Phenylalanine
|
WikiDoc Resources for Phenylalanine |
|
Articles |
|---|
|
Most recent articles on Phenylalanine Most cited articles on Phenylalanine |
|
Media |
|
Powerpoint slides on Phenylalanine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Phenylalanine at Clinical Trials.gov Trial results on Phenylalanine Clinical Trials on Phenylalanine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phenylalanine NICE Guidance on Phenylalanine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phenylalanine Discussion groups on Phenylalanine Patient Handouts on Phenylalanine Directions to Hospitals Treating Phenylalanine Risk calculators and risk factors for Phenylalanine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phenylalanine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Phenylalanine (abbreviated as Phe or F)[1] is an α-amino acid with the formula HO2CCH(NH2)CH2C6H5. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. The codons for L-phenylalanine are UUU and UUC. It is a white, powdery solid. L-Phenylalanine (LPA) is an electrically-neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA.
Biosynthesis
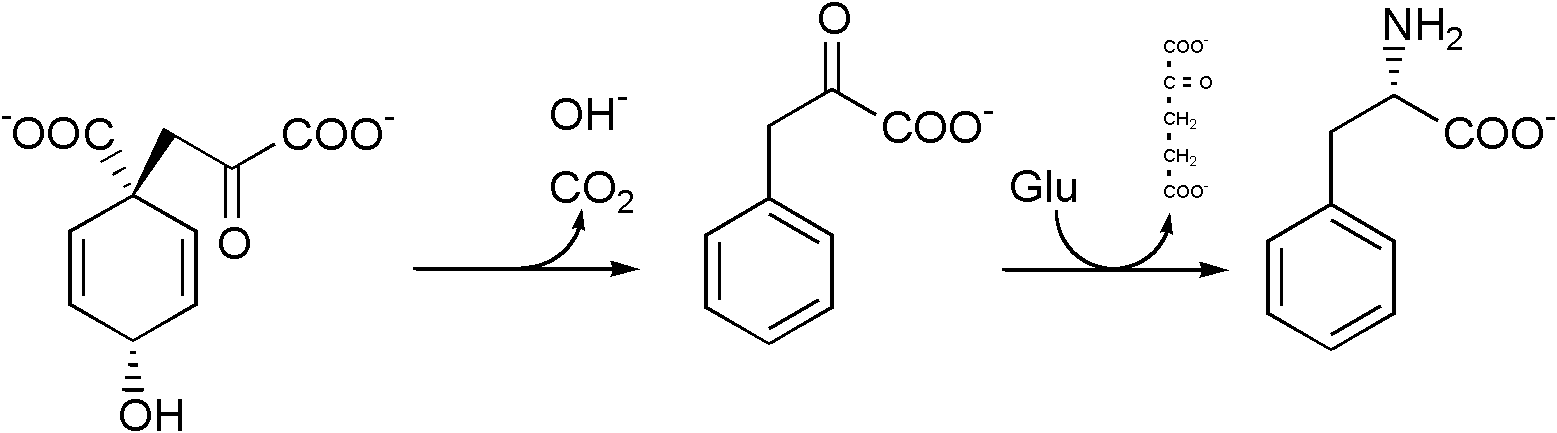
Phenylalanine cannot be made by animals, which have to obtain it from their diet. It is produced by plants and most microorganisms from prephenate, an intermediate on the shikimate pathway.[2]
Prephenate is decarboxylated with loss of the hydroxyl group to give phenylpyruvate. This species is transaminated using glutamate as the nitrogen source to give phenylalanine and α-ketoglutarate.
Other biological roles
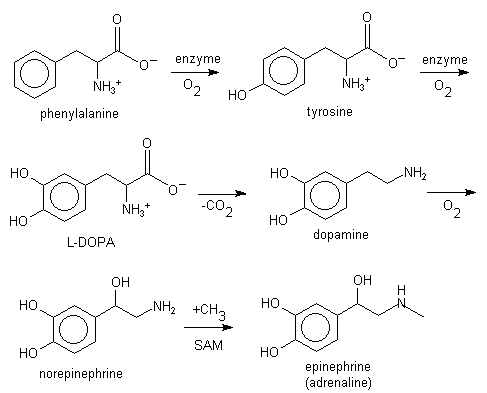
L-phenylalanine can also be converted into L-tyrosine, another one of the DNA-encoded amino acids. L-tyrosine in turn is converted into L-DOPA, which is further converted into dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) (the latter three are known as the catecholamines).
