Citric acid cycle
|
WikiDoc Resources for Citric acid cycle |
|
Articles |
|---|
|
Most recent articles on Citric acid cycle Most cited articles on Citric acid cycle |
|
Media |
|
Powerpoint slides on Citric acid cycle |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Citric acid cycle |
|
Clinical Trials |
|
Ongoing Trials on Citric acid cycle at Clinical Trials.gov Trial results on Citric acid cycle Clinical Trials on Citric acid cycle at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Citric acid cycle NICE Guidance on Citric acid cycle
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Citric acid cycle Discussion groups on Citric acid cycle Patient Handouts on Citric acid cycle Directions to Hospitals Treating Citric acid cycle Risk calculators and risk factors for Citric acid cycle
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Citric acid cycle |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
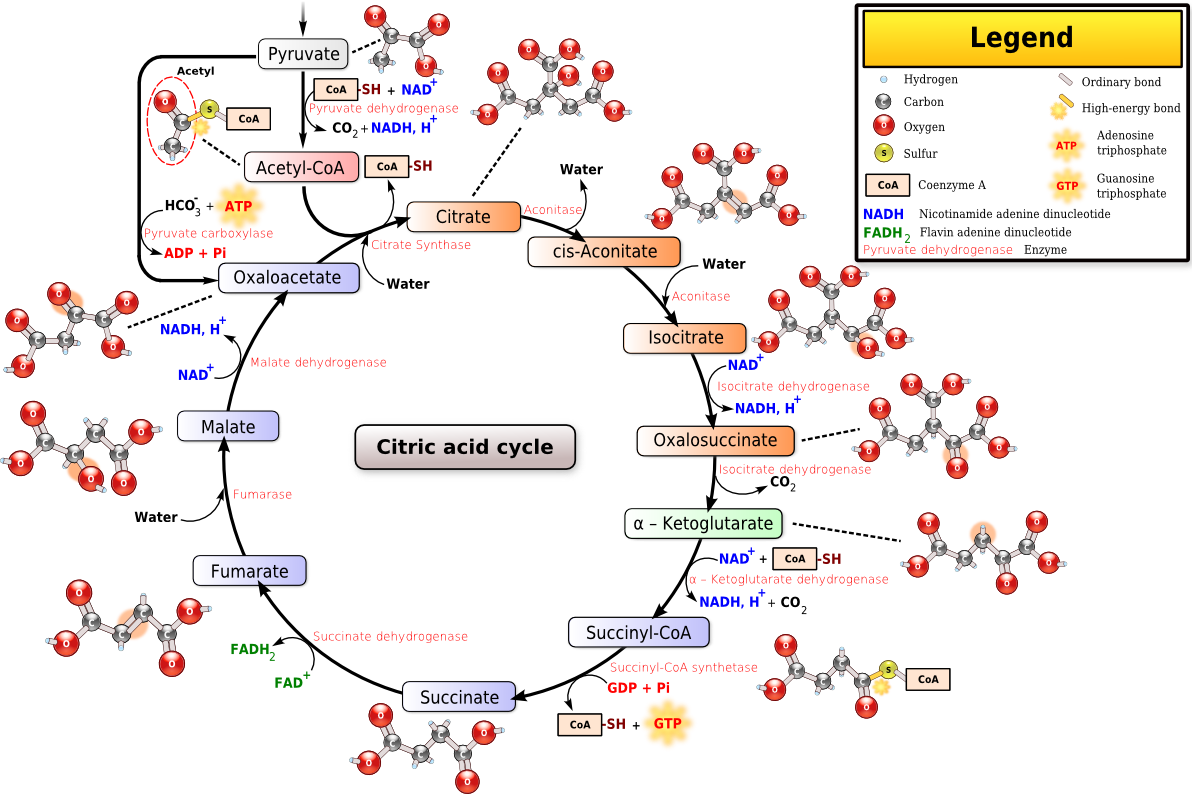
The citric acid cycle, also known as the tricarboxylic acid cycle (TCA cycle) or the Krebs cycle, (On rare occasions the citric acid cycle is known by a fourth name, the Szent-Györgyi-Krebs cycle) is a series of enzyme-catalysed chemical reactions of central importance in all living cells that use oxygen as part of cellular respiration. In eukaryotes, the citric acid cycle is located in the matrix of the mitochondrion. The components and reactions of the citric acid cycle were established by seminal work from both Albert Szent-Györgyi and Hans Krebs.
