis, rubella |
| Cerebral calcification |
|
| Congenital heart disease | Rubella |
| Hearing loss (commonly progressive) | Rubella, CMV, toxoplasmosis, syphilis |
| Hepatosplenomegaly | CMV, rubella, toxoplasmosis, HSV, syphilis, enterovirus, parvovirus B19 |
| Hydrocephalus | Toxoplasmosis, CMV, syphilis, possibly enterovirus |
| Hydrops, ascites, pleural effusions | Parvovirus B19, CMV, toxoplasmosis, syphilis |
| Jaundice with or without thrombocytopenia | CMV, toxoplasmosis, rubella, HSV, syphilis, enterovirus |
| Limb paralysis with atrophy and cicatrices | Varicella |
| Maculopapular exanthem | Syphilis, measles, rubella, enterovirus |
| Microcephaly | CMV, toxoplasmosis, rubella, varicella, HSV |
| Myocarditis/encephalomyocarditis | Echovirus, coxsackie B, other enterovirus |
| Ocular findings | CMV, toxoplasmosis, rubella, HSV, syphilis, enterovirus, parvovirus B19 |
| Progressive hepatic failure and clotting abnormalities | Echovirus, coxsackie B, other enterovirus, HSV, toxoplasmosis |
| Pseudoparalysis, pain | Syphilis |
| Vesicles | HSV, syphilis, varicella, enterovirus |
Laboratory Findings
- rubella may be diagnosed by detection of specific IgM, but virus detection is the technique of choice.
- VZV may be diagnosed by serological techniques in up to 71% of cases. Detection of virus in vesicle scrapings or swabs from the oropharynx is the technique of choice for neonatal HSV.
- enterovirus infections are best diagnosed by detection of viral RNA.
- HIV-1 may be diagnosed within 3 months of birth by testing serial blood samples with a combination of techniques. Maternal infection with HBV, HCV, HIV and HTLV1/11 may be diagnosed by serological techniques and genital PVs by detection of viral DNA. Chorionic villus samples, amniotic fluid and fetal blood may be obtained for prenatal diagnosis of infection.
- detection of virus in amniotic fluid is the technique of choice for prenatal diagnosis of CMV, insufficient data is currently available to determine whether it may be used for intrauterine rubella.
- The most reliable technique for diagnosis of fetal B19 infection is detection of viral DNA .
the use of TORCH screening should be discouraged.[39]
Electrocardiogram
There are no ECG findings associated with [perinatal inectioon].
X-ray
There are no x-ray findings associated with perinatal infection.
Echocardiography or Ultrasound
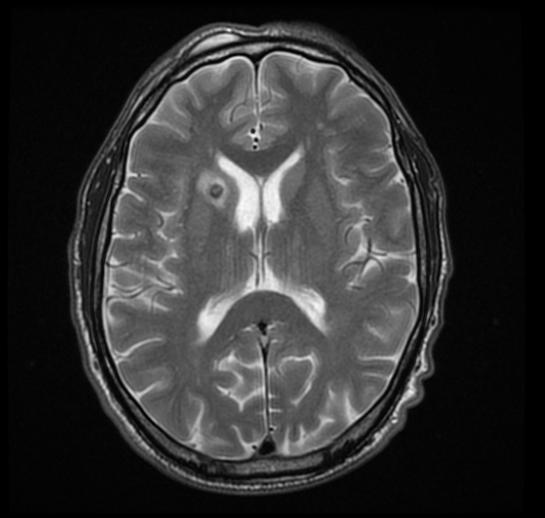
There are no echocardiography/ultrasound findings associated with perinatal infection. CT scan may be helpful in the diagnosis of Toxoplasmosis include dilated ventricles with multiple subependymal and parenchymal calcifications .
MRI may be helpful in the diagnosis of [[[Toxoplasmosis]]]. Findings on MRI suggestive of/diagnosis include ring enhanced lesion
-
Caption1
Imaging features of selected congenital infections [40]
| Imaging feature | CMV | LCM virus | Rubella virus | Toxoplasma gondii | Zika Virus |
|---|---|---|---|---|---|
| Calcifications | +++ | +++ | +++ | +++ | +++ |
| Polymicrogyria | +++ | ++ | - | - | +++ |
| Hydrocephalus | + (passive) | ++ (obstructive and passive) | - | ++ (obstructive) | ++ (obstructive and passive) |
| Lissencephaly | ++ | + | - | - | ++ |
| Cerebellar hypoplasia | ++ | + | - | - | ++ |
| White matter lesions | +++ | +/- | ++ | + | ++ |
| Fetal brain disruption | - | - | - | - | +++ |
CMV=cytomegalovirus; LCM virus =lymphocytic choriomeningitis virus. (-) absent; (+) uncommon or rare; (++) common; (+++) very common.
