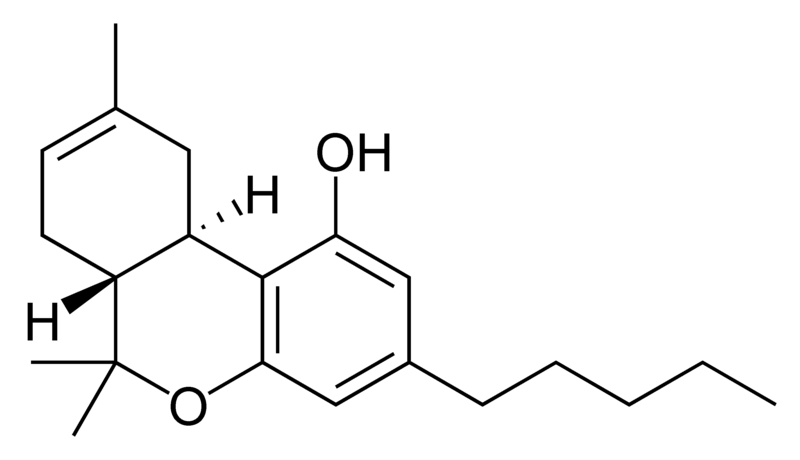
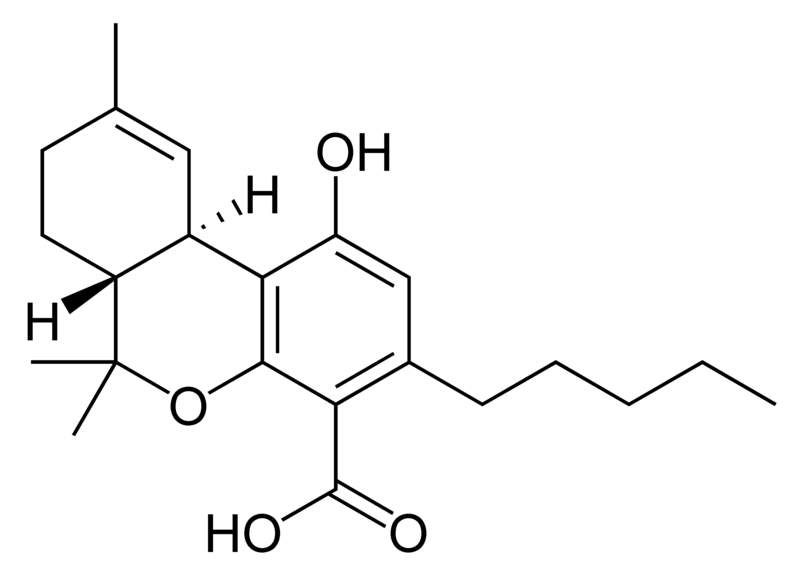
Cannabinoids
|
WikiDoc Resources for Cannabinoids |
|
Articles |
|---|
|
Most recent articles on Cannabinoids Most cited articles on Cannabinoids |
|
Media |
|
Powerpoint slides on Cannabinoids |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cannabinoids at Clinical Trials.gov Clinical Trials on Cannabinoids at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cannabinoids
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cannabinoids Discussion groups on Cannabinoids Patient Handouts on Cannabinoids Directions to Hospitals Treating Cannabinoids Risk calculators and risk factors for Cannabinoids
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cannabinoids |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
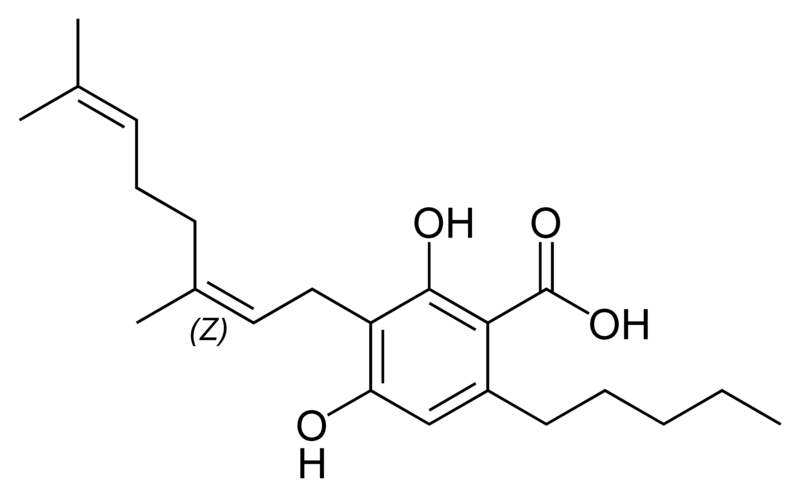
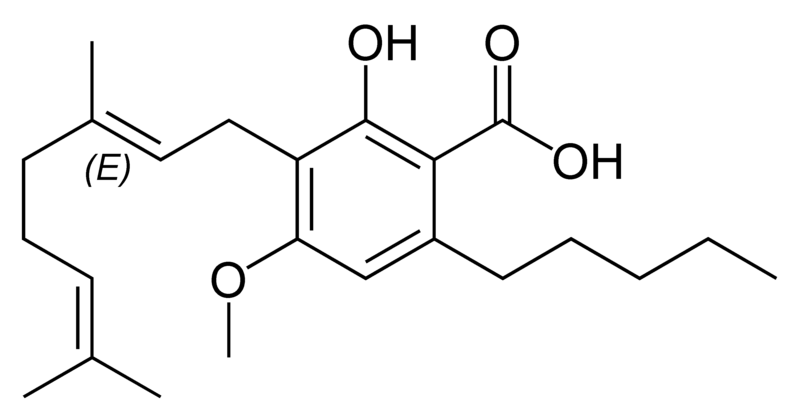
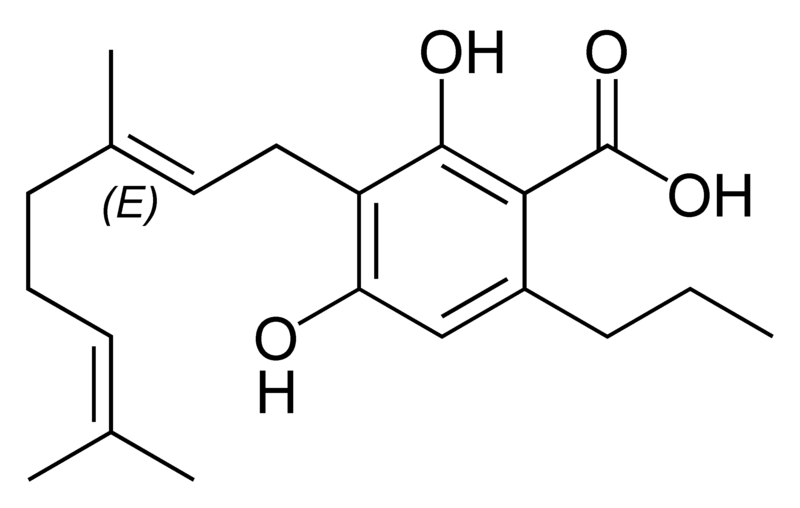
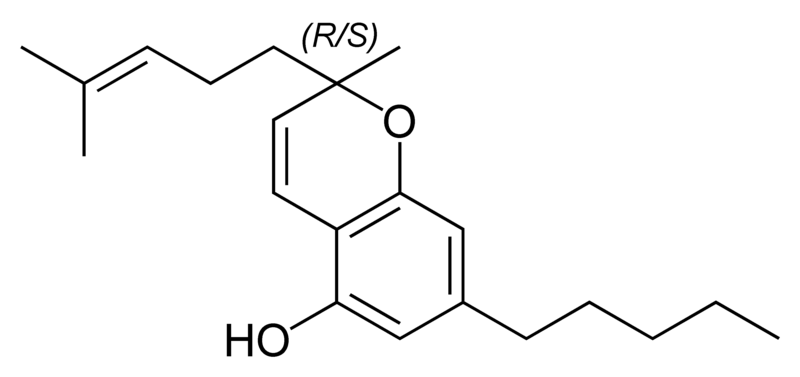
Cannabinoids are a group of terpenophenolic compounds present in Cannabis (Cannabis sativa L). The broader definition of cannabinoids refer to a group of substances that are structurally related to tetrahydrocannabinol (THC) or that bind to cannabinoid receptors. The chemical definition encompasses a variety of distinct chemical classes: the classical cannabinoids structurally related to THC, the nonclassical cannabinoids, the aminoalkylindoles, the eicosanoids related to the endocannabinoids, 1,5-diarylpyrazoles, quinolines and arylsulphonamides and additional compounds that do not fall into these standard classes but bind to cannabinoid receptors.[1] The term cannabinoids also refers to a unique group of secondary metabolites found in the cannabis plant, which are responsible for the plant's peculiar pharmacological effects. Currently, there are three general types of cannabinoids: herbal cannabinoids occur uniquely in the cannabis plant; endogenous cannabinoids are produced in the bodies of humans and other animals; and synthetic cannabinoids are similar compounds produced in a laboratory.
