Monoamine oxidase: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
imported>Vycl1994 |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{protein | {{redirect|MAO|other uses|Mao (disambiguation)}} | ||
| Name = monoamine oxidase A | {{enzyme | ||
| caption = Ribbon diagram of a [[monomer]] of human MAO-A, with [[FAD]] and [[clorgiline]] bound, oriented as if attached to the [[outer membrane]] of a [[mitochondrion]]. From {{PDB|2BXS}}. | | Name = Monoamine oxidase | ||
| EC_number = 1.4.3.4 | |||
| CAS_number = 9001-66-5 | |||
| IUBMB_EC_number = 1/4/3/4 | |||
| GO_code = 0008131 | |||
| image = | |||
| width = | |||
| caption = | |||
}} | |||
{{Pfam_box | |||
| Symbol = MAO | |||
| Name = Monoamine oxidase | |||
| image = | |||
| width = | |||
| caption = | |||
| Pfam= PF01593 | |||
| Pfam_clan = | |||
| InterPro= IPR001613 | |||
| SMART= | |||
| PROSITE = | |||
| SCOP = | |||
| TCDB = | |||
| OPM family= 119 | |||
| OPM protein= 2z5x | |||
| PDB= | |||
| Membranome family = 418 | |||
}} | |||
{{infobox protein | |||
| Name = [[MAOA|monoamine oxidase A]] | |||
| caption = Ribbon diagram of a [[monomer]] of human MAO-A, with [[Flavin adenine dinucleotide|FAD]] and [[clorgiline]] bound, oriented as if attached to the [[Outer mitochondrial membrane|outer membrane]] of a [[mitochondrion]]. From {{PDB|2BXS}}. | |||
| image = Monoamine oxidase A 2BXS.png | | image = Monoamine oxidase A 2BXS.png | ||
| width = | | width = | ||
| HGNCid = 6833 | | HGNCid = 6833 | ||
| Symbol = MAOA | | Symbol = [[MAOA]] | ||
| AltSymbols = | | AltSymbols = | ||
| EntrezGene = 4128 | | EntrezGene = 4128 | ||
| Line 12: | Line 41: | ||
| UniProt = P21397 | | UniProt = P21397 | ||
| PDB = | | PDB = | ||
| ECnumber = | | ECnumber = | ||
| Chromosome = X | | Chromosome = X | ||
| Arm = p | | Arm = p | ||
| Line 18: | Line 47: | ||
| LocusSupplementaryData = .4-p11.3 | | LocusSupplementaryData = .4-p11.3 | ||
}} | }} | ||
{{protein | {{infobox protein | ||
| Name = monoamine oxidase B | | Name = [[monoamine oxidase B]] | ||
| caption = | | caption = Ribbon diagram of human MAO-B. From {{PDB|1GOS}}. | ||
| image = MonoamineOxidase-1GOS.png | | image = MonoamineOxidase-1GOS.png | ||
| width = | | width = | ||
| HGNCid = 6834 | | HGNCid = 6834 | ||
| Symbol = MAOB | | Symbol = [[Monoamine oxidase B|MAOB]] | ||
| AltSymbols = | | AltSymbols = | ||
| EntrezGene = 4129 | | EntrezGene = 4129 | ||
| Line 31: | Line 60: | ||
| UniProt = P27338 | | UniProt = P27338 | ||
| PDB = | | PDB = | ||
| ECnumber = | | ECnumber = | ||
| Chromosome = X | | Chromosome = X | ||
| Arm = p | | Arm = p | ||
| Line 37: | Line 66: | ||
| LocusSupplementaryData = .4-p11.3 | | LocusSupplementaryData = .4-p11.3 | ||
}} | }} | ||
{{ | '''Monoamine oxidases''' ('''MAO''') ({{EC number|1.4.3.4}}) are a family of [[enzyme]]s that [[catalysis|catalyze]] the [[oxidation]] of [[monoamine]]s, employing oxygen to clip off their amine group.<ref name="pmid15279561">{{cite journal | vauthors = Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J | title = Monoamine oxidases: certainties and uncertainties | journal = Current Medicinal Chemistry | volume = 11 | issue = 15 | pages = 1965–82 | date = August 2004 | pmid = 15279561 | doi = 10.2174/0929867043364810 }}</ref><ref name="pmid15279562">{{cite journal | vauthors = Edmondson DE, Mattevi A, Binda C, Li M, Hubálek F | title = Structure and mechanism of monoamine oxidase | journal = Current Medicinal Chemistry | volume = 11 | issue = 15 | pages = 1983–93 | date = August 2004 | pmid = 15279562 | doi = 10.2174/0929867043364784 }}</ref> They are found bound to the outer membrane of [[mitochondria]] in most cell types of the body. The first such enzyme was discovered in 1928 by [[Mary Bernheim]] in the liver and was named tyramine oxidase.<ref name="pmid16744124">{{cite journal | vauthors = Hare ML | title = Tyramine oxidase: A new enzyme system in liver | journal = The Biochemical Journal | volume = 22 | issue = 4 | pages = 968–79 | year = 1928 | pmid = 16744124 | pmc = 1252213 | doi = 10.1042/bj0220968 }}</ref><ref name="pmid10643441">{{cite journal | vauthors = Slotkin TA | title = Mary Bernheim and the discovery of monoamine oxidase | journal = Brain Research Bulletin | volume = 50 | issue = 5–6 | pages = 373 | year = 1999 | pmid = 10643441 | doi = 10.1016/S0361-9230(99)00110-0 }}</ref> The MAOs belong to the [[protein family]] of [[flavin-containing amine oxidoreductase]]s. | ||
MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate [[monoamine neurotransmitter]]s. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with [[monoamine oxidase inhibitors]] (MAOIs) which block the action of MAOs. | |||