There are no other diagnostic studies associated with perinatal infection
Treatment
| Disease | Medical Therapy | Surgery | prevention |
|---|---|---|---|
| Toxoplasmosis | Mother: immediate administration of spiramycin
Fetus and Newborn: pyrimethamine, sulfadiazine, and folinic acid.[41] |
Avoid raw, undercooked, and cured meats avoid contact with cat litter
Wash hands frequently, especially after touching soil (e.g., during gardening). [41] | |
| Rubella | Intrauterine rubella infection > 16 weeks: reassurance
Congenital rubella syndrome: supportive care and surveillance |
Immunization of seronegative women before pregnancy
Nationally notifiable condition: Suspected congenital rubella syndrome must be reported to the local or state health department.[42] | |
| Cytomegalovirus | Fetus: Severe anemia: intrauterine blood transfusions and Thrombocytopenia: platelet transfusions
Newborn: Supportive therapy of symptoms,Ganciclovir, valganciclovir, or foscarnet Mother: valacyclovir. |
Frequent hand washing, Pregnant women with risk factors for TORCH infection should avoid potentially contaminated workplaces (e.g., schools, pediatric clinics) [43] | |
| Herpesvirus | Acyclovir & Supportive care | only for herpes simplex [44] | Antiviral therapy (acyclovir) beginning at 36 weeks of gestation for individuals with a known history of HSV lesions
Cesarean section in women with active genital lesions or prodromal symptoms (e.g., burning pain).[45] |
| Parvovirus | Intrauterine fetal blood transfusion in cases of severe fetal anemia | Hand hygiene (frequent hand washing),Pregnant women with risk factors for TORCH infection should avoid potentially contaminated workplaces (e.g., schools, pediatric clinics).[46] | |
| Acquired immunodeficiency syndrome (AIDS) | mother: As a result of the AIDS Clinical Trials Group (ACTG) , the US Public Health Service[47] published recommendations for the use of ZDV or AZT to reduce the risk of HIV transmission from infected women to their infants. These recommendations are as follows:
*Antepartum: ZDV, 100 mg orally five times per day, starting at 14–34 weeks. *Intrapartum: ZDV, 2 mg/kg intravenously (IV), loading dose, given over 1 hour, followed by 1 mg/kg/hr IV until delivery. Newborn: ZDV syrup 2 mg/kg orally every 6 hours, beginning 8–12 hours after birth for the first 6 weeks of life |
Vertical transmission reduced to 2% if a scheduled cesarean section is performed. It is not yet known if there is a significant benefit from cesarean delivery in patients who have viral loads of less than 1000 copies/ml who are on HAART. Maternal morbidity is greater with cesarean delivery, particularly in those women with low CD4 cell counts. Therefore, women who are HIV positive must be counseled about the maternal risks and potential benefits of both ZDV prophylaxis and cesarean delivery so that they can make informed choices. If cesarean delivery is chosen, it should be performed electively at 38 weeks of gestation. ZDV should begin 3 hours prior to delivery. It is important to use perioperative prophylactic antibiotics to reduce maternal infectious morbidity. The management of labor (if the patient chooses this option) should include avoidance of scalp electrodes and scalp sampling.[48] ,[49] | The newborn should be carefully cleaned of maternal blood and secretions. There is no evidence that the postpartum course is altered. The virus has been isolated from breast milk, and although the risk of transfer is not known, breastfeeding is not recommended when there is a suitable alternative, as exists in the developed world. A final step in the case of the HIV-infected patient is to see that the patient receives ongoing care. Even if she is asymptomatic after delivery, she will require support and surveillance for disease progression.[50] ,[51] |
| Varicella zoster virus | pregnant women or newborns with (severe) infection: acyclovir
Administer postexposure prophylaxis in newborns if mother displays symptoms of varicella < 5 days before delivery: IgG antibodies (varicella-zoster immune globulin, VZIG) |
Immunization of seronegative women before pregnancy
VZIG in pregnant women without immunity within 10 days of exposure.[52] | |