In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. Other relevant reactions in the pathway include those in glycolysis and pyruvate oxidation before the citric acid cycle, and oxidative phosphorylation after it. In addition, it provides precursors for many compounds including some amino acids and is therefore functional even in cells performing fermentation.
Two carbons are oxidized to CO2, and the energy from these reactions is transferred to other metabolic processes by GTP (or ATP), and as electrons in NADH and FADH2. The NADH generated in the TCA cycle may later donate its electrons in oxidative phosphorylation to drive ATP synthesis; FADH2 is covalently attached to succinate dehydrogenase, an enzyme functioning both in the TCA cycle and the mitochondrial electron transfer chain in oxidative phosphorylation. FADH2 thereby facilitates transfer of electrons to coenzyme Q, an intermediate in the electron transfer chain.[1]
The citric acid cycle is continuously supplied new carbons in the form of acetyl-CoA, entering at step 1 below.[2]
| S t e p |
Substrates | Products | Enzyme | Reaction type | Comment |
|---|---|---|---|---|---|
| 1 | Oxaloacetate + Acetyl CoA + H2O |
Citrate + CoA-SH |
Citrate synthase | Aldol condensation | rate limiting stage, extends the 4C oxaloacetate to a 6C molecule |
| 2 | Citrate | cis-Aconitate + H2O |
Aconitase | Dehydration | reversible isomerisation |
| 3 | cis-Aconitate + H2O |
Isocitrate | Hydration | ||
| 4 | Isocitrate + NAD+ |
Oxalosuccinate + NADH + H + |
Isocitrate dehydrogenase | Oxidation | generates NADH (equivalent of 3 ATP) |
| 5 | Oxalosuccinate | α-Ketoglutarate + CO2 |
Decarboxylation | irreversible stage, generates a 5C molecule | |
| 6 | α-Ketoglutarate + NAD+ + CoA-SH |
Succinyl-CoA + NADH + H+ + CO2 |
α-Ketoglutarate dehydrogenase | Oxidative decarboxylation |
generates NADH (equivalent of 3 ATP), regenerates the 4C chain (CoA excluded) |
| 7 | Succinyl-CoA + GDP + Pi |
Succinate + CoA-SH + GTP |
Succinyl-CoA synthetase | substrate level phosphorylation | or ADP->ATP,[1] generates 1 ATP or equivalent |
| 8 | Succinate + ubiquinone (Q) |
Fumarate + ubiquinol (QH2) |
Succinate dehydrogenase | Oxidation | uses FAD as a prosthetic group (FAD->FADH2 in the first step of the reaction) in the enzyme,[1] generates the equivalent of 2 ATP |
| 9 | Fumarate + H2O |
L-Malate | Fumarase | H2O addition (hydration) |
|
| 10 | L-Malate+ NAD+ |
Oxaloacetate + NADH + H+ |
Malate dehydrogenase | Oxidation | generates NADH (equivalent of 3 ATP) |
Mitochondria in animals including humans possess two succinyl-CoA synthetases, one that produces GTP from GDP, and another that produces ATP from ADP.[3] Plants have the type that produces ATP (ADP-forming succinyl-CoA synthetase).[2]
The GTP that is formed by GDP-forming succinyl-CoA synthetase may be utilized by nucleoside-diphosphate kinase to form ATP (the catalyzed reaction is GTP + ADP -> GDP + ATP).[1]
A simplified view of the process
- The citric acid cycle begins with acetyl-CoA transferring its two-carbon acetyl group to the four-carbon acceptor compound (oxaloacetate) to form a six-carbon compound (citrate).
- The citrate then goes through a series of chemical transformations, losing first one, then a second carboxyl group as CO2. The carbons lost as CO2 originate from what was oxaloacetate, not directly from acetyl-CoA. The carbons donated by acetyl-CoA become part of the oxaloacetate carbon backbone after the first turn of the citric acid cycle. Loss of the acetyl-CoA-donated carbons as CO2 requires several turns of the citric acid cycle. However, because of the role of the citric acid cycle in anabolism, they may not be lost since many TCA cycle intermediates are also used as precursors for the biosynthesis of other molecules.[4]
- Most of the energy made available by the oxidative steps of the cycle is transferred as energy-rich electrons to NAD+, forming NADH. For each acetyl group that enters the citric acid cycle, three molecules of NADH are produced.