== | == Subtypes and tissue distribution == | ||
In humans there are two types of MAO: [[Monoamine oxidase A|MAO-A]] and [[MAOB|MAO-B]].<ref name="pmid15279563">{{cite journal|authorlink1=Jean Chen Shih | vauthors = Shih JC, Chen K | title = Regulation of MAO-A and MAO-B gene expression | journal = Current Medicinal Chemistry | volume = 11 | issue = 15 | pages = 1995–2005 | date = August 2004 | pmid = 15279563 | doi = 10.2174/0929867043364757 }}</ref> | |||
In humans there are two types of MAO: [[Monoamine oxidase A|MAO-A]] and [[MAOB|MAO-B]]. | |||
* Both are found in [[neuron]]s and [[astroglia]]. | * Both are found in [[neuron]]s and [[astroglia]]. | ||
* Outside the [[central nervous system]]: | * Outside the [[central nervous system]]: | ||
** MAO-A is also found in the [[liver]], [[gastrointestinal tract]] and [[placenta]]. | ** MAO-A is also found in the [[liver]], [[pulmonary vascular system|pulmonary vascular]] [[endothelium]], [[Human gastrointestinal tract|gastrointestinal tract]], and [[placenta]]. | ||
** MAO-B is mostly found in [[blood]] [[platelet]]s. | ** MAO-B is mostly found in [[blood]] [[platelet]]s. | ||
MAO-A appears at roughly 80% of adulthood levels at birth, increasing very slightly after the first 4 years of life, while MAO-B is almost non-detectable in the infant brain. Regional distribution of the monoamine oxidases is characterized by extremely high levels of both MAOs in the [[hypothalamus]] and hippocampal uncus, as well as a large amount of MAO-B with very little MAO-A in the [[striatum]] and [[globus pallidus]]. The cortex has relatively high levels of only MAO-A, with the exception of areas of the [[cingulate cortex]], which contains a balance of both. Autopsied brains demonstrated the predicted increased concentration of MAO-A in regions dense in serotonergic neurotransmission, however MAO-B only correlated with norepinephrine.<ref name="pmid23403377">{{cite journal | vauthors = Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, Houle S, Kish SJ | title = Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies | journal = Journal of Cerebral Blood Flow and Metabolism | volume = 33 | issue = 6 | pages = 863–71 | date = June 2013 | pmid = 23403377 | pmc = 3677103 | doi = 10.1038/jcbfm.2013.19 }}</ref> | |||
== Function == | |||
[[File:Noradrenaline breakdown.svg|thumb|350px|Norepinephrine degradation. Monoamine oxidase is shown left in the blue box.<ref name=Rang&Dale6th-11-4>Figure 11-4 in: {{cite book | vauthors = Flower R, Rang HP, Dale MM, Ritter JM | title = Rang & Dale's pharmacology | publisher = Churchill Livingstone | location = Edinburgh | year = 2007 | pages = | isbn = 978-0-443-06911-6 }}</ref>]] | |||
Monoamine oxidases catalyze the [[oxidative deamination]] of monoamines. [[Oxygen]] is used to remove an [[amine]] group (plus the adjacent hydrogen atom) from a molecule, resulting in the corresponding [[ketone]] (or [[aldehyde]]) and [[ammonia]]. Monoamine [[oxidase]]s contain the covalently bound [[cofactor (biochemistry)|cofactor]] [[Flavin adenine dinucleotide|FAD]] and are, thus, classified as [[flavoprotein]]s. Monoamine oxidase A and B share roughly 70% of their structure and both have substrate binding sites that are predominantly [[hydrophobic]]. Two [[tyrosine]] residues (398, 435, 407 and 444) in the binding pocket that are commonly involved in inhibitor activity have been hypothesized to be relevant to orienting substrates, and mutations of these residues are relevant to mental health. Four main models have been proposed for the mechanism of [[electron transfer]] (single electron transfer, hydrogen atom transfer, nucleophilic model, and hydride transfer) although there is insufficient evidence to support any of them.<ref name="pmid22022344">{{cite journal | vauthors = Gaweska H, Fitzpatrick PF | title = Structures and Mechanism of the Monoamine Oxidase Family | journal = Biomolecular Concepts | volume = 2 | issue = 5 | pages = 365–377 | date = October 2011 | pmid = 22022344 | pmc = 3197729 | doi = 10.1515/BMC.2011.030 }}</ref> | |||
== Substrate specificities == | |||
They are well known [[enzymes]] in [[pharmacology]], since they are the target for the action of a number of [[monoamine oxidase inhibitor]] [[drugs]]. MAO-A is particularly important in the [[catabolism]] of monoamines ingested in food. Both MAOs are also vital to the inactivation of [[monoamine neurotransmitter]]s, for which they display different [[Enzyme#Specificity|specificities]]. | |||