| Hepatitis | Hepatitis A:pregnant or not, who is exposed by contact or travel in endemic areas should receive immune serum globulin (0.02–0.05 mL/kg). If exposure is prolonged and close, the higher dose should be used and repeated every 4–6 months. [53] Hepatitis B: infants born to mothers with circulating HBV. These infants, if chronically infected, are at high long-term risk for hepatic cancer. Because of this and because it is possible to prevent perinatal transmission, particularly if infection occurs in late pregnancy, testing for HBV surface antigen is recommended as a part of routine prenatal testing. [54]. Infants born to HBV-positive mothers should receive 0.5 mL of hepatitis B immune globulin within 12 hours of birth and simultaneously receive the first dose of HBV vaccine (half the adult dose). The remaining doses should follow the adult schedule. There is no reason to modify the obstetric management because cesarean delivery will not modify the risk. The HBV vaccine now in use is a recombinant product, poses no infectious risk, and can be used in pregnancy for women at risk. Complete immunization requires the initial dose with repeated doses at 1 and 6 months. Healthcare workers should know their HBV immune status and, if susceptible, should be vaccinated. Hepatitis C: At present, no vaccine is available for HCV, and there are insufficient data to recommend pregnancy termination. The management of the pregnant woman infected with HCV must be individualized until further evidence is available to make reasonable recommendations.[55] | ||
| Influenza | Prompt empiric treatment with appropriate neuraminidase inhibitors (oseltamivir and zanamivir) appeared to decrease the risk of severe disease. [56] | Preterm delivery and cesarean delivery were commonly associated with maternal illness with preterm birth rates as high as 30% and cesarean section rates of nearly double the current national baseline.[57] | Influenza vaccination is now an important component of antenatal care. The CDC recommends that women who will be pregnant during the flu season (October through mid May) be vaccinated. [58] Vaccination may be performed in all three trimesters. Specific vaccines prepared for epidemic strains are more effective than the poly antigenic preparations. Complications of vaccination are generally mild, except for Guillain-Barré syndrome. This is characterized by progressive ascending paralysis but fortunately is usually self-limited and reversible. Evidence from the swine flu epidemic of 1976 suggests that the incidence is approximately 1 in 100,000 vaccinations. The frequency of complications does not appear to be altered by pregnancy. The theoretical risks of vaccination are outweighed by its benefits. |
| Genital condylomata | The treatment of choice for large-volume and symptomatic disease is the carbon dioxide (CO2) laser, [59] and it is suggested that treatment with it be carried out in the third trimester to reduce the chances of recurrence from latent HPV infection at the time of delivery. Interferons have been used successfully [60] but are not yet approved for clinical use.Trichloroacetic acid is the best choice for isolated or small-volume genital disease.[61] | There is almost never a reason to perform a cesarean section for condylomata if the patient is seen sufficiently early in pregnancy to accomplish treatment. | |
| Group B streptococci | As stated earlier, penicillin is the drug of choice for GBS treatment and prophylaxis. Ampicillin is an acceptable alternative. Penicillin is preferred due to its narrow spectrum of activity. Five million units of penicillin G is given as the loading dose. This is followed with 2.5–3.0 million units every 4 hours until delivery. The dose of ampicillin is 2 g loading followed by 1 g every 4 hours. Increased resistance of GBS isolates to second-line therapies has been noted. Susceptibility testing should be ordered in patients who are allergic to penicillin. Cefazolin is recommended for patients that are not at high risk for anaphylaxis. Two grams are given intravenously followed by 1 g every 8 hours. If the patient is a high risk of anaphylaxis, treatment would depend on the susceptibility of the isolate. Clindamycin (900 mg IV every 8 hours). Erythromycin is no longer an acceptable alternative for penicillin allergic women at high risk for anaphylaxis. Patients at high risk of anaphylaxis with unknown susceptibility or resistance to clindamycin, should be treated with vancomycin. The dose of vancomycin is 1 g every 12 hours until delivery. It must be emphasized that vancomycin is reserved for patients at high risk for anaphylaxis.[62] | Women with intact membranes who present with threatened preterm delivery and unknown GBS status should receive IAP until GBS culture results are available. If GBS is negative, or the patient is not in true labor, IAP may be discontinued and re-screening should be done at 35–37 weeks.[63] | |
| Listeriosis | IV ampicillin and gentamicin (for both mother and newborn) | *Avoidance of soft cheeses
| |
| Tuberculosis | * recent converters should be treated with isoniazid, 300 mg/day, starting after the first trimester and continuing for 6–9 months. Women younger than 35 years of age with a positive PPD of unknown duration should receive isoniazid, 300 mg/day, for 6 months after delivery.
