Cannabinoid receptor: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (→Cardiovascular activity: task, replaced: journal = J Am Coll Cardiol. → journal = J Am Coll Cardiol using AWB) |
||
| Line 1: | Line 1: | ||
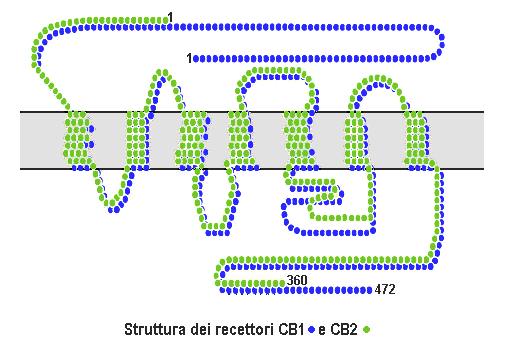
[[Image:Cb1 cb2 structure.png|thumb| | [[Image:Cb1 cb2 structure.png|thumb|237px|CB<sub>1</sub> and CB<sub>2</sub> structures.]] | ||
{{Infobox protein | |||
{{protein | | Name = [[cannabinoid receptor 1 (brain)]] | ||
|Name=[[cannabinoid receptor 1 (brain)]] | | image = WikiMedia_CB1_File.png | ||
| width = | |||
|image= | | caption = [[Protein nuclear magnetic resonance spectroscopy|NMR]] solution structure of a peptide mimetic of the fourth cytoplasmic loop of the CB<sub>1</sub> cannabinoid receptor based on the {{PDB|2b0y}} coordinates. | ||
|width= | | Symbol = CNR1 | ||
| | | AltSymbols = CNR | ||
|Symbol=CNR1 | | IUPHAR_id = | ||
|AltSymbols=CNR | | EntrezGene = 1268 | ||
|EntrezGene=1268 | | HGNCid = 2159 | ||
|OMIM=114610 | | OMIM = 114610 | ||
|RefSeq=NM_033181 | | HomoloGene = 7273 | ||
|UniProt=P21554 | | PDB = | ||
| RefSeq = NM_033181 | |||
|ECnumber= | | UniProt = P21554 | ||
|Chromosome=6 | | ECnumber = | ||
|Arm=q | | Chromosome = 6 | ||
|Band=14 | | Arm = q | ||
|LocusSupplementaryData=-q15 | | Band = 14 | ||
| LocusSupplementaryData = -q15 | |||
}} | }} | ||
{{protein | {{Infobox protein | ||
|Name=[[cannabinoid receptor 2 (macrophage)]] | | Name = [[cannabinoid receptor 2 (macrophage)]] | ||
| image = | |||
|image= | | width = | ||
|width= | | caption = | ||
| | | Symbol = CNR2 | ||
|Symbol=CNR2 | | AltSymbols = | ||
|AltSymbols= | | IUPHAR_id = | ||
|EntrezGene=1269 | | EntrezGene = 1269 | ||
|OMIM=605051 | | HGNCid = 2160 | ||
|RefSeq=NM_001841 | | OMIM = 605051 | ||
|UniProt=P34972 | | HomoloGene = 1389 | ||
| PDB = | |||
|ECnumber= | | RefSeq = NM_001841 | ||
|Chromosome=1 | | UniProt = P34972 | ||
|Arm=p | | ECnumber = | ||
|Band= | | Chromosome = 1 | ||
|LocusSupplementaryData= | | Arm = p | ||
| Band = | |||
| LocusSupplementaryData = | |||
}} | }} | ||
{{ | {{Cannabis sidebar}} | ||
'''Cannabinoid receptors''', located throughout the body, are part of the [[endocannabinoid system]], which is involved in a variety of physiological processes including [[appetite]], [[nociception|pain-sensation]], [[Mood (psychology)|mood]], and [[memory]].<ref>{{Cite journal|last=Aizpurua-Olaizola|first=Oier|last2=Elezgarai|first2=Izaskun|last3=Rico-Barrio|first3=Irantzu|last4=Zarandona|first4=Iratxe|last5=Etxebarria|first5=Nestor|last6=Usobiaga|first6=Aresatz|title=Targeting the endocannabinoid system: future therapeutic strategies|url=http://www.sciencedirect.com/science/article/pii/S1359644616302926|journal=Drug Discovery Today|doi=10.1016/j.drudis.2016.08.005|pmid=27554802|year=2016}}</ref> | |||
Cannabinoid receptors are of a class of [[cell membrane]] [[Receptor (biochemistry)|receptor]]s in the [[G protein-coupled receptor]] superfamily.<ref name="pmid12432948"/><ref name="pmid18426493"/><ref name="pmid19273110"/> As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains.<ref name="pmid7556170"/> Cannabinoid receptors are activated by three major groups of [[ligand (biochemistry)|ligands]]: [[endocannabinoids]], produced by the [[mammillary body]]; plant [[cannabinoids]] (such as [[cannabidiol]], produced by the [[cannabis]] plant); and [[Chemical synthesis|synthetic]] cannabinoids (such as [[HU-210]]). All of the endocannabinoids and plant cannabinoids are [[lipophilic]], such as fat soluble compounds. | |||
There are currently two known subtypes of cannabinoid receptors, termed [[cannabinoid receptor type 1|CB<sub>1</sub>]] and [[cannabinoid receptor type 2|CB<sub>2</sub>]].<ref name="pmid2165569"/><ref name="pmid1718258"/> The CB<sub>1</sub> receptor is expressed mainly in the [[human brain|brain]] ([[central nervous system]] or "CNS"), but also in the [[lung]]s, [[liver]] and [[kidney]]s. The CB<sub>2</sub> receptor is expressed mainly in the [[immune system]] and in [[Pluripotential hemopoietic stem cell|hematopoietic cells]].<ref name="pmid21295074">{{cite journal |vauthors=Pacher P, Mechoulam R | title = Is lipid signaling through cannabinoid 2 receptors part of a protective system? | journal = Prog Lipid Res. | year = 2011 | pmid =21295074 | doi = 10.1016/j.plipres.2011.01.001 | volume = 50 | issue = 2 | pages = 193–211 | pmc = 3062638 }}</ref> Mounting evidence suggests that there are novel cannabinoid receptors<ref name="pmid15866316"/> that is, non-CB<sub>1</sub> and non-CB<sub>2</sub>, which are expressed in [[endothelial]] cells and in the CNS. In 2007, the binding of several cannabinoids to the G protein-coupled receptor [[GPR55]] in the brain was described.<ref name="pmid17876302"/> | |||
The | The protein sequences of CB<sub>1</sub> and CB<sub>2</sub> receptors are about 44% similar.<ref name=latek>{{Cite journal | ||
| pmid = 21365223 | |||
| year = 2011 | |||
| author1 = Latek | |||
| first1 = D | |||
| title = Modeling of ligand binding to G protein coupled receptors: Cannabinoid CB1, CB2 and adrenergic β 2 AR | |||
| journal = Journal of Molecular Modeling | |||
| volume = 17 | |||
| issue = 9 | |||
| pages = 2353–66 | |||
| last2 = Kolinski | |||
| first2 = M | |||
| last3 = Ghoshdastider | |||
| first3 = U | |||
| last4 = Debinski | |||
| first4 = A | |||
| last5 = Bombolewski | |||
| first5 = R | |||
| last6 = Plazinska | |||
| first6 = A | |||
| last7 = Jozwiak | |||
| first7 = K | |||
| last8 = Filipek | |||
| first8 = S | |||
| doi = 10.1007/s00894-011-0986-7 | |||
}}</ref><ref name="pmid7689702"/> When only the transmembrane regions of the receptors are considered, amino acid similarity between the two receptor subtypes is approximately 68%.<ref name="pmid7556170"/> In addition, minor variations in each receptor have been identified. Cannabinoids bind reversibly and [[stereochemistry|stereo-selectively]] to the cannabinoid receptors. Subtype selective cannabinoids have been developed which theoretically may have advantages for treatment of certain diseases such as obesity.<ref name="pmid17148745">{{cite journal |vauthors=Kyrou I, Valsamakis G, Tsigos C | title = The endocannabinoid system as a target for the treatment of visceral obesity and metabolic syndrome | journal = Ann. N. Y. Acad. Sci. | volume = 1083 | issue = | pages = 270–305 |date=November 2006 | pmid = 17148745 | doi = 10.1196/annals.1367.024 }}</ref> | |||