- Electrons are also transferred to the electron acceptor FAD, forming FADH2.
- At the end of each cycle, the four-carbon oxaloacetate has been regenerated, and the cycle continues.
Products
Products of the first turn of the cycle are: one GTP, three NADH, one FADH2, two CO2.
Because two acetyl-CoA molecules are produced from each glucose molecule, two cycles are required per glucose molecule. Therefore, at the end of all cycles, the products are: two GTP, six NADH, two FADH2, and four CO2
| Description | Reactants | Products |
| The sum of all reactions in the citric acid cycle is: | Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2 H2O | → CoA-SH + 3 NADH + 3 H+ + FADH2 + GTP + 2 CO2 |
| Combining the reactions occurring during the pyruvate oxidation with those occurring during the citric acid cycle, the following overall pyruvate oxidation reaction is obtained: | Pyruvic acid + 4 NAD+ + FAD + GDP + Pi + 2 H2O | → 4 NADH + 4 H+ + FADH2 + GTP + 3 CO2 |
| Combining the above reaction with the ones occurring in the course of glycolysis, the following overall glucose oxidation reaction (excluding reactions in the respiratory chain) is obtained: | Glucose + 10 NAD+ + 2 FAD + 2 ADP + 2 GDP + 4 Pi + 2 H2O | → 10 NADH + 10 H+ + 2 FADH2 + 2 ATP + 2 GTP + 6 CO2 |
(the above reactions are equilibrated if Pi represents the H2PO4- ion, ADP and GDP the ADP2- and GDP2- ions, respectively, and ATP and GTP the ATP3- and GTP3- ions, respectively).
Considering the future conversion of GTP to ATP and the maximum 32 ATP produced by the 10 NADH and the 2 FADH2 (see the theoretical yields for cellular respiration), it follows that each glucose molecule is able to produce a maximum of 32 ATP.
Regulation
Although pyruvate dehydrogenase is not technically a part of the citric acid cycle, its regulation is included here.
The regulation of the TCA cycle is largely determined by substrate availability and product inhibition. NADH, a product of all dehydrogenases in the TCA cycle with the exception of succinate dehydrogenase, inhibits pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, and also citrate synthase. Acetyl-CoA inhibits pyruvate dehydrogenase, while succinyl-CoA inhibits succinyl-CoA synthase and citrate synthase. When tested in vitro with TCA enzymes, ATP inhibits citrate synthase and α-ketoglutarate dehydrogenase; however, ATP levels do not change more than 10% in vivo between rest and vigorous exercise. There is no known allosteric mechanism that can account for large changes in reaction rate from an allosteric effector whose concentration changes less than 10% [5].
Calcium is used as a regulator. It activates pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.[6] This increases the reaction rate of many of the steps in the cycle, and therefore increases flux throughout the pathway.
Citrate is used for feedback inhibition, as it inhibits phosphofructokinase, an enzyme involved in glycolysis that catalyses formation of fructose 1,6-bisphosphate, a precursor of pyruvate. This prevents a constant high rate of flux when there is an accumulation of citrate and a decrease in substrate for the enzyme.
Recent work has demonstrated an important link between intermediates of the citric acid cycle and the regulation of hypoxia inducible factors (HIF). HIF plays a role in the regulation of oxygen haemostasis, and is a transcription factor which targets angiogenesis, vascular remodelling, glucose ulitisation, iron transport and apoptosis. HIF is synthesized consititutively and hydroxylation of at least one of two critical proline residues mediates their interation with the von Hippel Lindau E3 ubiquitin ligase complex which targets them for rapid degradation. This reaction is calalysed by prolyl 4-hydroxylases. Fumarate and succinate have been identified as potent inhibitors of prolyl hydroxylases thus leading to the stabilisation of HIF.[7]
Major metabolic pathways converging on the TCA cycle
Most of the body's catabolic pathways converge on the TCA cycle, as the diagram shows. Reactions that form intermediates of the TCA cycle in order to replenish them (especially during the scarcity of the intermediates) are called anaplerotic reactions.
The citric acid cycle is the third step in carbohydrate catabolism (the breakdown of sugars). Glycolysis breaks glucose (a six-carbon-molecule) down into pyruvate (a three-carbon molecule). In eukaryotes, pyruvate moves into the mitochondria. It is converted into acetyl-CoA by decarboxylation and enters the citric acid cycle.