|
Prophylaxis is not recommended for women older than 35 years of age in the absence of active disease because of concern about hepatotoxicity. | |
| Syphilis | Therapy is indicated in the gravida with a positive FTA-ABS of recent onset, and the drug of choice is penicillin. [66] The regimen recommended is the same as in the nonpregnant woman. For early syphilis, a single dose of 2.4 million units of benzathine penicillin G is recommended. Some recommend a follow-up dose 1 week later, particularly in the third trimester. For late-stage syphilis (more than 1 year of duration), three doses are recommended. For the patient allergic to penicillin, treatment with penicillin after oral desensitization is recommended. This should be done in a facility that has appropriate provisions for resuscitation, if needed. [67] | *Maternal screening in early pregnancy
| |
| Gonorrhea | current recommendations include one of the following regimens:
In addition, treatment for Chlamydia should be administered because of the likelihood of coinfection. [69] Disseminated infection in the newborn requires high-dose treatment, and ophthalmic infection should be treated both locally and systemically. |
Prevention of perinatal infection is best accomplished by careful maternal screening and treatment.[70] | |
| Mycoplasmas | The treatment for the pregnant woman and the neonate is clindamycin for Mycoplasma hominis and erythromycin for M. pneumoniae and Ureaplasma urealyticum.[71] | These associations have not been conclusively established, and, consequently, treatment should be used only if there is reasonable evidence for causality in a given situation.[72] | |
| Chlamydia | Recommended treatment for pregnancy includes the following:[73]
Erythromycin base, 500 mg, or erythromycin ethylsuccinate, 800 mg orally four times daily for 7 days Amoxicillin, 500 mg orally three times daily for 7 days Azithromycin, 1 g orally as a single dose |
The question of maternal screening and prophylactic treatment to prevent neonatal infection is unsettled. As diagnostic studies have become more readily available, screening has become more practical. The decision to routinely screen a prenatal population should probably be based on a determination of the specific population prevalence.[74] | |
| Salmonella | Treatment is chloramphenicol, despite the existence of some resistant strains. Alternate antibiotics are ampicillin or amoxicillin (combination of trimethoprim and sulfamethoxazole is useful for resistant strains but avoided in pregnancy if possible). Aspirin should be avoided because patients with typhoid are extremely sensitive and severe hypothermia may result. [75] | Prevention is best accomplished by sanitation and hygienic processes and the control of faulty food processing. [76] | |
| Trichomonas vaginalis | Because of evidence of a possible relationship between vaginal trichomoniasis and adverse pregnancy outcomes, metronidazole, 2 g orally as a single dose, can be given after the first trimester.[77] | ||
| Malaria | Perhaps the most likely consideration is a pregnant woman who must travel to an endemic area. Chloroquine phosphate, 500 mg once a week starting 1 week before the trip and continuing for 6 weeks after, is the recommendation. This can be safely given to pregnant women. [78] | ||
| Zika virus | *Avoidance of travel to ZIKv endemic areas during pregnancy.
|
Prevention
Minimizing the risk of transmitting a maternal infection to a fetus is often a major concern for parents. The first step is identifying possible maternal infections. Proper prenatal care in many cases allows for early diagnosis and thus early treatment of certain infections, thus improving the newborn's prognosis [80]
A woman's nutritional status may contribute to her ability to fight off infections, particularly in cases of malnutrition . A well-balanced diet rich in nutrients such as folic acid , calcium, iron, zinc, vitamin D, and the B vitamins is recommended for pregnant women. Mothers are recommended to eat approximately 300 additional calories day (above and beyond a normal non pregnancy diet) to support the fetus's growth and development [81]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Ostrander B, Bale JF (2019). "Congenital and perinatal infections". Handb Clin Neurol. 162: 133–153. doi:10.1016/B978-0-444-64029-1.00006-0. PMID 31324308.