It appears that cannabinoid receptors are unique to the [[phylum]] [[Chordata]] and, as such, they have a rather restricted [[phylogenetic]] distribution in the animal kingdom. However, enzymes involved in biosynthesis/inactivation of [[endocannabinoid]]s and endocannabinoid signalling in general (involving targets other than CB1/2-type receptors) occur throughout the animal kingdom.<ref>{{citation | author1=Maurice R. Elphick | title=The evolution and comparative neurobiology of endocannabinoid signalling | journal=Philosophical Transactions of the Royal Society of London B | volume=367(1607) | year=2012 | pages=3201–3215 | doi=10.1098/rstb.2011.0394}}</ref> | |||
==CB<sub>1</sub>== | |||
{{Main|Cannabinoid receptor type 1}} | |||
Cannabinoid receptor type 1 (CB<sub>1</sub>) receptors are thought to be one of the most widely [[Gene expression|expressed]] G<sub>αi</sub> protein-coupled receptors in the brain. This is due to endocannabinoid-mediated [[depolarization-induced suppression of inhibition]], a very common form of [[retrograde signaling]], in which the depolarization of a single neuron induces a reduction in [[GABA]]-mediated neurotransmission. Endocannabinoids released from the depolarized post-synaptic neuron bind to CB<sub>1</sub> receptors in the pre-synaptic neuron and cause a reduction in GABA release. | |||
They are also found in other parts of the body. For instance, in the liver, activation of the CB<sub>1</sub> receptor is known to increase de novo [[lipogenesis]].<ref name="pmid15864349"/> | |||
A 2004 study suggested that the endocannabinoids and their cannabinoid receptors play a major role during pre- and postnatal development.<ref>{{cite journal|url = http://www.sciencedirect.com/science/article/pii/S0014299904007423 | doi=10.1016/j.ejphar.2004.07.033 | volume=500 | title=The endocannabinoid-CB1 receptor system in pre- and postnatal life | year=2004 | journal=European Journal of Pharmacology | pages=289–297 | vauthors=Fride E}}</ref><ref>[http://www.nel.edu/pdf_/25_12/NEL251204A01_Fride_.pdf The Endocannabinoid-CB Receptor System: Importance for development and in pediatric disease] Neuroendocrinology Letters Nos.1/2, Feb-Apr Vol.25, 2004.</ref> In another recent study a group of researchers combined stochastic optical reconstruction microscopy (STORM) and [[patch clamp]] in order to see CB1 distribution on a nano scale with incredible resolution.<ref>{{cite journal | doi = 10.1038/nn.3892 | title = Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling | volume=18 | journal=Nature Neuroscience | pages=75–86 | pmid=25485758 | pmc=4281300 | vauthors=Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R, Varga C, Lee SH, Matolcsi M, Cervenak J, Kacskovics I, Watanabe M, Sagheddu C, Melis M, Pistis M, Soltesz I, Katona I | year=2014}}</ref><ref>Cannabinoids take the brain by STORM (Summary in [http://www.sciguru.org/newsitem/18095/cannabinoids-take-brain-storm SciGuru Science News])</ref> | |||
== | ==CB<sub>2</sub>== | ||
{{Main|Cannabinoid receptor type 2}} | |||
[[Cannabinoid receptor 2 (macrophage)|CB<sub>2</sub>]] receptors are mainly expressed on [[T cell]]s of the [[immune system]], on [[macrophage]]s and [[B cell]]s, and in [[Pluripotential hemopoietic stem cell|hematopoietic cells]]. They also have a function in [[keratinocyte]]s. They are also expressed on peripheral [[nerve]] terminals. These receptors play a role in [[antinociception]], or the relief of [[pain]]. In the brain, they are mainly expressed by [[Microglia|microglial cells]], where their role remains unclear. While the most likely cellular targets and executors of the CB<sub>2</sub> receptor-mediated effects of endocannabinoids or synthetic agonists are the immune and immune-derived cells (e.g. leukocytes, various populations of T and B lymphocytes, monocytes/macrophages, dendritic cells, mast cells, microglia in the brain, Kupffer cells in the liver, astrocytes, etc.), the number of other potential cellular targets is expanding, now including endothelial and smooth muscle cells, fibroblasts of various origins, cardiomyocytes, and certain neuronal elements of the peripheral or central nervous systems.<ref name="pmid21295074"/> | |||
[[Cannabinoid receptor 2 (macrophage)|CB<sub>2</sub>]] receptors are mainly expressed on [[T cell]]s of the [[immune system]], on [[macrophage]]s and [[B cell]]s, and in [[Pluripotential hemopoietic stem cell|hematopoietic cells]]. They also have a function in [[keratinocyte]]s | |||
==Other cannabinoid receptors== | |||
The existence of additional cannabinoid receptors has long been suspected, due to the actions of compounds such as [[abnormal cannabidiol]] that produce cannabinoid-like effects on [[blood pressure]] and [[inflammation]], yet do not activate either CB<sub>1</sub> or CB<sub>2</sub>.<ref name="pmid10570211"/><ref name="pmid17965195"/> Recent research strongly supports the hypothesis that the ''N''-arachidonoyl glycine ([[NAGly]]) receptor [[GPR18]] is the molecular identity of the abnormal cannabidiol receptor and additionally suggests that NAGly, the endogenous lipid metabolite of [[anandamide]] (also known as arachidonoylethanolamide or AEA), initiates directed [[Microglia#Chemokines|microglial migration]] in the CNS through activation of [[GPR18]].<ref name="pmid20346144"/> Other molecular biology studies have suggested that the orphan receptor [[GPR55]] should in fact be characterised as a cannabinoid receptor, on the basis of sequence homology at the binding site. Subsequent studies showed that GPR55 does indeed respond to cannabinoid ligands.<ref name="pmid17876302"/><ref name="pmid17704827"/> This profile as a distinct non-CB<sub>1</sub>/CB<sub>2</sub> receptor that responds to a variety of both endogenous and exogenous cannabinoid ligands, has led some groups to suggest GPR55 should be categorized as the CB<sub>3</sub> receptor, and this re-classification may follow in time.<ref name="pmid16517404"/> However this is complicated by the fact that another possible cannabinoid receptor has been discovered in the [[hippocampus]], although its gene has not yet been cloned,<ref name="pmid18482429"/> suggesting that there may be at least two more cannabinoid receptors to be discovered, in addition to the two that are already known. [[GPR119]] has been suggested as a fifth possible cannabinoid receptor.<ref name="pmid17906678"/> | |||