* [[Serotonin]], [[melatonin]], [[norepinephrine]], and [[epinephrine]] are mainly broken down by MAO-A. | |||
MAO- | * [[Phenethylamine]] and [[benzylamine]] are mainly broken down by MAO-B. | ||
* Both forms break down [[dopamine]], [[tyramine]], and [[tryptamine]] equally.<ref name="pmid11559028">{{cite journal | vauthors = Kalgutkar AS, Dalvie DK, Castagnoli N, Taylor TJ | title = Interactions of nitrogen-containing xenobiotics with monoamine oxidase (MAO) isozymes A and B: SAR studies on MAO substrates and inhibitors | journal = Chemical Research in Toxicology | volume = 14 | issue = 9 | pages = 1139–62 | date = September 2001 | pmid = 11559028 | doi = 10.1021/tx010073b }}</ref> | |||
Specific reactions catalyzed by MAO include: | |||
* [[Adrenaline]] or [[noradrenaline]] to [[3,4-Dihydroxymandelic acid]] | |||
* [[Metanephrine]] or [[normetanephrine]] to [[vanillylmandelic acid|vanillylmandelic acid (VMA)]] | |||
* [[Dopamine]] to [[dihydroxyphenylacetic acid]] | |||
* [[3-Methoxytyramine]] to [[homovanillic acid]] | |||
== Clinical significance == | |||
==Genetics== | Because of the vital role that MAOs play in the inactivation of neurotransmitters, MAO dysfunction (too much or too little MAO activity) is thought to be responsible for a number of psychiatric and neurological disorders. For example, unusually high or low levels of MAOs in the body have been associated with [[schizophrenia]],<ref name="pmid943955">{{cite journal | vauthors = Domino EF, Khanna SS | title = Decreased blood platelet MAO activity in unmedicated chronic schizophrenic patients | journal = The American Journal of Psychiatry | volume = 133 | issue = 3 | pages = 323–6 | date = March 1976 | pmid = 943955 | doi = 10.1176/ajp.133.3.323 }}</ref><ref name="pmid1267046">{{cite journal | vauthors = Schildkraut JJ, Herzog JM, Orsulak PJ, Edelman SE, Shein HM, Frazier SH | title = Reduced platelet monoamine oxidase activity in a subgroup of schizophrenic patients | journal = The American Journal of Psychiatry | volume = 133 | issue = 4 | pages = 438–40 | date = April 1976 | pmid = 1267046 | doi = 10.1176/ajp.133.4.438 }}</ref> [[clinical depression|depression]],<ref name="pmid17088501">{{cite journal | vauthors = Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S | title = Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression | journal = Archives of General Psychiatry | volume = 63 | issue = 11 | pages = 1209–16 | date = November 2006 | pmid = 17088501 | doi = 10.1001/archpsyc.63.11.1209 }}</ref> [[attention deficit disorder]],<ref>{{cite journal | vauthors = Domschke K, Sheehan K, Lowe N, Kirley A, Mullins C, O'sullivan R, Freitag C, Becker T, Conroy J, Fitzgerald M, Gill M, Hawi Z | title = Association analysis of the monoamine oxidase A and B genes with attention deficit hyperactivity disorder (ADHD) in an Irish sample: preferential transmission of the MAO-A 941G allele to affected children | journal = American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics | volume = 134B | issue = 1 | pages = 110–4 | date = April 2005 | pmid = 15717295 | doi = 10.1002/ajmg.b.30158 }}</ref> [[substance abuse]],<ref>{{cite journal | vauthors = Oreland L | title = Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection | journal = Neurotoxicology | volume = 25 | issue = 1–2 | pages = 79–89 | date = January 2004 | pmid = 14697883 | doi = 10.1016/S0161-813X(03)00115-3 }}</ref> migraines,<ref>{{cite journal | vauthors = Bussone G, Boiardi A, Cerrati A, Girotti F, Merati B, Rivolta G | title = Monoamine oxidase activities in patients with migraine or with cluster headache during the acute phases and after treatment with L-5-hydroxytryptophan | journal = Rivista di Patologia Nervosa e Mentale | volume = 100 | issue = 5 | pages = 269–74 | date = 1 October 2016 | pmid = 318025 }}</ref><ref>{{cite journal | vauthors = Filic V, Vladic A, Stefulj J, Cicin-Sain L, Balija M, Sucic Z, Jernej B | title = Monoamine oxidases A and B gene polymorphisms in migraine patients | journal = Journal of the Neurological Sciences | volume = 228 | issue = 2 | pages = 149–53 | date = February 2005 | pmid = 15694196 | doi = 10.1016/j.jns.2004.11.045 }}</ref> and irregular sexual maturation.{{citation needed|date=April 2013}} [[Monoamine oxidase inhibitor]]s are one of the major classes of drug prescribed for the treatment of depression, although they are often last-line treatment due to risk of the drug's interaction with diet or other drugs. Excessive levels of [[catecholamine]]s ([[epinephrine]], [[norepinephrine]], and [[dopamine]]) may lead to a [[Hypertensive emergency|hypertensive crisis]], and excessive levels of [[serotonin]] may lead to [[serotonin syndrome]]. | ||