- ↑ 2.0 2.1 "Definitions and Indicators in Family Planning. Maternal & Child Health and Reproductive Health" (PDF). Archived from the original (PDF) on 25 January 2012. Unknown parameter
|url-status=ignored (help) By European Regional Office, World Health Organization. Revised March 1999 & January 2001. In turn citing: WHO Geneva, WHA20.19, WHA43.27, Article 23 - ↑ Singh, Meharban (2010). Care of the Newborn. p. 7. Edition 7. ISBN 9788170820536
- ↑ Stewart AD, Logsdon JM, Kelley SE (2005). "An empirical study of the evolution of virulence under both horizontal and vertical transmission". Evolution. 59 (4): 730–9. PMID 15926685.
- ↑ Joo E, Carmack A, Garcia-Buñuel E, Kelly CJ (2000). "Implementation of guidelines for HIV counseling and voluntary HIV testing of pregnant women". Am J Public Health. 90 (2): 273–6. doi:10.2105/ajph.90.2.273. PMC 1446152. PMID 10667191.
- ↑ Cavalli-Sforza LL, Feldman MW (1981). "Cultural transmission and evolution: a quantitative approach". Monogr Popul Biol. 16: 1–388. PMID 7300842.
- ↑ Neu N, Duchon J, Zachariah P (2015). "TORCH infections". Clin Perinatol. 42 (1): 77–103, viii. doi:10.1016/j.clp.2014.11.001. PMID 25677998.
- ↑ Li, Ding; Yang, Hao; Zhang, Wen-Hong; et al. (2006). "A Simple Parallel Analytical Method of Prenatal Screening". Gynecologic and Obstetric Investigation. 62 (4): 220–225. doi:10.1159/000094092. ISSN 1423-002X. PMID 16791006. Unknown parameter
|s2cid=ignored (help) - ↑ Ford-Jones EL, Kellner JD (1995). ""Cheap torches": an acronym for congenital and perinatal infections". Pediatr Infect Dis J. 14 (7): 638–40. PMID 7567307.
- ↑ Barbero S, Ponte PL (1977). "[Infectious diseases in the fetus and newborn infant]". Arch Sci Med (Torino). 134 (4): 413–35. PMID 610692.
- ↑ 11.0 11.1 Hampton MM (2015). "Congenital Toxoplasmosis: A Review". Neonatal Netw. 34 (5): 274–8. doi:10.1891/0730-0832.34.5.274. PMID 26802827.
- ↑ Kaushik A, Verma S, Kumar P (2018). "Congenital rubella syndrome: A brief review of public health perspectives". Indian J Public Health. 62 (1): 52–54. doi:10.4103/ijph.IJPH_275_16. PMID 29512566.
- ↑ Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group (2007). "Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States". JAMA. 298 (18): 2155–63. doi:10.1001/jama.298.18.2155. PMID 18000199.
- ↑ Bale JF (2014). "Congenital cytomegalovirus infection". Handb Clin Neurol. 123: 319–26. doi:10.1016/B978-0-444-53488-0.00015-8. PMID 25015493.
- ↑ Roome AP, Tinkler AE, Hilton AL, Montefiore DG, Waller D (1975). "Neutral red with photoinactivation in the treatment of herpes genitalis". Br J Vener Dis. 51 (2): 130–3. doi:10.1136/sti.51.2.130. PMC 1045129. PMID 165862.
- ↑ Cosmi E, Mari G, Delle Chiaie L, Detti L, Akiyama M, Murphy J; et al. (2002). "Noninvasive diagnosis by Doppler ultrasonography of fetal anemia resulting from parvovirus infection". Am J Obstet Gynecol. 187 (5): 1290–3. doi:10.1067/mob.2002.128024. PMID 12439522.
- ↑ Little J, Rhodus NL (2007). "HIV and AIDs: update for dentistry". Gen Dent. 55 (3): 184–96. PMID 17511359.
- ↑ Higa K, Dan K, Manabe H (1987). "Varicella-zoster virus infections during pregnancy: hypothesis concerning the mechanisms of congenital malformations". Obstet Gynecol. 69 (2): 214–22. PMID 3027637.
- ↑ Papadopoulos NG, Megremis S, Kitsioulis NA, Vangelatou O, West P, Xepapadaki P (2017). "Promising approaches for the treatment and prevention of viral respiratory illnesses". J Allergy Clin Immunol. 140 (4): 921–932. doi:10.1016/j.jaci.2017.07.001. PMC 7112313 Check
|pmc=value (help). PMID 28739285. - ↑ Meijer WJ, van Noortwijk AG, Bruinse HW, Wensing AM (2015). "Influenza virus infection in pregnancy: a review". Acta Obstet Gynecol Scand. 94 (8): 797–819. doi:10.1111/aogs.12680. PMID 26012384.