==Signaling== | ==Signaling== | ||
Cannabinoid receptors are activated by cannabinoids, generated naturally inside the body ([[Endocannabinoids | Cannabinoid receptors are activated by cannabinoids, generated naturally inside the body ([[Cannabinoid#Endocannabinoids|endocannabinoids]]) or introduced into the body as [[cannabis (drug)|cannabis]] or a related [[Chemical synthesis|synthetic]] compound.<ref name=latek /> | ||
After the receptor is engaged, multiple [[intracellular]] [[signal transduction]] pathways are activated. At first, it was thought that cannabinoid receptors mainly inhibited the [[enzyme]] [[adenylate cyclase]] (and thereby the production of the [[second messenger]] molecule [[cyclic AMP]]), and positively influenced [[Inward-rectifier potassium ion channel|inwardly rectifying potassium channels]] (=Kir or IRK).<ref name="pmid16109430" | After the receptor is engaged, multiple [[intracellular]] [[signal transduction]] pathways are activated. At first, it was thought that cannabinoid receptors mainly inhibited the [[enzyme]] [[adenylate cyclase]] (and thereby the production of the [[second messenger]] molecule [[cyclic AMP]]), and positively influenced [[Inward-rectifier potassium ion channel|inwardly rectifying potassium channels]] (=Kir or IRK).<ref name="pmid16109430"/> However, a much more complex picture has appeared in different cell types, implicating other [[potassium ion channels]], [[calcium channel]]s, [[protein kinase A]] and [[protein kinase C|C]], [[C-Raf|Raf-1]], [[Extracellular signal-regulated kinases|ERK]], [[JNK]], [[p38 mitogen-activated protein kinases|p38]], [[c-fos]], [[c-jun]] and many more.<ref name="pmid16109430"/> | ||
Separation between the therapeutically undesirable psychotropic effects, and the clinically desirable ones however, has not been reported with [[agonists]] that bind to cannabinoid receptors. THC, as well as the two major [[endogenous]] compounds identified so far that bind to the cannabinoid receptors —[[anandamide]] and [[2-arachidonylglycerol]] (2-AG)— produce most of their effects by binding to both the CB<sub>1</sub> and CB<sub>2</sub> cannabinoid receptors. While the effects mediated by CB<sub>1</sub>, mostly in the | Separation between the therapeutically undesirable psychotropic effects, and the clinically desirable ones, however, has not been reported with [[agonists]] that bind to cannabinoid receptors. [[THC]], as well as the two major [[endogenous]] compounds identified so far that bind to the cannabinoid receptors —[[anandamide]] and [[2-arachidonylglycerol]] (2-AG)— produce most of their effects by binding to both the CB<sub>1</sub> and CB<sub>2</sub> cannabinoid receptors. While the effects mediated by CB<sub>1</sub>, mostly in the central nervous system, have been thoroughly investigated, those mediated by CB<sub>2</sub> are not equally well defined. | ||
==Physiology== | ==Physiology== | ||
===Cardiovascular activity=== | ===Cardiovascular activity=== | ||
Studies have suggested that activation of CB<sub>1</sub> receptors in human and rodent cardiomyocytes,<ref name="pmid17678736">{{cite journal |vauthors=Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P | title = Pharmacological Inhibition of CB1 Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity | journal = J Am Coll Cardiol| year = 2007 | pmid =17678736 | doi = 10.1016/j.jacc.2007.03.057 | volume = 50 | issue = 6 | pages = 528–36 | pmc = 2239316 }}</ref><ref name="pmid19942623">{{cite journal |vauthors=Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P | title = CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes | journal = Cardiovasc Res.| year = 2010 | pmid =19942623 | doi = 10.1093/cvr/cvp369 | volume = 85 | issue = 4 | pages = 773–784 | pmc = 2819835 }}</ref> coronary artery endothelial and inflammatory cells<ref name="pmid19596672">{{cite journal |vauthors=Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ | title = CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages | journal = Cardiovasc Res.| year = 2009 | pmid =19596672 | volume = 84 | issue = 3 | pages = 378–86 | doi = 10.1093/cvr/cvp240 }}</ref><ref name="pmid19103987">{{cite journal |vauthors=Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H | title = Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages | journal = Circulation| year = 2009 | pmid =19103987 | doi = 10.1161/CIRCULATIONAHA.108.811992 | volume = 119 | pages = 28–36 | issue = 1 }}</ref><ref name="pmid21070851">{{cite journal |vauthors=Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Haskó G, Pacher P | title = Fatty acid amide hydrolase is a key regulator of the endocannabinoid-induced myocardial tissue injury | journal = Free Radic Biol Med.| year = 2011 | pmid =21070851 | doi = 10.1016/j.freeradbiomed.2010.11.002 | volume = 50 | pages = 179–195 | issue = 1 | pmc = 3022384 }}</ref> promotes activation of mitogen-activated protein (MAP) kinases p38 and JNK, reactive oxygen species generation, cell death, and cardiovascular inflammatory response both in vitro, as well as in models of heart failure, atherosclerosis and vascular inflammation.<ref name="pmid17678736"/><ref name="pmid19942623"/><ref name="pmid19103987"/><ref name="pmid21070851"/> | |||
==Cannabinoid treatments== | |||
{{main|Medical cannabis}} | |||
Synthetic [[tetrahydrocannabinol]] (THC) is prescribed under the [[International Nonproprietary Name|INN]] ''dronabinol'' or the brand name ''Marinol'', to treat [[vomiting]] and for enhancement of [[appetite]], mainly in people [[AIDS]] as well as for refractory [[nausea]] and [[vomiting]] in people undergoing [[chemotherapy]].<ref>{{cite journal|last1=Badowski|first1=ME|title=A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics.|journal=Cancer chemotherapy and pharmacology|date=5 August 2017|doi=10.1007/s00280-017-3387-5|pmid=28780725|pmc=5573753}}</ref> THC is also an [[active pharmaceutical ingredient|active ingredient]] in [[nabiximols]], a specific extract of ''[[Cannabis]]'' that was approved as a [[botanical drug]] in the United Kingdom in 2010 as a mouth spray for people with [[multiple sclerosis]] to alleviate [[neuropathic pain]], [[spasticity]], [[overactive bladder]], and other symptoms.<ref>{{cite web|title=Sativex Oromucosal Spray - Summary of Product Characteristics|url=http://www.medicines.org.uk/emc/medicine/23262|publisher=UK Electronic Medicines Compendium|language=en|date=March 2015}}</ref> | |||