In fact, MAO-A inhibitors act as antidepressant and antianxiety agents, whereas MAO-B inhibitors are used alone or in combination to treat [[Alzheimer's disease]] and [[Parkinson's disease]].<ref name="pmid15279566">{{cite journal | vauthors = Riederer P, Lachenmayer L, Laux G | title = Clinical applications of MAO-inhibitors | journal = Current Medicinal Chemistry | volume = 11 | issue = 15 | pages = 2033–43 | date = August 2004 | pmid = 15279566 | doi = 10.2174/0929867043364775 }}</ref> Some research suggests that certain phenotypes of depression, such as those with anxiety, and "atypical" symptoms involving psychomotor retardation, weight gain and interpersonal sensitivity. However the findings related to this have not been consistent. MAOIs may be effective in treatment resistant depression, especially those that do not respond to tricyclic antidepressants.<ref name="pmid15552546">{{cite journal | vauthors = Fiedorowicz JG, Swartz KL | title = The role of monoamine oxidase inhibitors in current psychiatric practice | journal = Journal of Psychiatric Practice | volume = 10 | issue = 4 | pages = 239–48 | date = July 2004 | pmid = 15552546 | pmc = 2075358 | doi = 10.1097/00131746-200407000-00005}}</ref> | |||
[[Positron emission tomography|PET]] research shows that use of [[tobacco]] cigarettes heavily depletes MAO-B, mimicking the action of an MAO-B inhibitor. Smokers who smoke for emotional relief may therefore be unintentionally treating depression and/or anxiety that is better addressed by an MAO-B inhibitor.<ref name="pmid9549600">{{cite journal | vauthors = Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I | title = Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition | journal = Journal of Addictive Diseases | volume = 17 | issue = 1 | pages = 23–34 | year = 1998 | pmid = 9549600 | doi = 10.1300/J069v17n01_03 }}</ref> | |||
===Animal models=== | |||
There are significant differences in MAO activity in different species. Dopamine is primarily deaminated by [[monoamine oxidase A|MAO-A]] in rats, but by [[Monoamine oxidase B|MAO-B]] in [[vervet monkey]]s and humans.<ref name="Garrick Murphy">{{cite journal | vauthors = Garrick NA, Murphy DL | title = Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain | journal = Psychopharmacology | volume = 72 | issue = 1 | pages = 27–33 | year = 1980 | pmid = 6781004 | doi = 10.1007/bf00433804 }}</ref> | |||
Mice unable to produce either MAO-A or MAO-B display [[autism spectrum|autistic-like]] traits.<ref name="pmid22850464">{{cite journal | vauthors = Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC | title = Monoamine oxidase A and A/B knockout mice display autistic-like features | journal = The International Journal of Neuropsychopharmacology | volume = 16 | issue = 4 | pages = 869–88 | date = May 2013 | pmid = 22850464 | pmc = 3517692 | doi = 10.1017/S1461145712000715 }}</ref> These [[Knockout mouse|knockout mice]] display an increased response to stress.<ref name="pmid14697877">{{cite journal | vauthors = Shih JC | title = Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B | journal = Neurotoxicology | volume = 25 | issue = 1–2 | pages = 21–30 | date = January 2004 | pmid = 14697877 | doi = 10.1016/s0161-813x(03)00112-8 }}</ref> | |||
== Genetics == | |||
The [[gene]]s encoding MAO-A and MAO-B are located side-by-side on the short arm of the [[X chromosome]], and have about 70% sequence similarity. Rare mutations in the gene are associated with [[Brunner syndrome]]. | The [[gene]]s encoding MAO-A and MAO-B are located side-by-side on the short arm of the [[X chromosome]], and have about 70% sequence similarity. Rare mutations in the gene are associated with [[Brunner syndrome]]. | ||