- ↑ Siegel M, Fuerst HT (1966). "Low birth weight and maternal virus diseases. A prospective study of rubella, measles, mumps, chickenpox, and hepatitis". JAMA. 197 (9): 680–4. PMID 5952908.
- ↑ Bentley PL, Coulter MJ, Nelson BL (2019). "Squamous Cell Papillomatosis in the Setting of Recurrent Respiratory Papillomatosis". Head Neck Pathol. 13 (2): 235–238. doi:10.1007/s12105-018-0912-8. PMC 6513981 Check
|pmc=value (help). PMID 29594918. - ↑ Howard JB, McCracken GH (1974). "The spectrum of group B streptococcal infections in infancy". Am J Dis Child. 128 (6): 815–8. doi:10.1001/archpedi.1974.02110310063011. PMID 4613165.
- ↑ Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B; et al. (2017). "Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study". Lancet Infect Dis. 17 (5): 510–519. doi:10.1016/S1473-3099(16)30521-7. PMID 28139432.
- ↑ PRIDIE RB, STRADLING P (1961). "Management of pulmonary tuberculosis during pregnancy". Br Med J. 2 (5244): 78–9. doi:10.1136/bmj.2.5244.78. PMC 1969052. PMID 13737994.
- ↑ Gould JM, Aronoff SC (2016). "Tuberculosis and Pregnancy-Maternal, Fetal, and Neonatal Considerations". Microbiol Spectr. 4 (6). doi:10.1128/microbiolspec.TNMI7-0016-2016. PMID 28084207.
- ↑ Singh AE, Romanowski B (1999). "Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features". Clin Microbiol Rev. 12 (2): 187–209. PMC 88914. PMID 10194456.
- ↑ Handsfield HH, Hodson WA, Holmes KK (1973). "Neonatal gonococcal infection. I. Orogastric contamination with Neisseria gonorrhoea". JAMA. 225 (7): 697–701. doi:10.1001/jama.225.7.697. PMID 4198114.
- ↑ Klein JO, Buckland D, Finland M (1969). "Colonization of newborn infants by mycoplasmas". N Engl J Med. 280 (19): 1025–30. doi:10.1056/NEJM196905082801901. PMID 5778411.
- ↑ Blanchard TJ, Mabey DC (1994). "Chlamydial infections". Br J Clin Pract. 48 (4): 201–5. PMID 7917800.
- ↑ Hohmann EL (2001). "Nontyphoidal salmonellosis". Clin Infect Dis. 32 (2): 263–9. doi:10.1086/318457. PMID 11170916.
- ↑ Ahmadal-Agroudi M, El-Mawla Megahed LA, Abdallah EM, Morsy TA (2017). "A MINI OVERVIEW OF MALARIA IN PREGNANCY". J Egypt Soc Parasitol. 47 (1): 177–196. PMID 30157347.
- ↑ Jin Y, Endo A, Shimada M, Minato M, Takada M, Takahashi S; et al. (1995). "Congenital systemic candidiasis". Pediatr Infect Dis J. 14 (9): 818–20. PMID 8559641.
- ↑ Hooper JE, Lu Q, Pepkowitz SH (2007). "Disseminated coccidioidomycosis in pregnancy". Arch Pathol Lab Med. 131 (4): 652–5. doi:10.1043/1543-2165(2007)131[652:DCIP]2.0.CO;2. PMID 17425401.
- ↑ Bevilacqua G, Braibanti S, Solari E, Anfuso S, Fragni G, Soncini E (2005). "[Perinatal risk factors for infection in the newborn. Multicenter clinico-epidemiologic investigation]". Pediatr Med Chir. 27 (3–4): 31–8. PMID 16910447.
- ↑ Fortner KB, Nieuwoudt C, Reeder CF, Swamy GK (2018). "Infections in Pregnancy and the Role of Vaccines". Obstet Gynecol Clin North Am. 45 (2): 369–388. doi:10.1016/j.ogc.2018.01.006. PMID 29747736.
- ↑ Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP (2011). "Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy". Clin Microbiol Infect. 17 (9): 1285–93. doi:10.1111/j.1469-0691.2011.03564.x. PMID 21631642.