== Ligands == | |||
:''See also: [[Cannabinoid receptor type 1#Ligands|cannabinoid receptor type 1 ligands]], [[Cannabinoid receptor type 2#Ligands|cannabinoid receptor type 2 ligands]] | |||
=== Binding affinity and selectivity of cannabinoid ligands === | |||
{| class="wikitable sortable" style="font-size: smaller; text-align: center; width: auto;" | |||
|- | |||
! style="width: 12em"| | |||
! CB<sub>1</sub> affinity (K<sub>i</sub>) | |||
! Efficacy towards CB<sub>1</sub> | |||
! CB<sub>2</sub> affinity (K<sub>i</sub>) | |||
! Efficacy towards CB<sub>2</sub> | |||
! Type | |||
! References | |||
|- | |||
! '''[[Anandamide]]''' | |||
| 78nM | |||
| Full agonist | |||
| 370nM | |||
| ? | |||
| Endogenous | |||
| | |||
|- | |||
! [[N-Arachidonoyl dopamine]] | |||
| ? | |||
| Agonist | |||
| ? | |||
| ? | |||
| Endogenous | |||
| | |||
|- | |||
! [[2-Arachidonoylglycerol]] | |||
| ? | |||
| Full agonist | |||
| ? | |||
| ? | |||
| Endogenous | |||
| | |||
|- | |||
! [[2-Arachidonyl glyceryl ether]] | |||
| 21 nM | |||
| Full agonist | |||
| 480nM | |||
| Full agonist | |||
| Endogenous | |||
| | |||
|- | |||
! '''[[Δ-9-Tetrahydrocannabinol]]''' | |||
| 10nM | |||
| Partial agonist | |||
| 24nM | |||
| Partial agonist | |||
| Phytogenic | |||
| <ref name="whoa">{{cite web|title=PDSP Database - UNC|url=http://pdsp.med.unc.edu/pdsp.php?|accessdate=11 June 2013|deadurl=yes|archiveurl=https://web.archive.org/web/20131108013656/http://pdsp.med.unc.edu/pdsp.php|archivedate=8 November 2013|df=}}</ref><ref name="whoa"/> | |||
|- | |||
! [[EGCG]] | |||
| 33.6μM | |||
| Agonist | |||
| >50μM | |||
| ? | |||
| Phytogenic | |||
| | |||
|- | |||
! [[Yangonin]] | |||
| 0.72 μM | |||
| ? | |||
| > 10 μM | |||
| ? | |||
| Phytogenic | |||
| <ref>{{Cite journal | |||
| last1 = Ligresti | first1 = A. | |||
| last2 = Villano | first2 = R. | |||
| last3 = Allarà | first3 = M. | |||
| last4 = Ujváry | first4 = I. N. | |||
| last5 = Di Marzo | first5 = V. | |||
| title = Kavalactones and the endocannabinoid system: The plant-derived yangonin is a novel CB1 receptor ligand | |||
| doi = 10.1016/j.phrs.2012.04.003 | |||
| journal = Pharmacological Research | |||
| volume = 66 | |||
| issue = 2 | |||
| pages = 163–169 | |||
| year = 2012 | |||
| pmid = 22525682 | |||
| pmc = | |||
}}</ref> | |||
|- | |||
! [[AM-1221]] | |||
| 52.3nM | |||
| Agonist | |||
| 0.28nM | |||
| Agonist | |||
| Synthetic | |||
| <ref name="dude">{{Ref patent2 |country= WO |number= 200128557 |status= granted |title= Cannabimimetic indole derivatives |pubdate= 2001-04-26 |gdate= 2001-06-07 |pridate= 1999-10-18 |inventor= Makriyannis A, Deng H }}</ref> | |||
|- | |||
! [[AM-1235]] | |||
| 1.5nM | |||
| Agonist | |||
| 20.4nM | |||
| Agonist | |||
| Synthetic | |||
| <ref name="like">{{Ref patent2 | country = US | number = 7241799 | status = granted | title = Cannabimimetic indole derivatives | pubdate = 2004-11-05 | gdate = 2007-07-10 | pridate= 2004-11-05 | inventor = Makriyannis A, Deng H | assign1= }}</ref> | |||
|- | |||
! [[AM-2232]] | |||
| 0.28nM | |||
| Agonist | |||
| 1.48nM | |||
| Agonist | |||
| Synthetic | |||
| <ref name="like"/> | |||
|- | |||
! [[UR-144]] | |||
| 150nM | |||
| Full agonist | |||
| 1.8nM | |||
| Full agonist | |||
| Synthetic | |||
| <ref name="myhandsareamazing">{{cite journal |vauthors=Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD | title = Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity | journal = J. Med. Chem. | volume = 53 | issue = 1 | pages = 295–315 |date=January 2010 | pmid = 19921781 | doi = 10.1021/jm901214q }}</ref> | |||
|- | |||
! [[JWH-007]] | |||
| 9.0nM | |||
| Agonist | |||
| 2.94nM | |||
| Agonist | |||
| Synthetic | |||
| <ref name="Aung_2000">{{cite journal |vauthors=Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR | title = Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB<sub>1</sub> and CB<sub>2</sub> receptor binding | journal = Drug Alcohol Depend | volume = 60 | issue = 2 | pages = 133–40 |date=August 2000 | pmid = 10940540 | doi =10.1016/S0376-8716(99)00152-0 }}</ref> | |||
|- | |||
! [[JWH-015]] | |||
| 383nM | |||
| Agonist | |||
| 13.8nM | |||
| Agonist | |||
| Synthetic | |||
| <ref name="Aung_2000"/> | |||
|- | |||
! [[JWH-018]] | |||
| 9.00 ± 5.00 nM | |||
| Full agonist | |||
| 2.94 ± 2.65 nM | |||
| Full agonist | |||
| Synthetic | |||
| <ref name="Aung_2000"/> | |||
|- | |||
|} | |||
== See also == | |||
* [[Cannabinoid receptor antagonist]] | |||
* [[Endocannabinoid enhancer]] | |||
* [[Endocannabinoid reuptake inhibitor]] | |||
* [[Cannabidiol]] | |||
* [[Effects of cannabis]] | |||
==References== | |||
{{reflist|2|refs= | |||
<ref name="pmid12432948">{{cite journal | author = Howlett AC | title = The cannabinoid receptors | journal = Prostaglandins Other Lipid Mediat. | volume = 68–69 | issue = | pages = 619–31 |date=August 2002 | pmid = 12432948 | doi = 10.1016/S0090-6980(02)00060-6| url = }}</ref> | |||
<ref name="pmid18426493">{{cite journal | author = Mackie K | title = Cannabinoid receptors: where they are and what they do | journal = J. Neuroendocrinol. | volume = 20 Suppl 1 | issue = | pages = 10–4 |date=May 2008 | pmid = 18426493 | doi = 10.1111/j.1365-2826.2008.01671.x | url = }}</ref> | |||
<ref name="pmid19273110">{{cite journal |vauthors=Graham ES, Ashton JC, Glass M | title = Cannabinoid receptors: a brief history and "what's hot" | journal = Front. Biosci. | volume = 14 | issue = 14| pages = 944–57 | year = 2009 | pmid = 19273110 | doi = 10.2741/3288| url = }}</ref> | |||
<ref name="pmid7556170">{{cite journal |vauthors=Sylvaine G, Sophie M, Marchand J, Dussossoy D, Carriere D, Carayon P, Monsif B, Shire D, LE Fur G, Casellas P | title = Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations | journal = Eur. J. Biochem. | volume = 232 | issue = 1 | pages = 54–61 | year = 1995 | pmid = 7556170 | doi = 10.1111/j.1432-1033.1995.tb20780.x| url = }}</ref> | |||
<ref name="pmid2165569">{{cite journal |vauthors=Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI | title = Structure of a cannabinoid receptor and functional expression of the cloned cDNA | journal = Nature | volume = 346 | issue = 6284 | pages = 561–4 | year = 1990 | pmid = 2165569 | doi = 10.1038/346561a0 }}</ref> | |||