Cannabinoid receptors
Before the 1980's, it was often speculated that cannabinoids produced their physiological and behavioral effects via nonspecific interaction with cell membranes, instead of interacting with specific membrane-bound receptors. The discovery of the first cannabinoid receptors in the 1980s helped to resolve this debate. These receptors are common in animals, and have been found in mammals, birds, fish, and reptiles. There are currently two known types of cannabinoid receptors, termed CB1 and CB2.
- CB1 receptors are found primarily in the brain, specifically in the basal ganglia and in the limbic system, including the hippocampus. They are also found in the cerebellum and in both male and female reproductive systems. CB1 receptors are essentially absent in the medulla oblongata, the part of the brain stem that is responsible for respiratory and cardiovascular functions. Thus, there is not a risk of respiratory or cardiovascular failure as there is with many other drugs. CB1 receptors appear to be responsible for the euphoric and anticonvulsive effects of cannabis.
- CB2 receptors are almost exclusively found in the immune system, with the greatest density in the spleen. CB2 receptors appear to be responsible for the anti-inflammatory and possibly other therapeutic effects of cannabis.
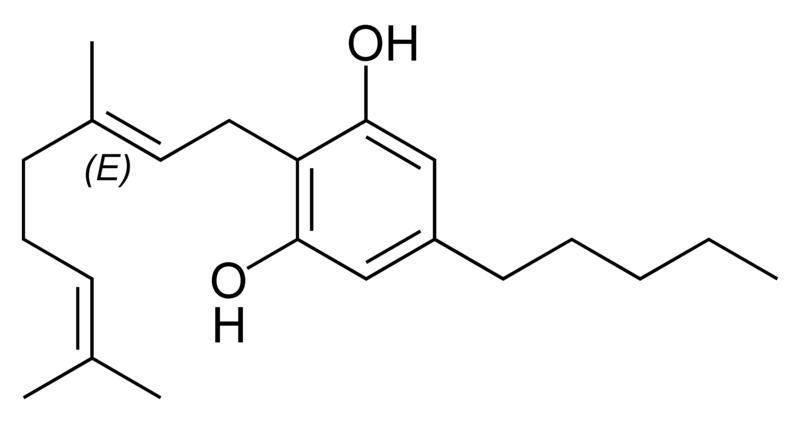