In protein catabolism, proteins are broken down by protease enzymes into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to Acetyl-CoA and entering into the citric acid cycle.
In fat catabolism, triglycerides are hydrolyzed to break them into fatty acids and glycerol. In the liver the glycerol can be converted into glucose via dihydroxyacetone phosphate and glyceraldehyde-3-phosphate by way of gluconeogenesis. In many tissues, especially heart tissue, fatty acids are broken down through a process known as beta oxidation which results in acetyl-CoA which can be used in the citric acid cycle. Sometimes beta oxidation can yield propionyl CoA which can result in further glucose production by gluconeogenesis in the liver.
The citric acid cycle is always followed by oxidative phosphorylation. This process extracts the energy (as electrons) from NADH and FADH2, oxidizing them to NAD+ and FAD, respectively, so that the cycle can continue. Whereas the citric acid cycle does not use oxygen, oxidative phosphorylation does.
The total energy gained from the complete breakdown of one molecule of glucose by glycolysis, the citric acid cycle and oxidative phosphorylation equals about 36 ATP molecules. The citric acid cycle is called an amphibolic pathway because it participates in both catabolism and anabolism.
See also
- Calvin cycle
- Oxidative decarboxylation
- Citric acid
- Glycolysis
- Pyruvate decarboxylation
- Oxidative phosphorylation
- Reverse (Reductive) Krebs cycle
- Glyoxylate cycle
- Hans Adolf Krebs
References
- ↑ 1.0 1.1 1.2 1.3 Berg, JM (2002). Biochemistry - 5th Edition. WH Freeman and Company. pp. 465–484, 498–501. ISBN 0-7167-4684-0. Unknown parameter
|coauthors=ignored (help) - ↑ 2.0 2.1 Buchanan (2000). Biochemistry & molecular biology of plants (1st Edition ed.). American society of plant physiology. ISBN 0-943088-39-9. Unknown parameter
|coauthors=ignored (help) - ↑ Johnson JD, Mehus JG, Tews K, Milavetz BI, Lambeth DO (1998). "Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes". J Biol Chem. 273 (42): 27580–6.
- ↑ Wolfe RR, Jahoor F. (1990) Recovery of labeled CO2 during the infusion of C-1- vs C-2-labeled acetate: implications for tracer studies of substrate oxidation. Am J Clin Nutr. 51(2):248-52. PMID 2106256
- ↑ Voet, D. & Voet, J. G. (2004) Biochemistry 3rd Edition (John Wiley & Sons, Inc., New York) p. 615
- ↑ Denton RM (1975). "Regulation of mammalian pyruvate dehydrogenase". Mol Cell Biochem. 9 (1): 27–53. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J (2007). "Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF". J. Biol. Chem. 282 (7): 4524–32. PMID 17182618.
Additional Resources
- Neil A. Campbell (2005). Biology (7th ed. ed.). Benjamin Cummings. ISBN 978-0805371468. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - Solomon, E.P. (2005). Biology. Brooks Cole. ISBN 978-0534495480. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help); Unknown parameter|editions=ignored (help)
External links
- An animation of the citric acid cycle at Smith College
- A video of members of The Ohio State Marching Band enacting the Krebs cycle at YouTube
- Notes on citric acid cycle at rahulgladwin.com
- A more detailed tutorial animation at johnkyrk.com
- A citric-acid cycle self quiz flash applet at University of Pittsburgh
- The chemical logic behind the citric acid cycle at ufp.pt
ar:دورة حمض الستريك bg:Цикъл на Кребс cs:Citrátový cyklus da:Citronsyrecyklus de:Citratzyklus eo:Ciklo de Krebs ko:TCA 회로 hr:Krebsov ciklus it:Ciclo di Krebs he:מעגל קרבס lv:Trikarbonskābju cikls lb:Zitratzyklus lt:Krebso ciklas hu:Citromsavciklus mk:Кребсов циклус nl:Citroenzuurcyclus no:Sitronsyresyklus oc:Cicle de Krebs ps:د سېټريک اسيد څرخ sk:Krebsov cyklus sl:Krebsov cikel sr:Кребсов циклус su:Daur asam sitrat fi:Sitruunahappokierto sv:Citronsyracykeln tl:Pag-ikot ng asido sitriko