A study | A study based on the [[Dunedin cohort]] concluded that maltreated children with a low-activity polymorphism in the [[promoter (biology)|promoter]] region of the MAO-A gene were more likely to develop [[conduct disorder|antisocial conduct disorders]] than maltreated children with the high-activity variant.<ref name="pmid12161658">{{cite journal | vauthors = Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R | title = Role of genotype in the cycle of violence in maltreated children | journal = Science | volume = 297 | issue = 5582 | pages = 851–4 | date = August 2002 | pmid = 12161658 | doi = 10.1126/science.1072290 }}</ref> Out of the 442 total males in the study (maltreated or not), 37% had the low activity variant. Of the 13 maltreated males with low MAO-A activity, 11 had been assessed as exhibiting [[conduct disorder|adolescent conduct disorder]] and 4 were convicted for violent offenses. The suggested mechanism for this effect is the decreased ability of those with low MAO-A activity to quickly degrade norepinephrine, the synaptic neurotransmitter involved in [[sympathetic nervous system|sympathetic]] arousal and rage. This is argued to provide direct support for the idea that genetic susceptibility to disease is not determined at birth, but varies with exposure to environmental influences. However, most individuals with conduct disorder or convictions did not have low activity of MAO-A; maltreatment was found to have caused stronger predisposition for antisocial behavior than differences in MAO-A activity. | ||
The claim that an interaction between low MAO-A activity and maltreatment would cause anti-social behavior has been criticized since the predisposition towards anti-social behavior could equally well have been caused by ''other'' genes inherited from abusive parents.<ref name="isbn0-521-82818-X">{{cite book | last = Sesardic | first = Neven | name-list-format = vanc | title = Making sense of heritability | edition = | publisher = Cambridge University Press | location = Cambridge, UK | year = 2005 | pages = | quote = | isbn = 978-0-521-82818-5 }}</ref> | |||
A possible link between predisposition to [[neophilia|novelty seeking]] and a [[genotype]] of the MAO-A gene has been found.<ref name="pmid16538181">{{cite journal | vauthors = Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, Otani K | title = Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants | journal = Psychiatric Genetics | volume = 16 | issue = 2 | pages = 55–8 | date = April 2006 | pmid = 16538181 | doi = 10.1097/01.ypg.0000199447.62044.ef | laysummary = http://www.medialifemagazine.com/cgi-bin/artman/exec/view.cgi?archive=226&num=5439 | laysource = medialifemagazine.com }}</ref> | |||
A particular variant (or [[genotype]]), dubbed "[[warrior gene]]" in the popular press, was over-represented in [[Māori people|Māori]]. This supported earlier studies finding different proportions of variants in different ethnic groups. This is the case for many genetic variants, with 33% White/Non-Hispanic, 61% Asian/Pacific Islanders having the low-activity MAO-A [[promoter (biology)|promoter]] variant.<ref name="pmid9799080">{{cite journal | vauthors = Sabol SZ, Hu S, Hamer D | title = A functional polymorphism in the monoamine oxidase A gene promoter | journal = Human Genetics | volume = 103 | issue = 3 | pages = 273–9 | date = September 1998 | pmid = 9799080 | doi = 10.1007/s004390050816 }}</ref> | |||
== Aging == | |||
Unlike many other enzymes, MAO-B activity is increased during aging in the brain of humans and other mammals.<ref>{{cite journal | vauthors = Nicotra A, Pierucci F, Parvez H, Senatori O | title = Monoamine oxidase expression during development and aging | journal = Neurotoxicology | volume = 25 | issue = 1–2 | pages = 155–65 | date = January 2004 | pmid = 14697890 | doi = 10.1016/S0161-813X(03)00095-0 }}</ref> Increased MAO-B activity was also found in the pineal gland of aging rats.<ref name="Razygraev_2016">{{cite journal | vauthors = Razygraev AV, Taborskaya KI, Volovik KY, Bunina AA, Petrosyan MA |date=2016-07-14|title=Monoamine oxidase activity in the rat pineal gland: Comparison with brain areas and alteration during aging|journal=Advances in Gerontology|volume=6|issue=2|pages=111–116|doi=10.1134/S2079057016020120|issn=2079-0570}}</ref> This may contribute to lowered levels of monoamines in aged brain and pineal gland.<ref name="Razygraev_2016"/> | |||
==See also== | == See also == | ||
*[[ | *[[Cheese effect]] | ||
*[[ | *[[Imidazoline receptor|I<sub>2</sub> receptor]] | ||
*[[ | *[[Monoamine oxidase inhibitor]] | ||
{{Clear}} | |||
{{ | |||
== | == References == | ||
{{Reflist|32em}} | |||
{{Mitochondrial enzymes}} | |||
{{Neurotransmitter metabolism enzymes}} | |||
{{CH-NH2 oxidoreductases}} | {{CH-NH2 oxidoreductases}} | ||
{{ | {{Enzymes}} | ||
{{Monoamine metabolism modulators}} | |||
{{Portal bar|Molecular and Cellular Biology|border=no}} | |||
[[Category:EC 1.4.3]] | [[Category:EC 1.4.3]] | ||
[[Category: | [[Category:Single-pass transmembrane proteins]] | ||
[[Category:Amphetamine]] | |||
[[ | |||
Latest revision as of 19:19, 13 December 2018
Lua error in Module:Redirect at line 65: could not parse redirect on page "MAO".