<ref name="pmid1718258">{{cite journal |vauthors=Gérard CM, Mollereau C, Vassart G, Parmentier M | title = Molecular cloning of a human cannabinoid receptor which is also expressed in testis | journal = Biochem. J. | volume = 279 | issue = Pt 1| pages = 129–34 | year = 1991 | pmid = 1718258 | doi = 10.1042/bj2790129| pmc = 1151556}}</ref> | |||
<ref name="pmid15866316">{{cite journal |vauthors=Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, Liu J, Kunos G | title = Evidence for novel cannabinoid receptors | journal = Pharmacol. Ther. | volume = 106 | issue = 2 | pages = 133–45 | year = 2005 | pmid = 15866316 | doi = 10.1016/j.pharmthera.2004.11.005 }}</ref> | |||
<ref name="pmid17876302">{{cite journal |vauthors=Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ | title = The orphan receptor GPR55 is a novel cannabinoid receptor | journal = Br. J. Pharmacol. | volume = 152 | issue = 7 | pages = 1092–1101 | year = 2007 | pmid = 17876302 | doi = 10.1038/sj.bjp.0707460 | pmc = 2095107 }}</ref> | |||
<ref name="pmid7689702">{{cite journal |vauthors=Munro S, Thomas KL, Abu-Shaar M | title = Molecular characterization of a peripheral receptor for cannabinoids | journal = Nature | volume = 365| issue = 6441 | pages = 61–65 | year = 1993 | pmid = 7689702 | doi = 10.1038/365061a0 }}</ref> | |||
<ref name="pmid15864349">{{cite journal |vauthors=Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G | title = Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity | journal = J. Clin. Invest. | volume = 115 | issue = 5 | pages = 1298–305 | year = 2005 | pmid = 15864349 | doi = 10.1172/JCI23057 | pmc = 1087161 }}</ref> | |||
<ref name="pmid10570211">{{cite journal |vauthors=Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G | title = Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 96 | issue = 24 | pages = 14136–41 |date=November 1999 | pmid = 10570211 | pmc = 24203 | doi = 10.1073/pnas.96.24.14136| url = }}</ref> | |||
<ref name="pmid17965195">{{cite journal |vauthors=McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA | title = Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2 | journal = Mol. Pharmacol. | volume = 73 | issue = 2 | pages = 441–50 |date=February 2008 | pmid = 17965195 | doi = 10.1124/mol.107.041863 | url = }}</ref> | |||
=== | <ref name="pmid20346144">{{cite journal |author1=McHugh D |author2=Hu SS-J |author3=Rimmerman N |author4=Juknat A |author5=Vogel Z |author6=Walker JM |author7=Bradshaw HB | title = N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor | journal = BMC Neuroscience | volume = 11 | pages = 44 |date=March 2010 | pmid = 20346144 | pmc = 2865488 | doi = 10.1186/1471-2202-11-44 | url = }}</ref> | ||
<ref name="pmid17704827">{{cite journal |vauthors=Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA | title = The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects | journal = Br. J. Pharmacol. | volume = 152 | issue = 5 | pages = 825–31 |date=November 2007 | pmid = 17704827 | pmc = 2190033 | doi = 10.1038/sj.bjp.0707419 | url = }}</ref> | |||
<ref name="pmid16517404">{{cite journal |vauthors=Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C | title = Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents | journal = Cell Metab. | volume = 3 | issue = 3 | pages = 167–75 |date=March 2006 | pmid = 16517404 | doi = 10.1016/j.cmet.2006.02.004 | url = }}</ref> | |||
<ref name="pmid18482429">{{cite journal | vauthors = de Fonseca FR, Schneider M | title = The endogenous cannabinoid system and drug addiction: 20 years after the discovery of the CB1 receptor | journal = Addict Biol | volume = 13 | issue = 2 | pages = 143–6 | date = June 2008 | pmid = 18482429 | doi = 10.1111/j.1369-1600.2008.00116.x | url = http://www.zi-mannheim.de/fileadmin/user_upload/redakteure/psychopharma/De_Fonseca_2008.pdf | deadurl = yes | archiveurl = https://web.archive.org/web/20110718033850/http://www.zi-mannheim.de/fileadmin/user_upload/redakteure/psychopharma/De_Fonseca_2008.pdf | archivedate = 2011-07-18 | df = }}</ref> | |||
<ref name=" | <ref name="pmid17906678">{{cite journal | author = Brown AJ | title = Novel cannabinoid receptors | journal = Br. J. Pharmacol. | volume = 152 | issue = 5 | pages = 567–75 |date=November 2007 | pmid = 17906678 | pmc = 2190013 | doi = 10.1038/sj.bjp.0707481 | url = }}</ref> | ||
== | <ref name="pmid16109430">{{cite journal |vauthors=Demuth DG, Molleman A | title = Cannabinoid signalling | journal = Life Sci. | volume = 78 | issue = 6 | pages = 549–63 | year = 2006 | pmid = 16109430 | doi = 10.1016/j.lfs.2005.05.055 }}</ref> | ||
}} | |||
==External links== | ==External links== | ||
*[ | * {{MeshName|Cannabinoid+Receptors}} | ||
* [https://web.archive.org/web/20061107223341/http://www.endocannabinoid.net/ The Endocannabinoid System Network (ECSN) - CB<sub>1</sub> receptor] | |||
* {{cite web | url = http://www.iuphar-db.org/GPCR/ChapterMenuForward?chapterID=1279 | title = Cannabinoid Receptors | accessdate = | date = | format = | work = IUPHAR Database of Receptors and Ion Channels | publisher = International Union of Basic and Clinical Pharmacology | pages = | language = | quote = }} | |||
*{{ | |||
{{G protein-coupled receptors}} | {{G protein-coupled receptors}} | ||
{{Cannabinoidergics}} | |||
{{DEFAULTSORT:Cannabinoid Receptor}} | |||
[[Category:G protein coupled receptors]] | [[Category:G protein coupled receptors]] | ||
Revision as of 22:50, 14 November 2017
| cannabinoid receptor 1 (brain) | |
|---|---|
| File:WikiMedia CB1 File.png | |
| Identifiers | |
| Symbol | CNR1 |
| Alt. symbols | CNR |
| Entrez | 1268 |
| HUGO | 2159 |
| OMIM | 114610 |
| Orthologs | 7273 |
| RefSeq | NM_033181 |
| UniProt | P21554 |
| Other data | |
| Locus | Chr. 6 q14-q15 |
| cannabinoid receptor 2 (macrophage) | |
|---|---|
| Identifiers | |
| Symbol | CNR2 |
| Entrez | 1269 |
| HUGO | 2160 |
| OMIM | 605051 |
| Orthologs | 1389 |
| RefSeq | NM_001841 |
| UniProt | P34972 |
| Other data | |
| Locus | Chr. 1 p |
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system, which is involved in a variety of physiological processes including appetite, pain-sensation, mood, and memory.[1]
Cannabinoid receptors are of a class of cell membrane receptors in the G protein-coupled receptor superfamily.[2][3][4] As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains.[5] Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids, produced by the mammillary body; plant cannabinoids (such as cannabidiol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and plant cannabinoids are lipophilic, such as fat soluble compounds.