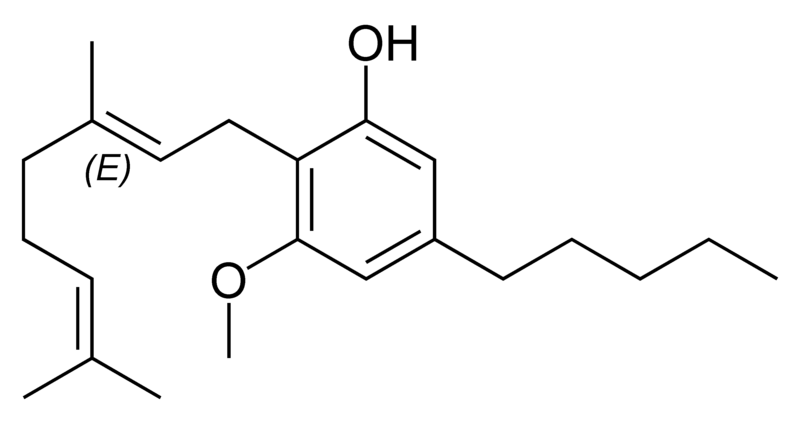
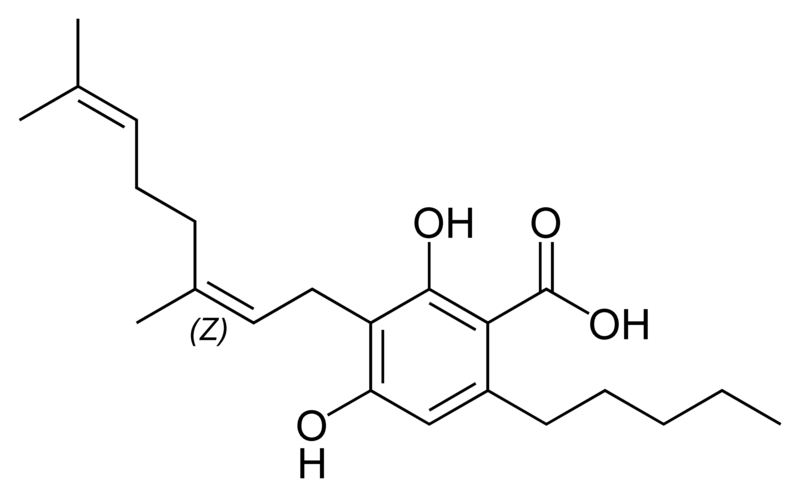
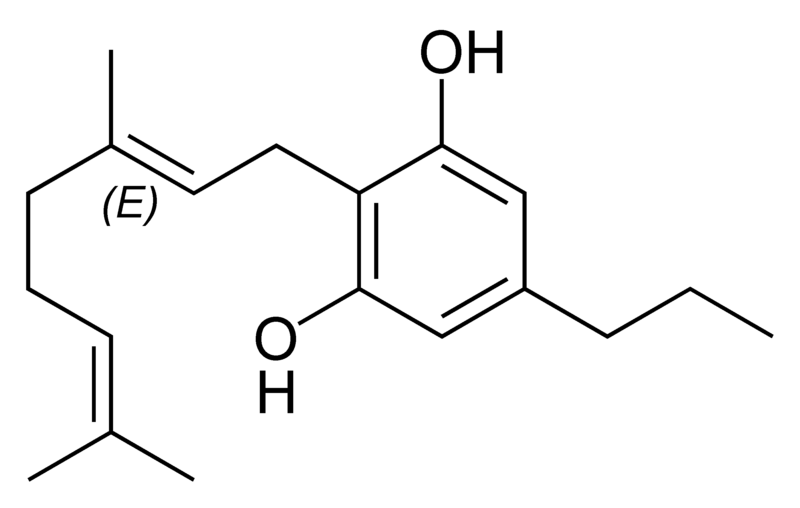
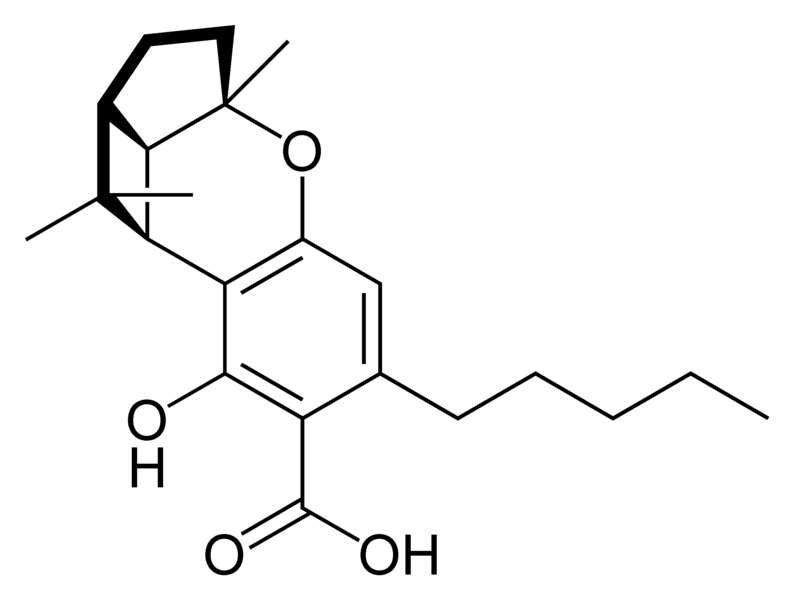
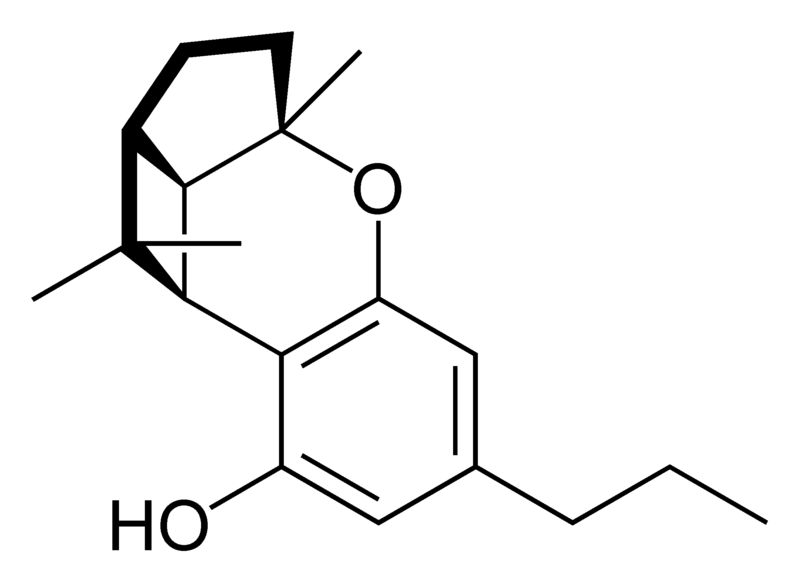
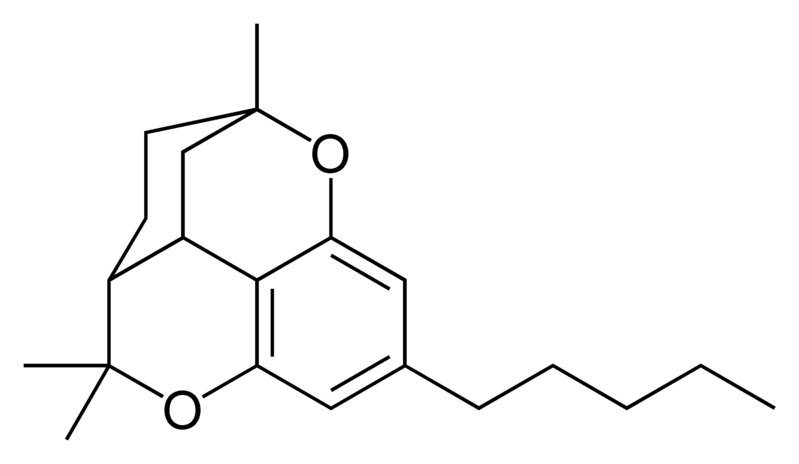
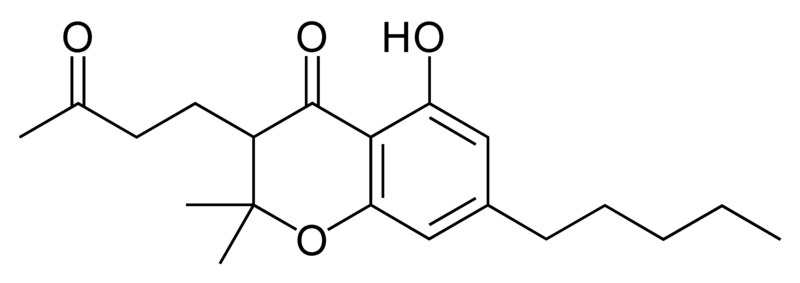
Natural cannabinoids
| Type | Skeleton | Cyclization |
|---|---|---|
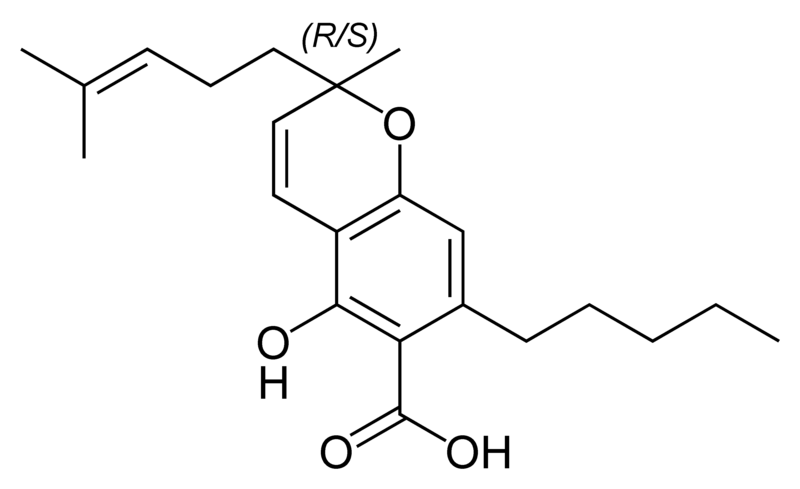
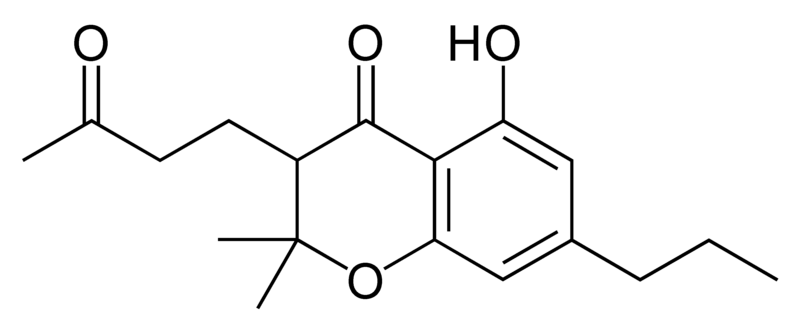
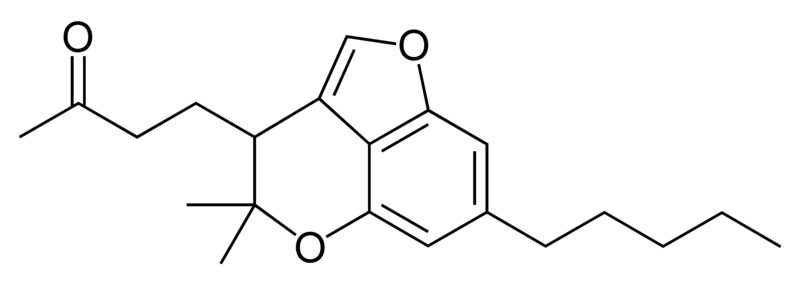
| Cannabigerol-type CBG |
|
|
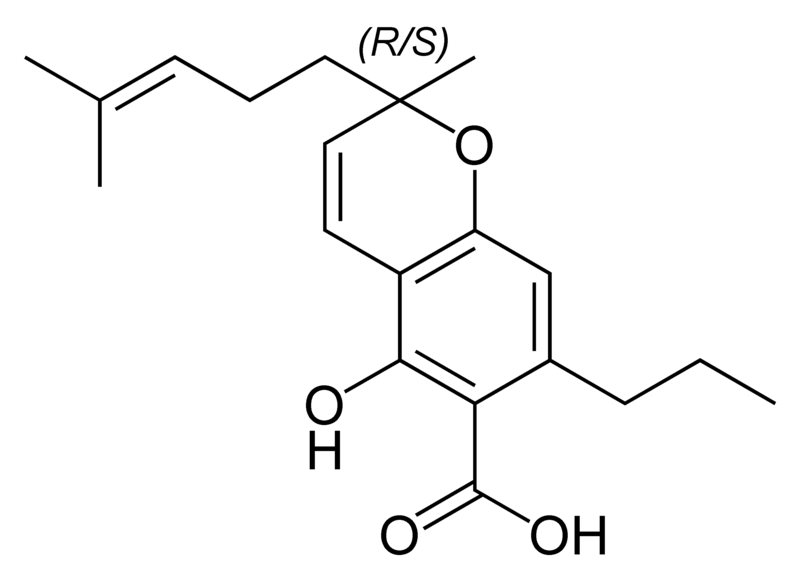
| Cannabichromene-type CBC |
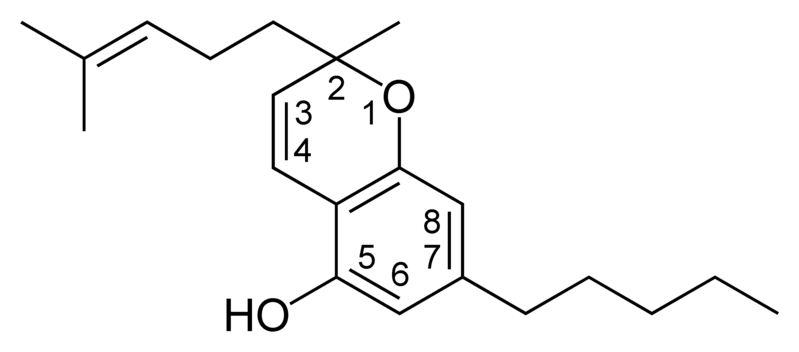
|
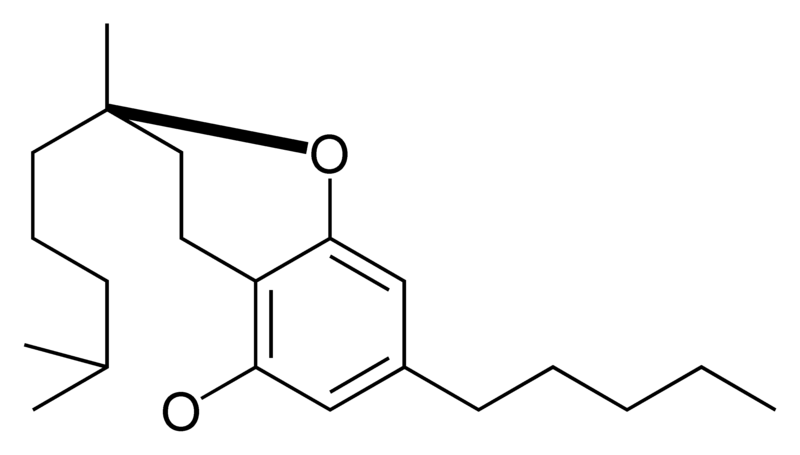
|
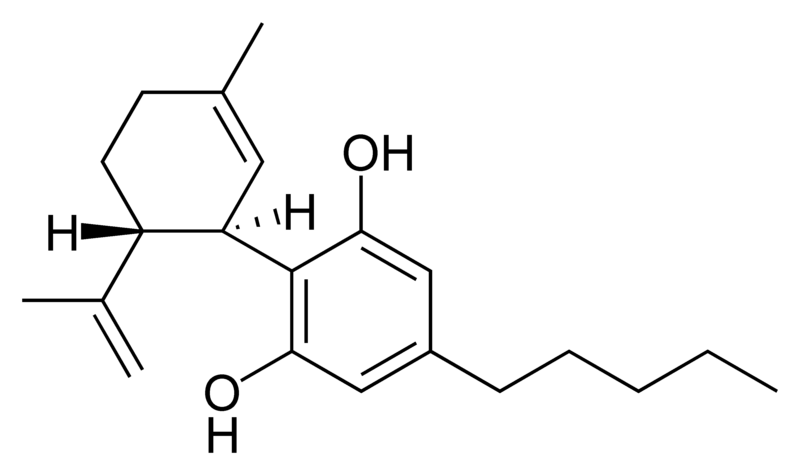