- ↑ [ Rabe IB, Staples JE, Villanueva J, et al. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 2016;65:543–6. ], additional text.
- ↑ [Best, J. M. (1996). Laboratory diagnosis of intrauterine and perinatal virus infections. Clinical and Diagnostic Virology, 5(2-3), 121–129. doi:10.1016/0928-0197(96)00213-9 ], additional text.
- ↑ [Ostrander, B., & Bale, J. F. (2019). Congenital and perinatal infections. Neonatal Neurology, 133–153. doi:10.1016/b978-0-444-64029-1.00006-0 ], additional text.
- ↑ 41.0 41.1 [ Cline, Matthew K., Chasse Bailey-Dorton, and Maria Cayelli. "Update in Maternity Care: Maternal Infections." Clinics in Office Practice 27, no. 1 (March 2000): 13–33 ], additional text.
- ↑ [Centers for Disease Control and Prevention. Three Cases of Congenital Rubella Syndrome in the Post elimination Era: Maryland, Alabama, and Illinois, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62(12): pp. 226–229. url: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6212a3.htm. ], additional text.
- ↑ [Julie Johnson, MD, Brenna Anderson, MD, MSc, and Robert F. Pass, MD. Prevention of Maternal and Congenital Cytomegalovirus Infection. Clinical Obstetrics and Gynecology. 2013. url:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3347968/], additional text.
- ↑ [ Riley LE, Wald A. Genital herpes simplex virus infection and pregnancy. In: Post TW, ed. UpToDate. Waltham, MA: UpToDate. http://www.uptodate.com/contents/genital-herpes-simplex-virus-infection-and-pregnancy. Last updated June 18, 2016. Accessed March 22, 2017.], additional text.
- ↑ [ Demmler-Harrison GJ. Neonatal herpes simplex virus infection: Management and prevention. In: Post TW, ed. UpToDate. Waltham, MA: UpToDate. http://www.uptodate.com/contents/neonatal-herpes-simplex-virus-infection-management-and-prevention. Last updated February 16, 2016. Accessed March 22, 2017. ], additional text.
- ↑ [Lamont RF, Sobel JD, Vaisbuch E, et al. Parvovirus B19 infection in human pregnancy. BJOG. 2010; 118(2): pp. 175–186. doi: 10.1111/j.1471-0528.2010.02749.x ], additional text.
- ↑ [Centers for Disease Control and Prevention: Recommendations of the US Public Health Service Task Force on use of zidovudine to reduce perinatal transmission of human immunodeficiency virus. MMWR Morb Mortal Wkly Rep 43:1, 1994 ], additional text.
- ↑ [ The International Perinatal HIV Group: The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1: a meta-analysis of 15 prospective cohort studies. N Engl J Med 340: 977, 1999 ], additional text.
- ↑ [ACOG Committee Opinion no. 234, May 2000 ], additional text.
- ↑ [ Ziegler JB, Cooper DA, Johnson RO: Postnatal transmission of AIDS-associated retrovirus from mother to infant. Lancet 1: 896, 1985 ], additional text.
- ↑ [Centers for Disease Control: Recommendations for assisting in the prevention of perinatal transmission of human T lymphocyte virus type lymphadenopathy- associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep 34: 721, 1985 ], additional text.
- ↑ [ Centers for Disease Control and Prevention. 2017 Nationally Notifiable Conditions. https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/. Updated January 1, 2017. Accessed March 22, 2017], additional text.
- ↑ [ Amstey MS: Treatment and prevention of viral infections. Clin Obstet Gynecol 31: 501, 1988 ], additional text.
- ↑ [ American College of Obstetricians and Gynecologists: Guidelines for Hepatitis B Virus Screening and Vaccination During Pregnancy. ACOG Committee Opinion 78. Washington DC, ACOG, 1990 ], additional text.
- ↑ [ Duff P. Hepatitis in pregnancy. Semin Perinatol 1998; 22: 277-83 ], additional text.
- ↑ [Mosby LG, Ellington SR, Forhan SE, Yeung LF, Perez M, Shah MM, MacFarlane K, Laird SK, House LD, Jamieson DJ. The Centers for Disease Control and Prevention's maternal health response to 2009 H1N1 influenza. Am J Obstet Gynecol. 2011 Jun;204(6 Suppl 1):S7-12. Epub 2011 Mar 31], additional text.