There are currently two known subtypes of cannabinoid receptors, termed CB1 and CB2.[6][7] The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system and in hematopoietic cells.[8] Mounting evidence suggests that there are novel cannabinoid receptors[9] that is, non-CB1 and non-CB2, which are expressed in endothelial cells and in the CNS. In 2007, the binding of several cannabinoids to the G protein-coupled receptor GPR55 in the brain was described.[10]
The protein sequences of CB1 and CB2 receptors are about 44% similar.[11][12] When only the transmembrane regions of the receptors are considered, amino acid similarity between the two receptor subtypes is approximately 68%.[5] In addition, minor variations in each receptor have been identified. Cannabinoids bind reversibly and stereo-selectively to the cannabinoid receptors. Subtype selective cannabinoids have been developed which theoretically may have advantages for treatment of certain diseases such as obesity.[13]
It appears that cannabinoid receptors are unique to the phylum Chordata and, as such, they have a rather restricted phylogenetic distribution in the animal kingdom. However, enzymes involved in biosynthesis/inactivation of endocannabinoids and endocannabinoid signalling in general (involving targets other than CB1/2-type receptors) occur throughout the animal kingdom.[14]
CB1
Cannabinoid receptor type 1 (CB1) receptors are thought to be one of the most widely expressed Gαi protein-coupled receptors in the brain. This is due to endocannabinoid-mediated depolarization-induced suppression of inhibition, a very common form of retrograde signaling, in which the depolarization of a single neuron induces a reduction in GABA-mediated neurotransmission. Endocannabinoids released from the depolarized post-synaptic neuron bind to CB1 receptors in the pre-synaptic neuron and cause a reduction in GABA release.
They are also found in other parts of the body. For instance, in the liver, activation of the CB1 receptor is known to increase de novo lipogenesis.[15]
A 2004 study suggested that the endocannabinoids and their cannabinoid receptors play a major role during pre- and postnatal development.[16][17] In another recent study a group of researchers combined stochastic optical reconstruction microscopy (STORM) and patch clamp in order to see CB1 distribution on a nano scale with incredible resolution.[18][19]
CB2
CB2 receptors are mainly expressed on T cells of the immune system, on macrophages and B cells, and in hematopoietic cells. They also have a function in keratinocytes. They are also expressed on peripheral nerve terminals. These receptors play a role in antinociception, or the relief of pain. In the brain, they are mainly expressed by microglial cells, where their role remains unclear. While the most likely cellular targets and executors of the CB2 receptor-mediated effects of endocannabinoids or synthetic agonists are the immune and immune-derived cells (e.g. leukocytes, various populations of T and B lymphocytes, monocytes/macrophages, dendritic cells, mast cells, microglia in the brain, Kupffer cells in the liver, astrocytes, etc.), the number of other potential cellular targets is expanding, now including endothelial and smooth muscle cells, fibroblasts of various origins, cardiomyocytes, and certain neuronal elements of the peripheral or central nervous systems.[8]
Other cannabinoid receptors
The existence of additional cannabinoid receptors has long been suspected, due to the actions of compounds such as abnormal cannabidiol that produce cannabinoid-like effects on blood pressure and inflammation, yet do not activate either CB1 or CB2.[20][21] Recent research strongly supports the hypothesis that the N-arachidonoyl glycine (NAGly) receptor GPR18 is the molecular identity of the abnormal cannabidiol receptor and additionally suggests that NAGly, the endogenous lipid metabolite of anandamide (also known as arachidonoylethanolamide or AEA), initiates directed microglial migration in the CNS through activation of GPR18.[22] Other molecular biology studies have suggested that the orphan receptor GPR55 should in fact be characterised as a cannabinoid receptor, on the basis of sequence homology at the binding site. Subsequent studies showed that GPR55 does indeed respond to cannabinoid ligands.[10][23] This profile as a distinct non-CB1/CB2 receptor that responds to a variety of both endogenous and exogenous cannabinoid ligands, has led some groups to suggest GPR55 should be categorized as the CB3 receptor, and this re-classification may follow in time.[24] However this is complicated by the fact that another possible cannabinoid receptor has been discovered in the hippocampus, although its gene has not yet been cloned,[25] suggesting that there may be at least two more cannabinoid receptors to be discovered, in addition to the two that are already known. GPR119 has been suggested as a fifth possible cannabinoid receptor.[26]
Signaling
Cannabinoid receptors are activated by cannabinoids, generated naturally inside the body (endocannabinoids) or introduced into the body as cannabis or a related synthetic compound.[11]
After the receptor is engaged, multiple intracellular signal transduction pathways are activated. At first, it was thought that cannabinoid receptors mainly inhibited the enzyme adenylate cyclase (and thereby the production of the second messenger molecule cyclic AMP), and positively influenced inwardly rectifying potassium channels (=Kir or IRK).[27] However, a much more complex picture has appeared in different cell types, implicating other potassium ion channels, calcium channels, protein kinase A and C, Raf-1, ERK, JNK, p38, c-fos, c-jun and many more.[27]
Separation between the therapeutically undesirable psychotropic effects, and the clinically desirable ones, however, has not been reported with agonists that bind to cannabinoid receptors. THC, as well as the two major endogenous compounds identified so far that bind to the cannabinoid receptors —anandamide and 2-arachidonylglycerol (2-AG)— produce most of their effects by binding to both the CB1 and CB2 cannabinoid receptors. While the effects mediated by CB1, mostly in the central nervous system, have been thoroughly investigated, those mediated by CB2 are not equally well defined.