| Monoamine oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.4.3.4 | ||||||||
| CAS number | 9001-66-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Monoamine oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | MAO | ||||||||
| Pfam | PF01593 | ||||||||
| InterPro | IPR001613 | ||||||||
| OPM superfamily | 119 | ||||||||
| OPM protein | 2z5x | ||||||||
| Membranome | 418 | ||||||||
| |||||||||
| monoamine oxidase A | |
|---|---|
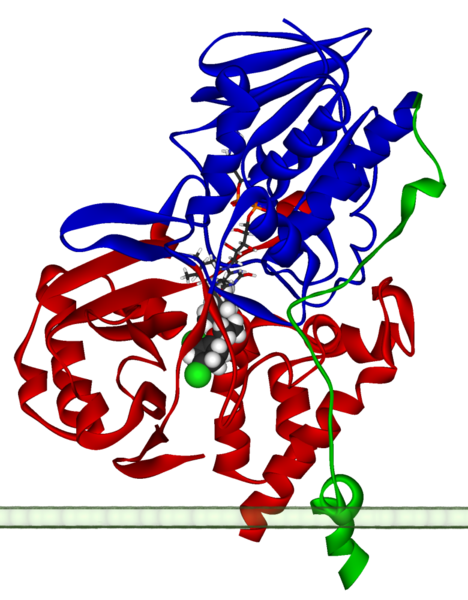 Ribbon diagram of a monomer of human MAO-A, with FAD and clorgiline bound, oriented as if attached to the outer membrane of a mitochondrion. From PDB: 2BXS. | |
| Identifiers | |
| Symbol | MAOA |
| Entrez | 4128 |
| HUGO | 6833 |
| OMIM | 309850 |
| RefSeq | NM_000240 |
| UniProt | P21397 |
| Other data | |
| Locus | Chr. X p11.4-p11.3 |
| monoamine oxidase B | |
|---|---|
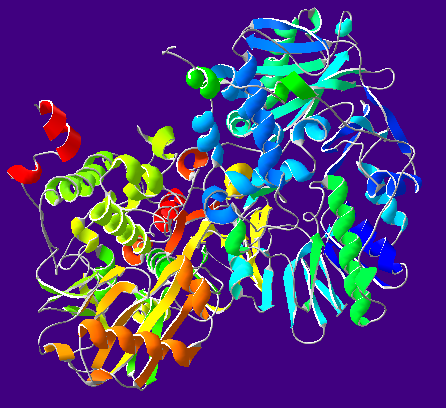 | |
| Identifiers | |
| Symbol | MAOB |
| Entrez | 4129 |
| HUGO | 6834 |
| OMIM | 309860 |
| RefSeq | NM_000898 |
| UniProt | P27338 |
| Other data | |
| Locus | Chr. X p11.4-p11.3 |
Monoamine oxidases (MAO) (EC 1.4.3.4) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group.[1][2] They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase.[3][4] The MAOs belong to the protein family of flavin-containing amine oxidoreductases.
MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs.
Subtypes and tissue distribution
In humans there are two types of MAO: MAO-A and MAO-B.[5]
- Both are found in neurons and astroglia.
- Outside the central nervous system:
- MAO-A is also found in the liver, pulmonary vascular endothelium, gastrointestinal tract, and placenta.
- MAO-B is mostly found in blood platelets.
MAO-A appears at roughly 80% of adulthood levels at birth, increasing very slightly after the first 4 years of life, while MAO-B is almost non-detectable in the infant brain. Regional distribution of the monoamine oxidases is characterized by extremely high levels of both MAOs in the hypothalamus and hippocampal uncus, as well as a large amount of MAO-B with very little MAO-A in the striatum and globus pallidus. The cortex has relatively high levels of only MAO-A, with the exception of areas of the cingulate cortex, which contains a balance of both. Autopsied brains demonstrated the predicted increased concentration of MAO-A in regions dense in serotonergic neurotransmission, however MAO-B only correlated with norepinephrine.[6]
Function
Monoamine oxidases catalyze the oxidative deamination of monoamines. Oxygen is used to remove an amine group (plus the adjacent hydrogen atom) from a molecule, resulting in the corresponding ketone (or aldehyde) and ammonia. Monoamine oxidases contain the covalently bound cofactor FAD and are, thus, classified as flavoproteins. Monoamine oxidase A and B share roughly 70% of their structure and both have substrate binding sites that are predominantly hydrophobic. Two tyrosine residues (398, 435, 407 and 444) in the binding pocket that are commonly involved in inhibitor activity have been hypothesized to be relevant to orienting substrates, and mutations of these residues are relevant to mental health. Four main models have been proposed for the mechanism of electron transfer (single electron transfer, hydrogen atom transfer, nucleophilic model, and hydride transfer) although there is insufficient evidence to support any of them.[8]
Substrate specificities
They are well known enzymes in pharmacology, since they are the target for the action of a number of monoamine oxidase inhibitor drugs. MAO-A is particularly important in the catabolism of monoamines ingested in food. Both MAOs are also vital to the inactivation of monoamine neurotransmitters, for which they display different specificities.
- Serotonin, melatonin, norepinephrine, and epinephrine are mainly broken down by MAO-A.
- Phenethylamine and benzylamine are mainly broken down by MAO-B.
- Both forms break down dopamine, tyramine, and tryptamine equally.[9]
Specific reactions catalyzed by MAO include:
- Adrenaline or noradrenaline to 3,4-Dihydroxymandelic acid
- Metanephrine or normetanephrine to vanillylmandelic acid (VMA)
- Dopamine to dihydroxyphenylacetic acid
- 3-Methoxytyramine to homovanillic acid
Clinical significance
Because of the vital role that MAOs play in the inactivation of neurotransmitters, MAO dysfunction (too much or too little MAO activity) is thought to be responsible for a number of psychiatric and neurological disorders. For example, unusually high or low levels of MAOs in the body have been associated with schizophrenia,[10][11] depression,[12] attention deficit disorder,[13] substance abuse,[14] migraines,[15][16] and irregular sexual maturation.[citation needed] Monoamine oxidase inhibitors are one of the major classes of drug prescribed for the treatment of depression, although they are often last-line treatment due to risk of the drug's interaction with diet or other drugs. Excessive levels of catecholamines (epinephrine, norepinephrine, and dopamine) may lead to a hypertensive crisis, and excessive levels of serotonin may lead to serotonin syndrome.