- ↑ [ Mosby LG, Ellington SR, Forhan SE, Yeung LF, Perez M, Shah MM, MacFarlane K, Laird SK, House LD, Jamieson DJ. The Centers for Disease Control and Prevention's maternal health response to 2009 H1N1 influenza. Am J Obstet Gynecol. 2011 Jun;204(6 Suppl 1):S7-12. Epub 2011 Mar 31. ], additional text.
- ↑ [ Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization practices (ACIP) MMWR. Recomm Rep 2004; 53(RR-6): 1-40 ], additional text.
- ↑ [Ferenczy A: Treating genital condyloma during pregnancy with the carbon dioxide laser. Am J Obstet Gynecol 148: 9, 1989], additional text.
- ↑ [ Friedman-Kien AE, Eron LJ, Conant M: Natural interferon alfa for treatment of condylomata acuminata. JAMA 259: 533, 1988], additional text.
- ↑ [Choo QL, Kuo G, Weiner, AJ et al: Isolation of a DNA clone derived from blood-borne non-A, non-B viral hepatitis genome. Science 244: 359, 1989 ], additional text.
- ↑ [Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010 Nov 19;59(RR-10):1-36], additional text.
- ↑ [ Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010 Nov 19;59(RR-10):1-36. ], additional text.
- ↑ [ Janakiraman V. Listeriosis in pregnancy: diagnosis, treatment, and prevention. Rev Obstet Gynecol. 2008; 1(4): pp. 179–85. pmid: 19173022. ], additional text.
- ↑ [ Ricci JM, Fojaco RM, Fojaco RM, O'sullivan MJ: Congenital syphillis: The University of Miami/Jackson Memorial Medical Center Experience, 1986-1988. Obstet Gynecol 74: 687, 1989 ], additional text.
- ↑ [ Centers for Disease Control: Syphilis: CDC recommended treatment schedules. J Infect Dis 134: 97, 1976], additional text.
- ↑ [ ACOG Educational Bulletin No 245, March 1998, American College of Obstetricians and Gynecologists ], additional text.
- ↑ [Centers for Disease Control and Prevention. 2017 Nationally Notifiable Conditions. https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/. Updated January 1, 2017. Accessed March 22, 2017. ], additional text.
- ↑ [ACOG Educational Bulletin No. 245, March 1998], additional text.
- ↑ [ACOG Educational Bulletin No. 245, March 1998], additional text.
- ↑ [Shurin PA, Alpert S, Rosner B et al: Chorioamnionitis and colonization of the newborn infant with genital mycoplasmas. N Engl J Med 293: 5, 1975 ], additional text.
- ↑ [Shurin PA, Alpert S, Rosner B et al: Chorioamnionitis and colonization of the newborn infant with genital mycoplasmas. N Engl J Med 293: 5, 1975 ], additional text.
- ↑ [ACOG Educational Bulletin No. 245, March 1998], additional text.
- ↑ [ACOG Educational Bulletin No. 245, March 1998], additional text.
- ↑ [ In Hoeprich PD (ed): Infectious Diseases, pp 555–561. Philadelphia, Harper & Row, 1977 ], additional text
- ↑ [ Hornick RB: Nontyphoidal salmonellosis. In Hoeprich PD (ed): Infectious Diseases, pp 555–561. Philadelphia, Harper & Row, 1977 ], additional text.
- ↑ [ Sever JL, Larsen JN, Grossman JH: Toxoplasmosis. In: Handbook of Perinatal Infections, pp 157–163. Boston, Little, Brown, 1979 ], additional text.
- ↑ [ Sever JL, Larsen JN, Grossman JH: Toxoplasmosis. In: Handbook of Perinatal Infections, pp 157–163. Boston, Little, Brown, 1979 ], additional text.
- ↑ [ Centers for Disease Control and Prevention (CDC). Zika virus prevention. Available at: https://www.cdc.gov/zika/prevention/index.html. Accessed April 24,2017], additional text.
- ↑ [Ford-Jones, E. Lee, and Greg Ryan. "Implications for the Fetus of Maternal Infections in Pregnancy." In Infectious Diseases , 2nd ed. Edited by Jonathan Cohen et all. New York: Mosby, 2004. ], additional text.
- ↑ [Ford-Jones, E. Lee, and Greg Ryan. "Implications for the Fetus of Maternal Infections in Pregnancy." In Infectious Diseases , 2nd ed. Edited by Jonathan Cohen et all. New York: Mosby, 2004 ], additional text.