Physiology
Cardiovascular activity
Studies have suggested that activation of CB1 receptors in human and rodent cardiomyocytes,[28][29] coronary artery endothelial and inflammatory cells[30][31][32] promotes activation of mitogen-activated protein (MAP) kinases p38 and JNK, reactive oxygen species generation, cell death, and cardiovascular inflammatory response both in vitro, as well as in models of heart failure, atherosclerosis and vascular inflammation.[28][29][31][32]
Cannabinoid treatments
Synthetic tetrahydrocannabinol (THC) is prescribed under the INN dronabinol or the brand name Marinol, to treat vomiting and for enhancement of appetite, mainly in people AIDS as well as for refractory nausea and vomiting in people undergoing chemotherapy.[33] THC is also an active ingredient in nabiximols, a specific extract of Cannabis that was approved as a botanical drug in the United Kingdom in 2010 as a mouth spray for people with multiple sclerosis to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms.[34]
Ligands
Binding affinity and selectivity of cannabinoid ligands
| CB1 affinity (Ki) | Efficacy towards CB1 | CB2 affinity (Ki) | Efficacy towards CB2 | Type | References | |
|---|---|---|---|---|---|---|
| Anandamide | 78nM | Full agonist | 370nM | ? | Endogenous | |
| N-Arachidonoyl dopamine | ? | Agonist | ? | ? | Endogenous | |
| 2-Arachidonoylglycerol | ? | Full agonist | ? | ? | Endogenous | |
| 2-Arachidonyl glyceryl ether | 21 nM | Full agonist | 480nM | Full agonist | Endogenous | |
| Δ-9-Tetrahydrocannabinol | 10nM | Partial agonist | 24nM | Partial agonist | Phytogenic | [35][35] |
| EGCG | 33.6μM | Agonist | >50μM | ? | Phytogenic | |
| Yangonin | 0.72 μM | ? | > 10 μM | ? | Phytogenic | [36] |
| AM-1221 | 52.3nM | Agonist | 0.28nM | Agonist | Synthetic | [37] |
| AM-1235 | 1.5nM | Agonist | 20.4nM | Agonist | Synthetic | [38] |
| AM-2232 | 0.28nM | Agonist | 1.48nM | Agonist | Synthetic | [38] |
| UR-144 | 150nM | Full agonist | 1.8nM | Full agonist | Synthetic | [39] |
| JWH-007 | 9.0nM | Agonist | 2.94nM | Agonist | Synthetic | [40] |
| JWH-015 | 383nM | Agonist | 13.8nM | Agonist | Synthetic | [40] |
| JWH-018 | 9.00 ± 5.00 nM | Full agonist | 2.94 ± 2.65 nM | Full agonist | Synthetic | [40] |
See also
- Cannabinoid receptor antagonist
- Endocannabinoid enhancer
- Endocannabinoid reuptake inhibitor
- Cannabidiol
- Effects of cannabis
References
- ↑ Aizpurua-Olaizola, Oier; Elezgarai, Izaskun; Rico-Barrio, Irantzu; Zarandona, Iratxe; Etxebarria, Nestor; Usobiaga, Aresatz (2016). "Targeting the endocannabinoid system: future therapeutic strategies". Drug Discovery Today. doi:10.1016/j.drudis.2016.08.005. PMID 27554802.
- ↑ Howlett AC (August 2002). "The cannabinoid receptors". Prostaglandins Other Lipid Mediat. 68–69: 619–31. doi:10.1016/S0090-6980(02)00060-6. PMID 12432948.
- ↑ Mackie K (May 2008). "Cannabinoid receptors: where they are and what they do". J. Neuroendocrinol. 20 Suppl 1: 10–4. doi:10.1111/j.1365-2826.2008.01671.x. PMID 18426493.
- ↑ Graham ES, Ashton JC, Glass M (2009). "Cannabinoid receptors: a brief history and "what's hot"". Front. Biosci. 14 (14): 944–57. doi:10.2741/3288. PMID 19273110.
- ↑ 5.0 5.1 Sylvaine G, Sophie M, Marchand J, Dussossoy D, Carriere D, Carayon P, Monsif B, Shire D, LE Fur G, Casellas P (1995). "Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations". Eur. J. Biochem. 232 (1): 54–61. doi:10.1111/j.1432-1033.1995.tb20780.x. PMID 7556170.
- ↑ Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990). "Structure of a cannabinoid receptor and functional expression of the cloned cDNA". Nature. 346 (6284): 561–4. doi:10.1038/346561a0. PMID 2165569.
- ↑ Gérard CM, Mollereau C, Vassart G, Parmentier M (1991). "Molecular cloning of a human cannabinoid receptor which is also expressed in testis". Biochem. J. 279 (Pt 1): 129–34. doi:10.1042/bj2790129. PMC 1151556. PMID 1718258.
- ↑ 8.0 8.1 Pacher P, Mechoulam R (2011). "Is lipid signaling through cannabinoid 2 receptors part of a protective system?". Prog Lipid Res. 50 (2): 193–211. doi:10.1016/j.plipres.2011.01.001. PMC 3062638. PMID 21295074.
