Catecholamine



Catecholamines are sympathomimetic[1] "fight-or-flight" hormones released by the adrenal glands in response to stress.[2] They are part of the sympathetic nervous system.
They are called catecholamines because they contain a catechol or 3,4-dihydroxylphenyl group. They are derived from the amino acid tyrosine.[3]
In the human body, the most abundant catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine, all of which are produced from phenylalanine and tyrosine. Various stimulant drugs are catecholamine analogs.
Catecholamines are water-soluble and are 50% bound to plasma proteins, so they circulate in the bloodstream.
Tyrosine is created from phenylalanine by hydroxylation by the enzyme phenylalanine hydroxylase. (Tyrosine is also ingested directly from dietary protein). It is then sent to catecholamine-secreting neurons. Here, several reactions serially convert tyrosine to L-DOPA, to dopamine, to norepinephrine, and eventually to epinephrine.[4]
Structure
Catecholamines have the distinct structure of a benzene ring with two hydroxyl groups, an intermediate ethyl chain, and a terminal amine group. Phenylethanolamines such as norepinephrine have a hydroxyl group on the ethyl chain.
Production and degradation
Location
Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system. Dopamine, which acts as a neurotransmitter in the central nervous system, is largely produced in neuronal cell bodies in two areas of the brainstem: the substantia nigra and the ventral tegmental area. The similarly melanin-pigmented cell bodies of the locus ceruleus produce norepinephrine.
Synthesis
Dopamine is the first catecholamine synthesized from DOPA. In turn, norepinephrine and epinephrine are derived from further metabolic modification of dopamine. The enzyme dopamine hydroxylase requires copper as a cofactor (not shown) and DOPA decarboxylase requires PLP (not shown). The rate limiting step in catecholamine biosynthesis is hydroxylation of tyrosine.
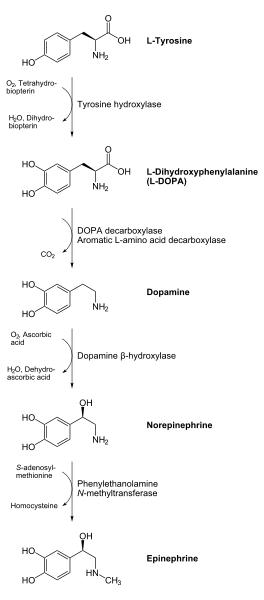
Catecholamine synthesis is inhibited by alpha-methyl-p-tyrosine (AMPT), which inhibits tyrosine hydroxylase. [5]
Degradation
Catecholamines have a half-life of a few minutes when circulating in the blood. They can be degraded either by methylation by catechol-O-methyltransferases (COMT) or by deamination by monoamine oxidases (MAO).
Amphetamines and MAOIs bind to MAO in order to inhibit its action of breaking down catecholamines. This is the primary reason why the effects of amphetamines have a longer lifespan than those of cocaine and other substances. Amphetamines not only cause a release of dopamine, epinephrine, and norepinephrine into the blood stream but also suppress re-absorption.
Function
Modality
Two catecholamines, norepinephrine and dopamine, act as neuromodulators in the central nervous system and as hormones in the blood circulation. The catecholamine norepinephrine is a neuromodulator of the peripheral sympathetic nervous system but is also present in the blood (mostly through "spillover" from the synapses of the sympathetic system).
High catecholamine levels in blood are associated with stress, which can be induced from psychological reactions or environmental stressors such as elevated sound levels, intense light, or low blood sugar levels.
Extremely high levels of catecholamines (also known as catecholamine toxicity) can occur in central nervous system trauma due to stimulation and/or damage of nuclei in the brainstem, in particular those nuclei affecting the sympathetic nervous system. In emergency medicine, this occurrence is widely known as catecholamine dump.
Extremely high levels of catecholamine can also be caused by neuroendocrine tumors in the adrenal medulla, a treatable condition known as pheochromocytoma.
High levels of catecholamines can also be caused by monoamine oxidase A deficiency. This is one of the enzymes responsible for degradation of these neurotransmitters and thus increases the bioavailability of them considerably. It occurs in the absence of pheochromocytoma, neuroendocrine tumors, and carcinoid syndrome, but it looks similar to carcinoid syndrome such as facial flushing and aggression.[6][7]
Effects
Catecholamines cause general physiological changes that prepare the body for physical activity (fight-or-flight response). Some typical effects are increases in heart rate, blood pressure, blood glucose levels, and a general reaction of the sympathetic nervous system. Some drugs, like tolcapone (a central COMT-inhibitor), raise the levels of all the catecholamines.
Function in plants
"They have been found in 44 plant families, but no essential metabolic function has been established for them. They are precursors of benzo[c]phenanthridine alkaloids, which are the active principal ingredients of many medicinal plant extracts. CAs have been implicated to have a possible protective role against insect predators, injuries, and nitrogen detoxification. They have been shown to promote plant tissue growth, somatic embryogenesis from in vitro cultures, and flowering. CAs inhibit indole-3-acetic acid oxidation and enhance ethylene biosynthesis. They have also been shown to enhance synergistically various effects of gibberellins."[8]
See also
References
- ↑ Template:DorlandsDict
- ↑ University of California, San Diego, Health Library, catecholamines.
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 137–8. ISBN 978-0-87893-697-7.
- ↑ Joh TH, Hwang O (1987). "Dopamine beta-hydroxylase: biochemistry and molecular biology". Annals of the New York Academy of Sciences. 493: 342–50. doi:10.1111/j.1749-6632.1987.tb27217.x. PMID 3473965.
- ↑ wrongdiagnosis.com - Description of Alpha-Methyltyrosine
- ↑ Manor I, Tyano S, Mel E; et al. (2002). "Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA)". Molecular Psychiatry. 7 (6): 626–32. doi:10.1038/sj.mp.4001037. PMID 12140786.
- ↑ Brunner HG (1996). "MAOA deficiency and abnormal behaviour: perspectives on an association". Ciba Foundation Symposium. 194: 155–64, discussion 164–7. PMID 8862875.
- ↑ A. I. Kuklin and B. V. Conger, "Catecholamines in plants," Journal of Plant Growth Regulation, Springer New York ISSN 0721-7595 (Print), ISSN 1435-8107 (Online), Issue Volume 14, Number 2 / June, 1995 DOI 10.1007/BF00203119, pp. 91-97.
External links
- Catecholamines at the US National Library of Medicine Medical Subject Headings (MeSH)
Template:Neurotransmitter metabolism intermediates
bg:Катехоламини ca:Catecolamina cs:Katecholamin de:Katecholamine eu:Katekolamina is:Katekólamín it:Catecolamina mk:Катехоламини nl:Catecholamine no:Katekolaminer sk:Katecholamín sl:Kateholamini fi:Katekoliamiini sv:Katekolamin uk:Катехоламіни