Phenylalanine uses the same active transport channel as tryptophan to cross the blood-brain barrier, and, in large quantities, interferes with the production of serotonin.
Lignin is derived from phenylalanine and from tyrosine. Phenylalanine is converted to cinnamic acid by the enzyme phenylalanine ammonia lyase.[2]
Phenylketonuria
The genetic disorder phenylketonuria (PKU) is the inability to metabolize phenylalanine. Individuals with this disorder are known as "phenylketonurics" and must abstain from consumption of phenylalanine. This dietary restriction also applies to pregnant women with hyperphenylalanine (high levels of phenylalanine in blood) because they do not properly metabolize the amino acid phenylalanine. Persons suffering from PKU must monitor their intake of protein to control the buildup of phenylalanine as their bodies convert protein into its component amino acids.
A related issue is the compound present in many sugarless gums and mints, snack foods, sugarless soft drinks (such as diet sodas including CocaCola Zero, Pepsi Max, some forms of Lipton Tea, diet Nestea, Clear Splash flavored water), and a number of other low calorie food products. The artificial sweetener aspartame, sold under the names "Equal" and "NutraSweet", is an ester that is hydrolyzed in the body to give phenylalanine, aspartic acid, and methanol (wood alcohol). The breakdown problems phenylketonurics have with protein and the attendant build up of phenylalanine in the body also occurs with the ingestion of aspartame, although to a lesser degree. Accordingly, all products in the U.S. and Canada that contain aspartame must be labeled: "Phenylketonurics: Contains phenylalanine." In the UK, foods containing aspartame must carry ingredients panels that refer to the presence of 'aspartame or E951', [2]and they must be labeled with a warning "Contains a source of phenylalanine". These warnings are specifically placed to aid individuals who suffer from PKU so that they can avoid such foods.
Interestingly, the macaque genome was recently sequenced and it was found that macaques naturally have a mutation that is found in humans who have PKU.[3]
D- and DL-phenylalanine
D-phenylalanine (DPA) either as a single enantiomer or as a component of the racemic mixture is available through conventional organic synthesis. It does not participate in protein biosynthesis although it is found in proteins, in small amounts, particularly aged proteins and food proteins that have been processed. The biological functions of D-amino acids remain unclear. Some D-amino acids, such as D-phenylalanine, may have pharmacological activity.
DL-Phenylalanine is marketed as a nutritional supplement for its putative analgesic and antidepressant activities. The putative analgesic activity of DL-phenylalanine may be explained by the possible blockage by D-phenylalanine of enkephalin degradation by the enzyme carboxypeptidase A. The mechanism of DL-phenylalanine's putative antidepressant activity may be accounted for by the precursor role of L-phenylalanine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects. D-phenylalanine is absorbed from the small intestine, following ingestion, and transported to the liver via the portal circulation. A fraction of D-phenylalanine appears to be converted to L-phenylalanine. D-phenylalanine is distributed to the various tissues of the body via the systemic circulation. D-phenylalanine appears to cross the blood-brain barrier with less efficiency than L-phenylalanine. A fraction of an ingested dose of D-phenylalanine is excreted in the urine.
History
The genetic codon for phenylalanine was the first to be discovered. Marshall W. Nirenberg discovered that insertion of m-RNA made up of multiple uracil repeats into E. coli, the bacterium produced a new protein, made up solely of repeated phenylalanine amino acids.
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
- ↑ 2.0 2.1 Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
External links
- Phenylalanine and tyrosine biosynthesis
- Computational Chemistry Wiki
- Nitrogen Order's Molecule of the Week
- DL-phenylalanine versus imipramine in depression
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) |