- ↑ Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, Liu J, Kunos G (2005). "Evidence for novel cannabinoid receptors". Pharmacol. Ther. 106 (2): 133–45. doi:10.1016/j.pharmthera.2004.11.005. PMID 15866316.
- ↑ 10.0 10.1 Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". Br. J. Pharmacol. 152 (7): 1092–1101. doi:10.1038/sj.bjp.0707460. PMC 2095107. PMID 17876302.
- ↑ 11.0 11.1 Latek, D; Kolinski, M; Ghoshdastider, U; Debinski, A; Bombolewski, R; Plazinska, A; Jozwiak, K; Filipek, S (2011). "Modeling of ligand binding to G protein coupled receptors: Cannabinoid CB1, CB2 and adrenergic β 2 AR". Journal of Molecular Modeling. 17 (9): 2353–66. doi:10.1007/s00894-011-0986-7. PMID 21365223.
- ↑ Munro S, Thomas KL, Abu-Shaar M (1993). "Molecular characterization of a peripheral receptor for cannabinoids". Nature. 365 (6441): 61–65. doi:10.1038/365061a0. PMID 7689702.
- ↑ Kyrou I, Valsamakis G, Tsigos C (November 2006). "The endocannabinoid system as a target for the treatment of visceral obesity and metabolic syndrome". Ann. N. Y. Acad. Sci. 1083: 270–305. doi:10.1196/annals.1367.024. PMID 17148745.
- ↑ Maurice R. Elphick (2012), "The evolution and comparative neurobiology of endocannabinoid signalling", Philosophical Transactions of the Royal Society of London B, 367(1607): 3201–3215, doi:10.1098/rstb.2011.0394
- ↑ Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G (2005). "Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity". J. Clin. Invest. 115 (5): 1298–305. doi:10.1172/JCI23057. PMC 1087161. PMID 15864349.
- ↑ Fride E (2004). "The endocannabinoid-CB1 receptor system in pre- and postnatal life". European Journal of Pharmacology. 500: 289–297. doi:10.1016/j.ejphar.2004.07.033.
- ↑ The Endocannabinoid-CB Receptor System: Importance for development and in pediatric disease Neuroendocrinology Letters Nos.1/2, Feb-Apr Vol.25, 2004.
- ↑ Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R, Varga C, Lee SH, Matolcsi M, Cervenak J, Kacskovics I, Watanabe M, Sagheddu C, Melis M, Pistis M, Soltesz I, Katona I (2014). "Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling". Nature Neuroscience. 18: 75–86. doi:10.1038/nn.3892. PMC 4281300. PMID 25485758.
- ↑ Cannabinoids take the brain by STORM (Summary in SciGuru Science News)
- ↑ Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G (November 1999). "Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors". Proc. Natl. Acad. Sci. U.S.A. 96 (24): 14136–41. doi:10.1073/pnas.96.24.14136. PMC 24203. PMID 10570211.
- ↑ McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA (February 2008). "Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2". Mol. Pharmacol. 73 (2): 441–50. doi:10.1124/mol.107.041863. PMID 17965195.
- ↑ McHugh D; Hu SS-J; Rimmerman N; Juknat A; Vogel Z; Walker JM; Bradshaw HB (March 2010). "N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor". BMC Neuroscience. 11: 44. doi:10.1186/1471-2202-11-44. PMC 2865488. PMID 20346144.
- ↑ Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA (November 2007). "The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects". Br. J. Pharmacol. 152 (5): 825–31. doi:10.1038/sj.bjp.0707419. PMC 2190033. PMID 17704827.
- ↑ Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C (March 2006). "Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents". Cell Metab. 3 (3): 167–75. doi:10.1016/j.cmet.2006.02.004. PMID 16517404.
- ↑ de Fonseca FR, Schneider M (June 2008). "The endogenous cannabinoid system and drug addiction: 20 years after the discovery of the CB1 receptor" (PDF). Addict Biol. 13 (2): 143–6. doi:10.1111/j.1369-1600.2008.00116.x. PMID 18482429. Archived from the original (PDF) on 2011-07-18.
- ↑ Brown AJ (November 2007). "Novel cannabinoid receptors". Br. J. Pharmacol. 152 (5): 567–75. doi:10.1038/sj.bjp.0707481. PMC 2190013. PMID 17906678.
- ↑ 27.0 27.1 Demuth DG, Molleman A (2006). "Cannabinoid signalling". Life Sci. 78 (6): 549–63. doi:10.1016/j.lfs.2005.05.055. PMID 16109430.
- ↑ 28.0 28.1 Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P (2007). "Pharmacological Inhibition of CB1 Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity". J Am Coll Cardiol. 50 (6): 528–36. doi:10.1016/j.jacc.2007.03.057. PMC 2239316. PMID 17678736.
- ↑ 29.0 29.1 Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P (2010). "CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes". Cardiovasc Res. 85 (4): 773–784. doi:10.1093/cvr/cvp369. PMC 2819835. PMID 19942623.
- ↑ Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ (2009). "CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages". Cardiovasc Res. 84 (3): 378–86. doi:10.1093/cvr/cvp240. PMID 19596672.
- ↑ 31.0 31.1 Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H (2009). "Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages". Circulation. 119 (1): 28–36. doi:10.1161/CIRCULATIONAHA.108.811992. PMID 19103987.
- ↑ 32.0 32.1 Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Haskó G, Pacher P (2011). "Fatty acid amide hydrolase is a key regulator of the endocannabinoid-induced myocardial tissue injury". Free Radic Biol Med. 50 (1): 179–195. doi:10.1016/j.freeradbiomed.2010.11.002. PMC 3022384. PMID 21070851.
- ↑ Badowski, ME (5 August 2017). "A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics". Cancer chemotherapy and pharmacology. doi:10.1007/s00280-017-3387-5. PMC 5573753. PMID 28780725.
- ↑ "Sativex Oromucosal Spray - Summary of Product Characteristics". UK Electronic Medicines Compendium. March 2015.
- ↑ 35.0 35.1 "PDSP Database - UNC". Archived from the original on 8 November 2013. Retrieved 11 June 2013.
- ↑ Ligresti, A.; Villano, R.; Allarà, M.; Ujváry, I. N.; Di Marzo, V. (2012). "Kavalactones and the endocannabinoid system: The plant-derived yangonin is a novel CB1 receptor ligand". Pharmacological Research. 66 (2): 163–169. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- ↑ WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ↑ 38.0 38.1 US patent 7241799, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2007-07-10
- ↑ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". J. Med. Chem. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- ↑ 40.0 40.1 40.2 Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR (August 2000). "Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding". Drug Alcohol Depend. 60 (2): 133–40. doi:10.1016/S0376-8716(99)00152-0. PMID 10940540.
External links
- Cannabinoid+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)
- The Endocannabinoid System Network (ECSN) - CB1 receptor
- "Cannabinoid Receptors". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.