In fact, MAO-A inhibitors act as antidepressant and antianxiety agents, whereas MAO-B inhibitors are used alone or in combination to treat Alzheimer's disease and Parkinson's disease.[17] Some research suggests that certain phenotypes of depression, such as those with anxiety, and "atypical" symptoms involving psychomotor retardation, weight gain and interpersonal sensitivity. However the findings related to this have not been consistent. MAOIs may be effective in treatment resistant depression, especially those that do not respond to tricyclic antidepressants.[18]
PET research shows that use of tobacco cigarettes heavily depletes MAO-B, mimicking the action of an MAO-B inhibitor. Smokers who smoke for emotional relief may therefore be unintentionally treating depression and/or anxiety that is better addressed by an MAO-B inhibitor.[19]
Animal models
There are significant differences in MAO activity in different species. Dopamine is primarily deaminated by MAO-A in rats, but by MAO-B in vervet monkeys and humans.[20]
Mice unable to produce either MAO-A or MAO-B display autistic-like traits.[21] These knockout mice display an increased response to stress.[22]
Genetics
The genes encoding MAO-A and MAO-B are located side-by-side on the short arm of the X chromosome, and have about 70% sequence similarity. Rare mutations in the gene are associated with Brunner syndrome.
A study based on the Dunedin cohort concluded that maltreated children with a low-activity polymorphism in the promoter region of the MAO-A gene were more likely to develop antisocial conduct disorders than maltreated children with the high-activity variant.[23] Out of the 442 total males in the study (maltreated or not), 37% had the low activity variant. Of the 13 maltreated males with low MAO-A activity, 11 had been assessed as exhibiting adolescent conduct disorder and 4 were convicted for violent offenses. The suggested mechanism for this effect is the decreased ability of those with low MAO-A activity to quickly degrade norepinephrine, the synaptic neurotransmitter involved in sympathetic arousal and rage. This is argued to provide direct support for the idea that genetic susceptibility to disease is not determined at birth, but varies with exposure to environmental influences. However, most individuals with conduct disorder or convictions did not have low activity of MAO-A; maltreatment was found to have caused stronger predisposition for antisocial behavior than differences in MAO-A activity.
The claim that an interaction between low MAO-A activity and maltreatment would cause anti-social behavior has been criticized since the predisposition towards anti-social behavior could equally well have been caused by other genes inherited from abusive parents.[24]
A possible link between predisposition to novelty seeking and a genotype of the MAO-A gene has been found.[25]
A particular variant (or genotype), dubbed "warrior gene" in the popular press, was over-represented in Māori. This supported earlier studies finding different proportions of variants in different ethnic groups. This is the case for many genetic variants, with 33% White/Non-Hispanic, 61% Asian/Pacific Islanders having the low-activity MAO-A promoter variant.[26]
Aging
Unlike many other enzymes, MAO-B activity is increased during aging in the brain of humans and other mammals.[27] Increased MAO-B activity was also found in the pineal gland of aging rats.[28] This may contribute to lowered levels of monoamines in aged brain and pineal gland.[28]
See also
References
- ↑ Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J (August 2004). "Monoamine oxidases: certainties and uncertainties". Current Medicinal Chemistry. 11 (15): 1965–82. doi:10.2174/0929867043364810. PMID 15279561.
- ↑ Edmondson DE, Mattevi A, Binda C, Li M, Hubálek F (August 2004). "Structure and mechanism of monoamine oxidase". Current Medicinal Chemistry. 11 (15): 1983–93. doi:10.2174/0929867043364784. PMID 15279562.
- ↑ Hare ML (1928). "Tyramine oxidase: A new enzyme system in liver". The Biochemical Journal. 22 (4): 968–79. doi:10.1042/bj0220968. PMC 1252213. PMID 16744124.
- ↑ Slotkin TA (1999). "Mary Bernheim and the discovery of monoamine oxidase". Brain Research Bulletin. 50 (5–6): 373. doi:10.1016/S0361-9230(99)00110-0. PMID 10643441.
- ↑ Shih JC, Chen K (August 2004). "Regulation of MAO-A and MAO-B gene expression". Current Medicinal Chemistry. 11 (15): 1995–2005. doi:10.2174/0929867043364757. PMID 15279563.
- ↑ Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, Houle S, Kish SJ (June 2013). "Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies". Journal of Cerebral Blood Flow and Metabolism. 33 (6): 863–71. doi:10.1038/jcbfm.2013.19. PMC 3677103. PMID 23403377.
- ↑ Figure 11-4 in: Flower R, Rang HP, Dale MM, Ritter JM (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-06911-6.
- ↑ Gaweska H, Fitzpatrick PF (October 2011). "Structures and Mechanism of the Monoamine Oxidase Family". Biomolecular Concepts. 2 (5): 365–377. doi:10.1515/BMC.2011.030. PMC 3197729. PMID 22022344.
- ↑ Kalgutkar AS, Dalvie DK, Castagnoli N, Taylor TJ (September 2001). "Interactions of nitrogen-containing xenobiotics with monoamine oxidase (MAO) isozymes A and B: SAR studies on MAO substrates and inhibitors". Chemical Research in Toxicology. 14 (9): 1139–62. doi:10.1021/tx010073b. PMID 11559028.