| Cannabidiol-type CBD |
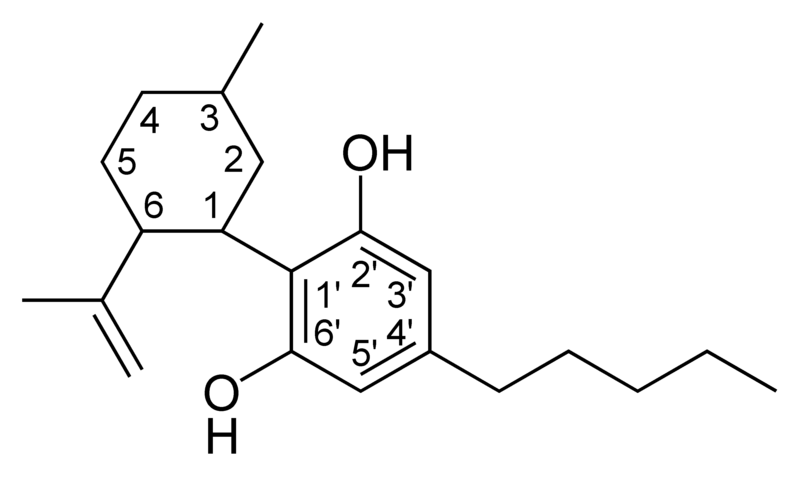
|
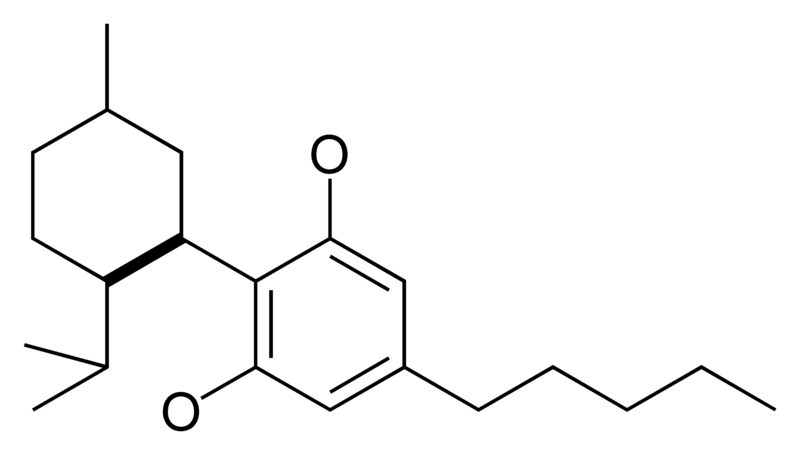
|
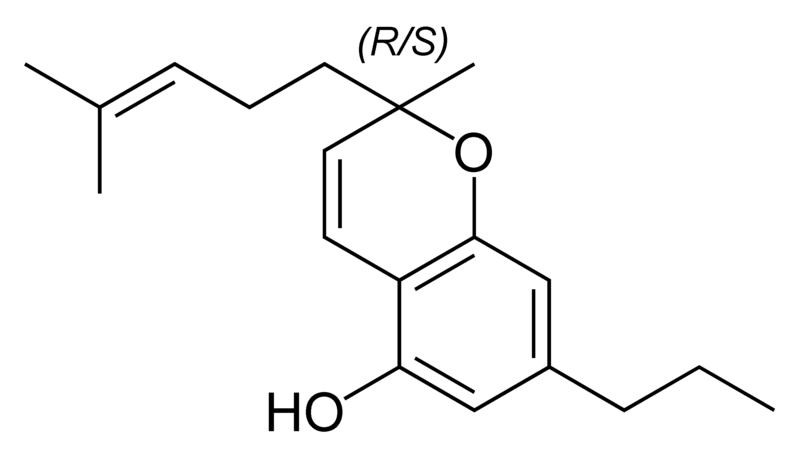
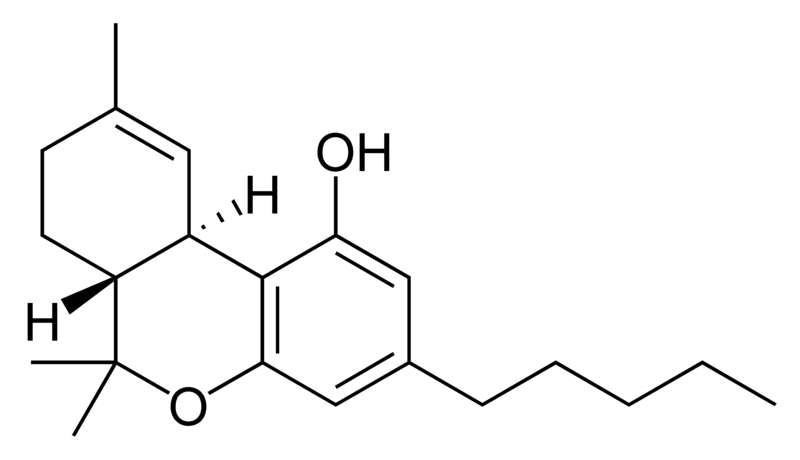
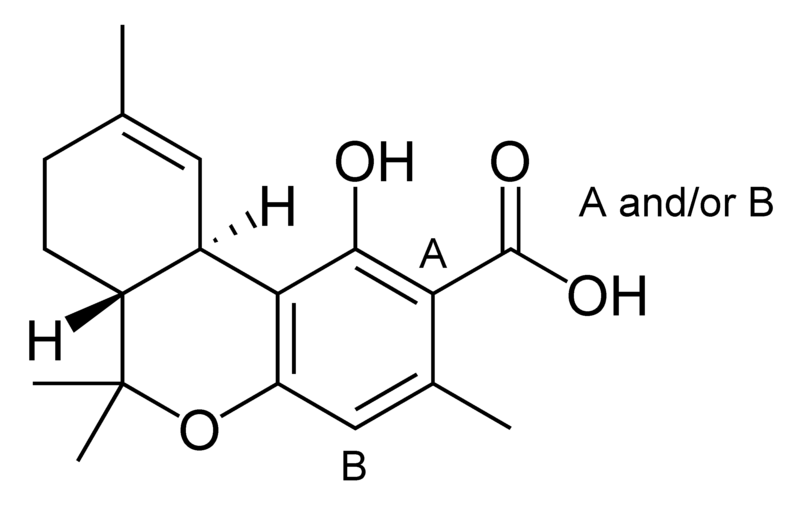
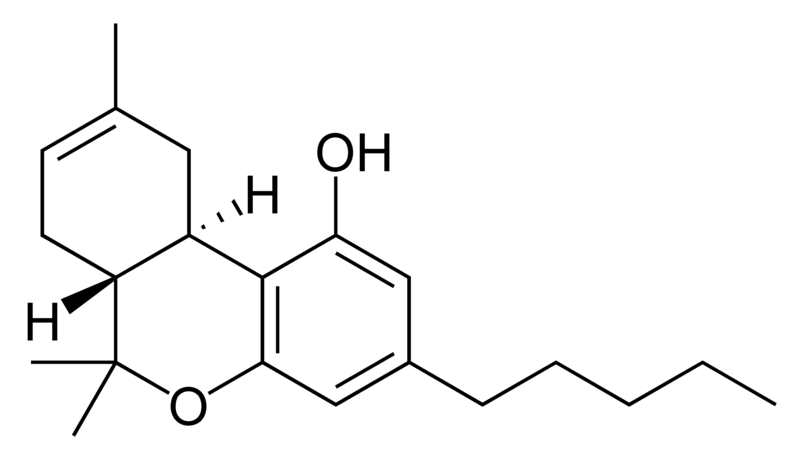
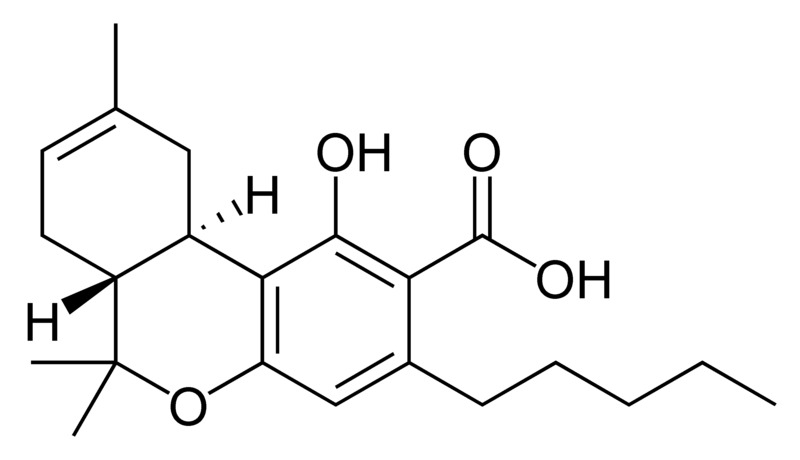
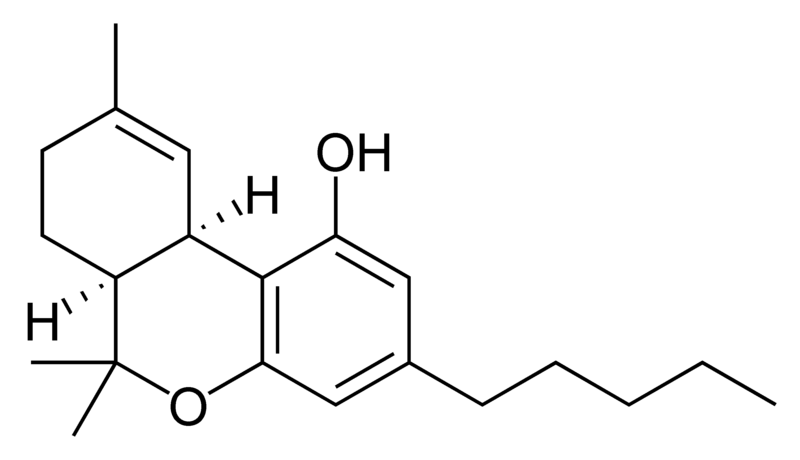
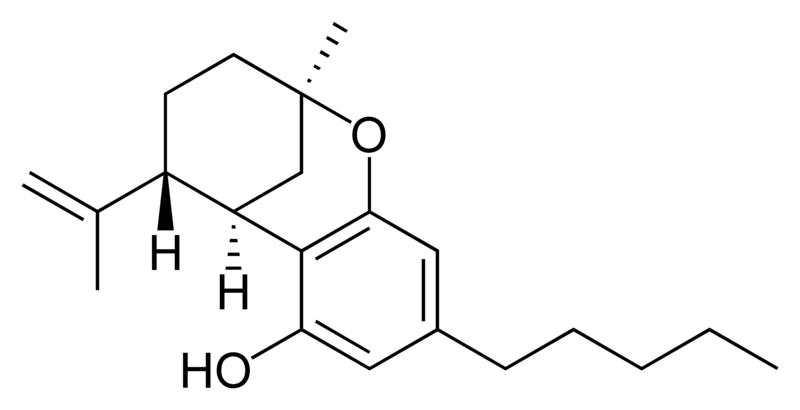
| Tetrahydrocannabinol- and Cannabinol-type THC, CBN |
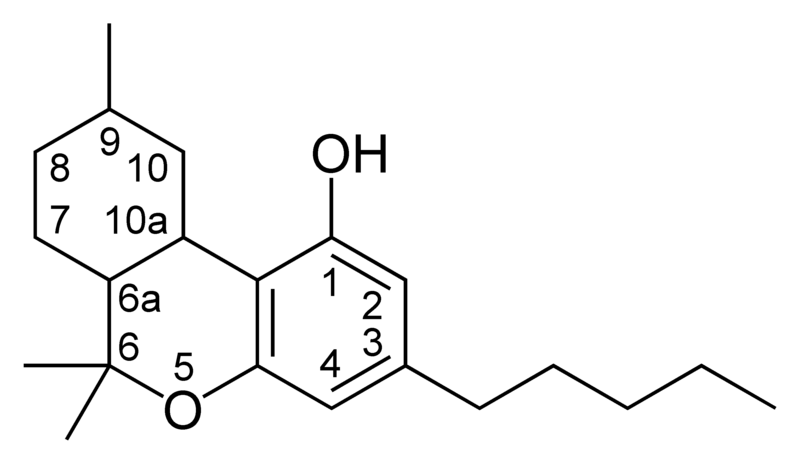
|
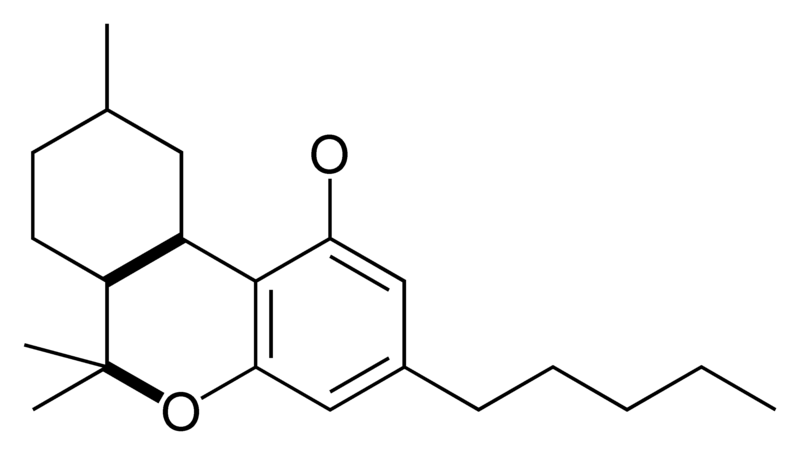
|
| Cannabielsoin-type CBE |
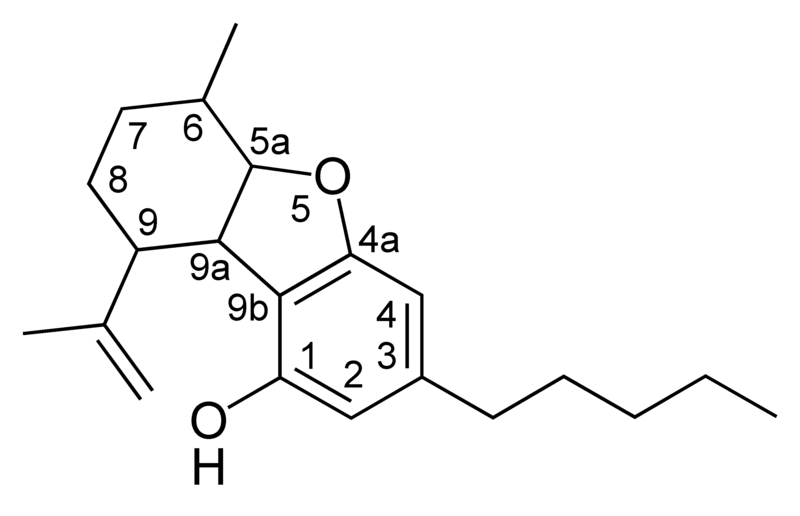
|
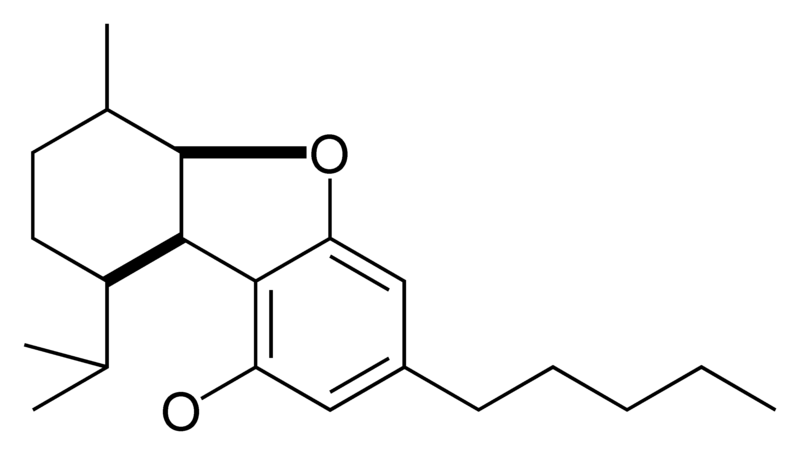
|
| iso- Tetrahydrocannabinol- type iso-THC |
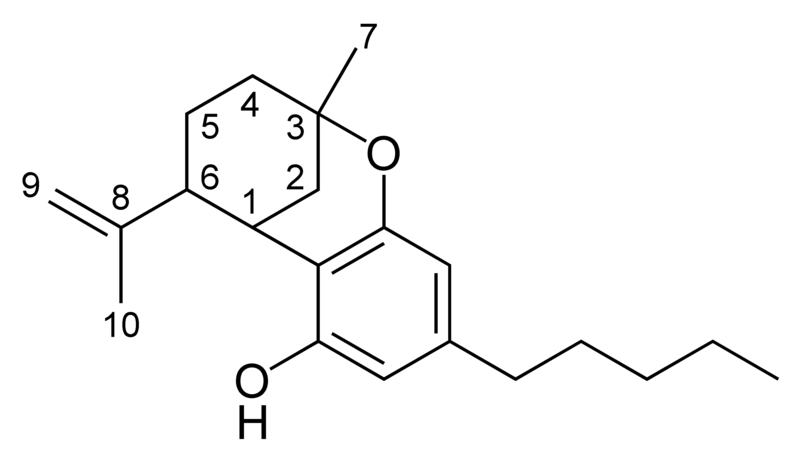
|
Chemical structure of the iso-CBN-type cyclization of cannabinoids. |
| Cannabicyclol-type CBL |
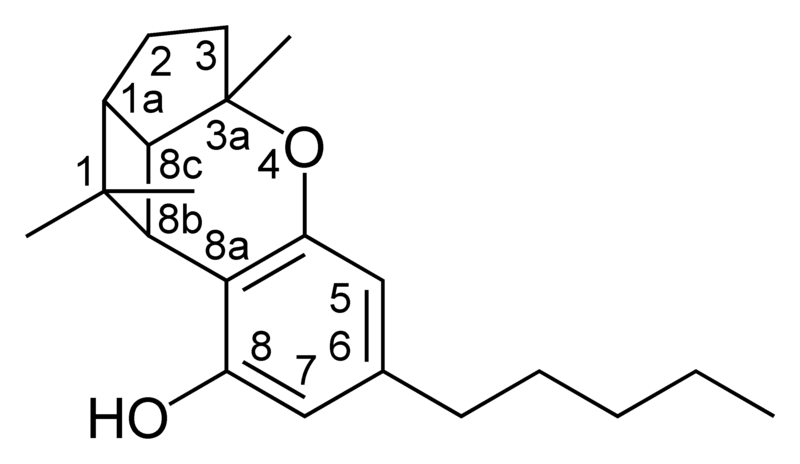
|
|
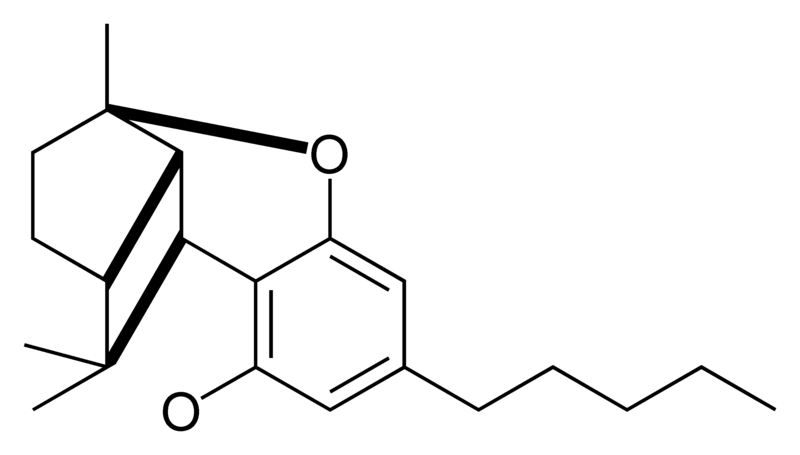
| Cannabicitran-type CBT |
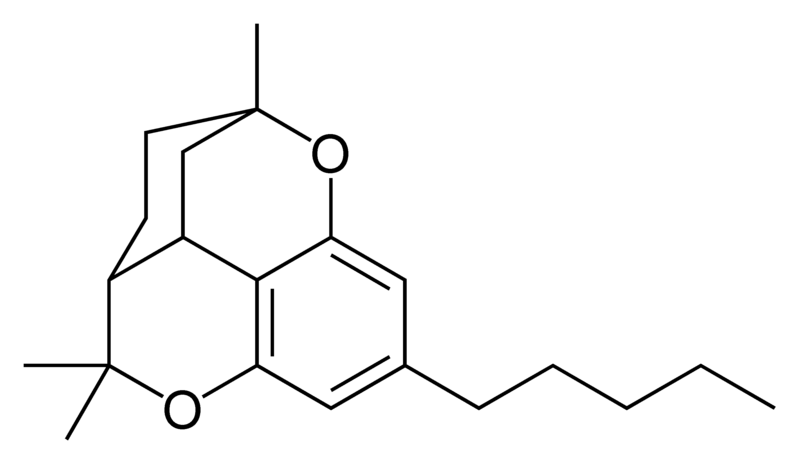
|
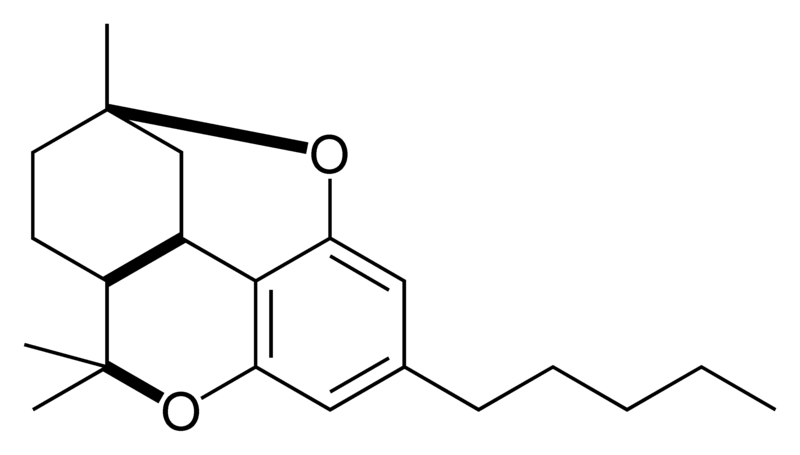
|
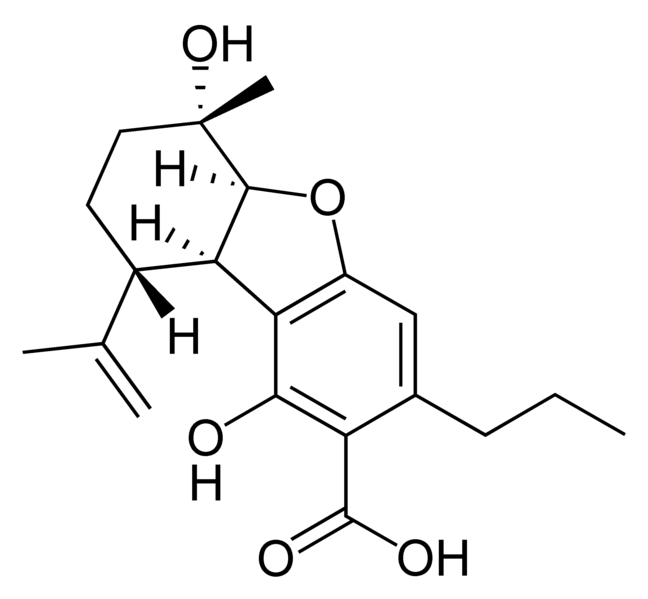
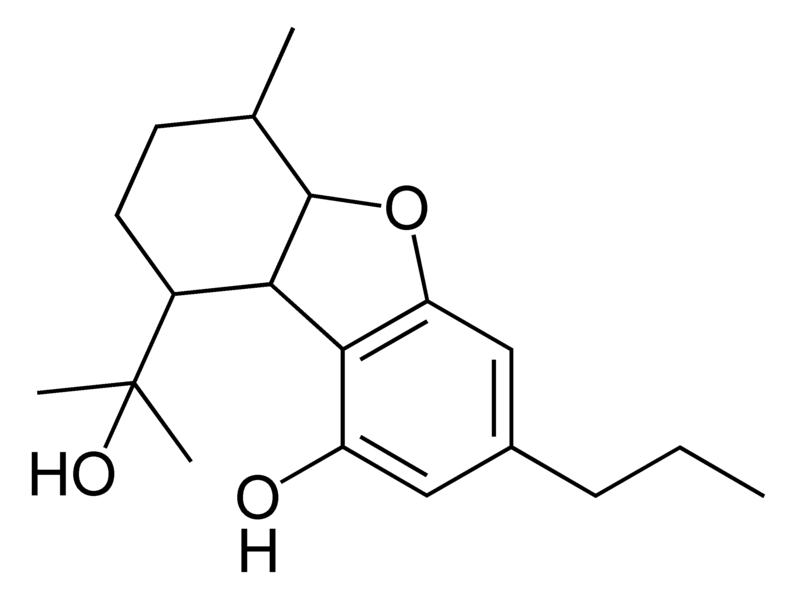
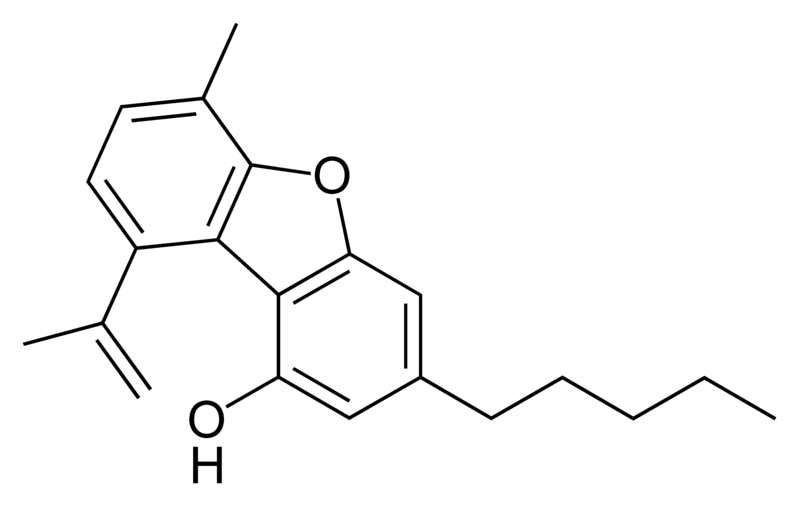
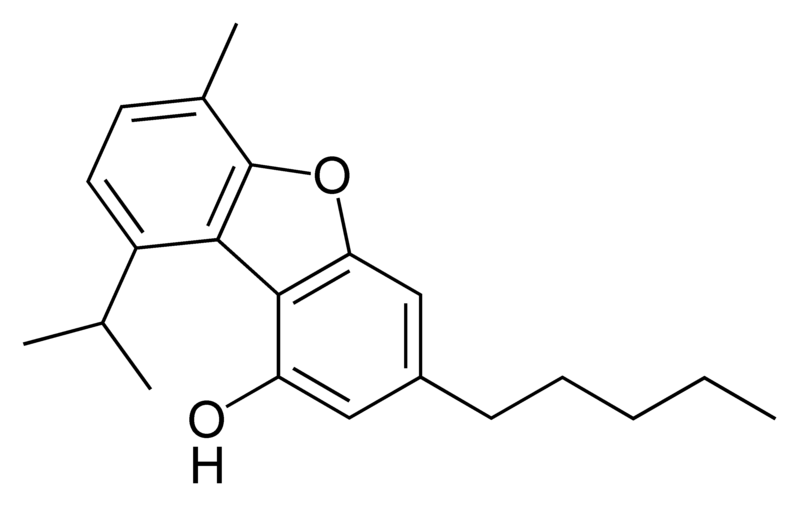
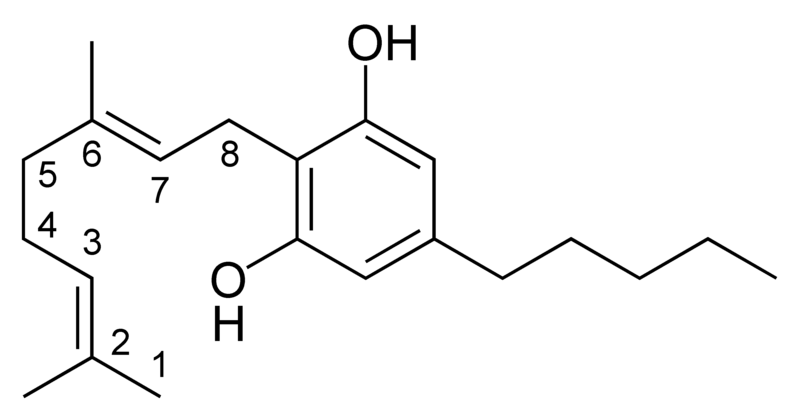
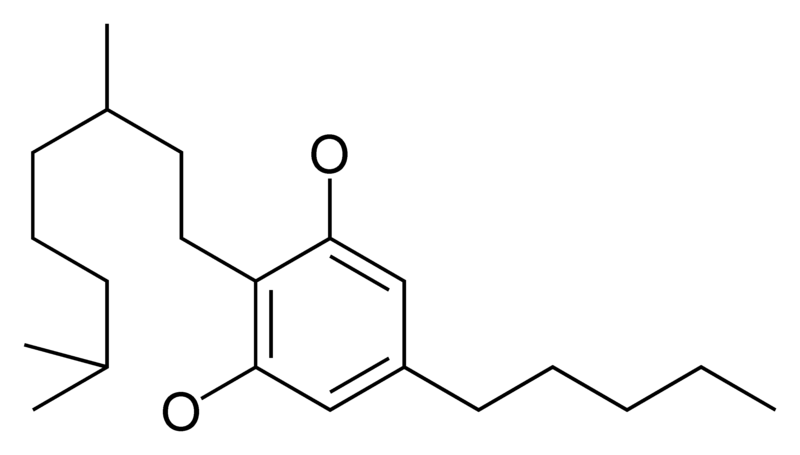
Natural cannabinoids, also called herbal cannabinoids and classical cannabinoids, are nearly insoluble in water but soluble in lipids, alcohols, and other non-polar organic solvents. However, as phenols they form more water-soluble phenolate salts under strongly alkaline conditions. All natural cannabinoids are derived from their respective 2-carboxylic acids (2-COOH) by decarboxylation; that is, catalyzed by heat, light, or alkaline conditions. Natural cannabinoids are only known to occur naturally in the cannabis plant, and are concentrated in a viscous resin that is produced in glandular structures known as trichomes. In addition to cannabinoids, the resin is rich in terpenes, which are largely responsible for the odour of the cannabis plant.