- ↑ Domino EF, Khanna SS (March 1976). "Decreased blood platelet MAO activity in unmedicated chronic schizophrenic patients". The American Journal of Psychiatry. 133 (3): 323–6. doi:10.1176/ajp.133.3.323. PMID 943955.
- ↑ Schildkraut JJ, Herzog JM, Orsulak PJ, Edelman SE, Shein HM, Frazier SH (April 1976). "Reduced platelet monoamine oxidase activity in a subgroup of schizophrenic patients". The American Journal of Psychiatry. 133 (4): 438–40. doi:10.1176/ajp.133.4.438. PMID 1267046.
- ↑ Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S (November 2006). "Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression". Archives of General Psychiatry. 63 (11): 1209–16. doi:10.1001/archpsyc.63.11.1209. PMID 17088501.
- ↑ Domschke K, Sheehan K, Lowe N, Kirley A, Mullins C, O'sullivan R, Freitag C, Becker T, Conroy J, Fitzgerald M, Gill M, Hawi Z (April 2005). "Association analysis of the monoamine oxidase A and B genes with attention deficit hyperactivity disorder (ADHD) in an Irish sample: preferential transmission of the MAO-A 941G allele to affected children". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 134B (1): 110–4. doi:10.1002/ajmg.b.30158. PMID 15717295.
- ↑ Oreland L (January 2004). "Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection". Neurotoxicology. 25 (1–2): 79–89. doi:10.1016/S0161-813X(03)00115-3. PMID 14697883.
- ↑ Bussone G, Boiardi A, Cerrati A, Girotti F, Merati B, Rivolta G (1 October 2016). "Monoamine oxidase activities in patients with migraine or with cluster headache during the acute phases and after treatment with L-5-hydroxytryptophan". Rivista di Patologia Nervosa e Mentale. 100 (5): 269–74. PMID 318025.
- ↑ Filic V, Vladic A, Stefulj J, Cicin-Sain L, Balija M, Sucic Z, Jernej B (February 2005). "Monoamine oxidases A and B gene polymorphisms in migraine patients". Journal of the Neurological Sciences. 228 (2): 149–53. doi:10.1016/j.jns.2004.11.045. PMID 15694196.
- ↑ Riederer P, Lachenmayer L, Laux G (August 2004). "Clinical applications of MAO-inhibitors". Current Medicinal Chemistry. 11 (15): 2033–43. doi:10.2174/0929867043364775. PMID 15279566.
- ↑ Fiedorowicz JG, Swartz KL (July 2004). "The role of monoamine oxidase inhibitors in current psychiatric practice". Journal of Psychiatric Practice. 10 (4): 239–48. doi:10.1097/00131746-200407000-00005. PMC 2075358. PMID 15552546.
- ↑ Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I (1998). "Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition". Journal of Addictive Diseases. 17 (1): 23–34. doi:10.1300/J069v17n01_03. PMID 9549600.
- ↑ Garrick NA, Murphy DL (1980). "Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain". Psychopharmacology. 72 (1): 27–33. doi:10.1007/bf00433804. PMID 6781004.
- ↑ Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC (May 2013). "Monoamine oxidase A and A/B knockout mice display autistic-like features". The International Journal of Neuropsychopharmacology. 16 (4): 869–88. doi:10.1017/S1461145712000715. PMC 3517692. PMID 22850464.
- ↑ Shih JC (January 2004). "Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B". Neurotoxicology. 25 (1–2): 21–30. doi:10.1016/s0161-813x(03)00112-8. PMID 14697877.
- ↑ Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R (August 2002). "Role of genotype in the cycle of violence in maltreated children". Science. 297 (5582): 851–4. doi:10.1126/science.1072290. PMID 12161658.
- ↑ Sesardic N (2005). Making sense of heritability. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-82818-5.
- ↑ Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, Otani K (April 2006). "Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants". Psychiatric Genetics. 16 (2): 55–8. doi:10.1097/01.ypg.0000199447.62044.ef. PMID 16538181. Lay summary – medialifemagazine.com.
- ↑ Sabol SZ, Hu S, Hamer D (September 1998). "A functional polymorphism in the monoamine oxidase A gene promoter". Human Genetics. 103 (3): 273–9. doi:10.1007/s004390050816. PMID 9799080.
- ↑ Nicotra A, Pierucci F, Parvez H, Senatori O (January 2004). "Monoamine oxidase expression during development and aging". Neurotoxicology. 25 (1–2): 155–65. doi:10.1016/S0161-813X(03)00095-0. PMID 14697890.
- ↑ 28.0 28.1 Razygraev AV, Taborskaya KI, Volovik KY, Bunina AA, Petrosyan MA (2016-07-14). "Monoamine oxidase activity in the rat pineal gland: Comparison with brain areas and alteration during aging". Advances in Gerontology. 6 (2): 111–116. doi:10.1134/S2079057016020120. ISSN 2079-0570.
- Pages with script errors
- Protein pages needing a picture
- Genes on human chromosome X
- Pages with broken file links
- All articles with unsourced statements
- Articles with unsourced statements from April 2013
- Articles with invalid date parameter in template
- Portal templates with all redlinked portals
- EC 1.4.3
- Single-pass transmembrane proteins
- Amphetamine