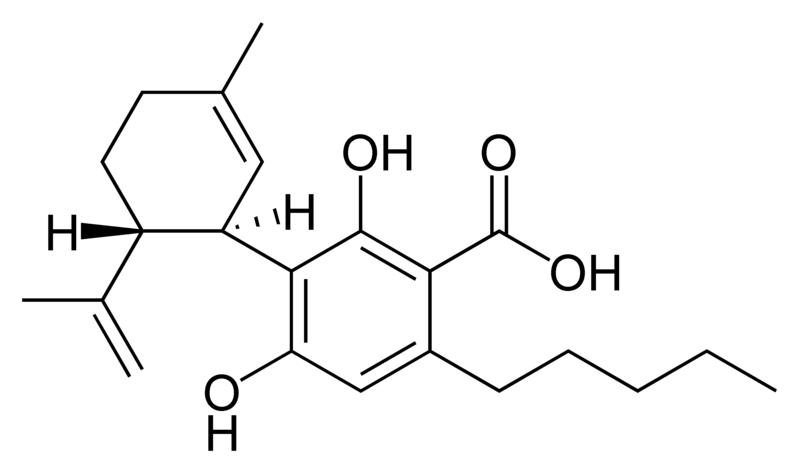
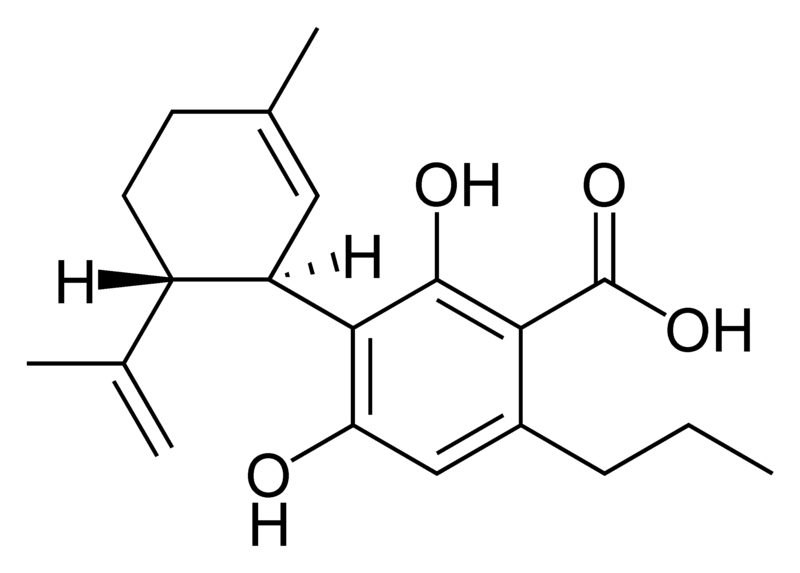
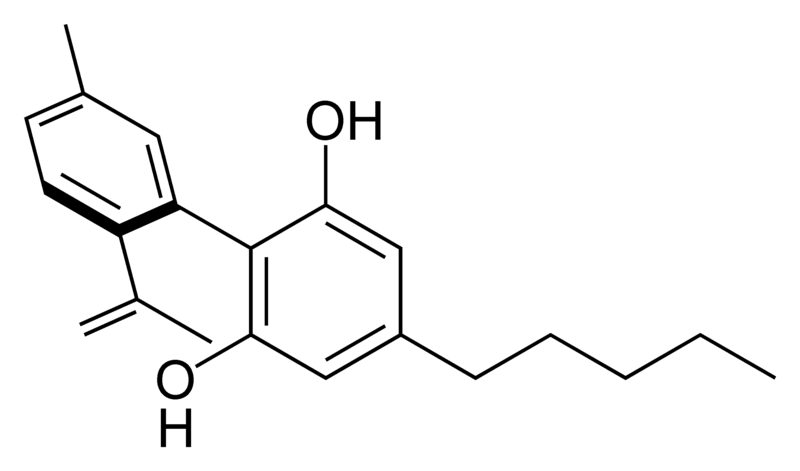
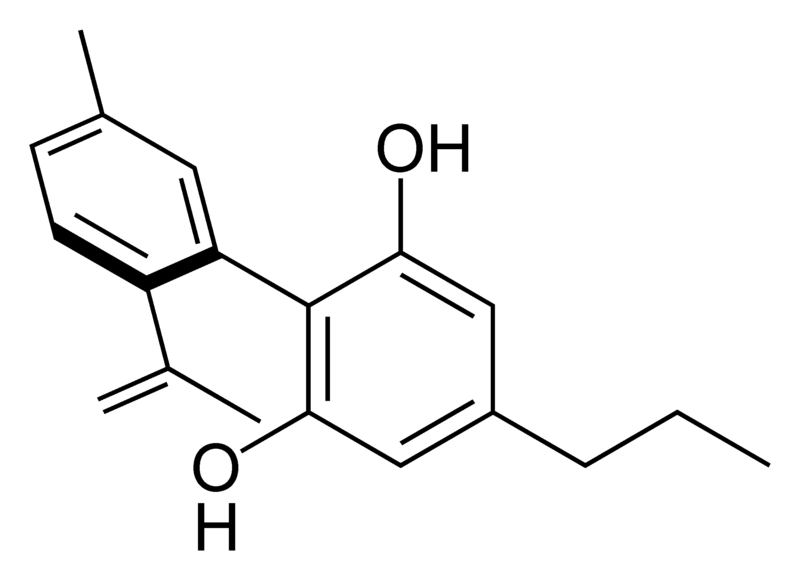
There are today seventy known herbal cannabinoids. To the right the main classes of natural cannabinoids are shown. All classes derive from cannabigerol-type compounds and differ mainly in the way this precursor is cyclized.
Tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN) are the most prevalent natural cannabinoids and have received the most study. Other common ones are listed below:
- CBG Cannabigerol
- CBC Cannabichromene
- CBL Cannabicyclol
- CBV Cannabivarin
- THCV Tetrahydrocannabivarin
- CBDV Cannabidivarin
- CBCV Cannabichromevarin
- CBGV Cannabigerovarin
- CBGM Cannabigerol Monoethyl Ether
THC is the primary psychoactive component of the plant. Medically, it appears to ease moderate pain and to be neuroprotective. THC has approximately equal affinity for the CB1 and CB2 receptors.[2] Its effects are perceived to be more cerebral.
CBD is not psychoactive, and appears to moderate the euphoric effects of THC. It may decrease the rate of THC clearance from the body, perhaps by interfering with the metabolism of THC in the liver. Medically, it appears to relieve convulsion, inflammation, anxiety, and nausea. CBD has a greater affinity for the CB2 receptor than for the CB1 receptor. It is perceived to have more effect on the body.
CBN is the primary product of THC degradation, and there is usually little of it in a fresh plant. CBN content increases as THC degrades in storage, and with exposure to light and air. It is only mildly psychoactive, and is perceived to be sedative or stupefying.
These compounds may be in different forms depending on the position of the double bond in the alicyclic carbon ring. There is potential for confusion because there are different numbering systems used to describe the position of this double bond. Under the dibenzopyran numbering system widely used today, the major form of THC is called delta-9-THC, while the minor form is called delta-8-THC. Under the alternate terpene numbering system, these same compounds are called delta-1-THC and delta-6-THC, respectively.
Most herbal cannabinoid compounds are 21 carbon compounds. However, some do not follow this rule, primarily because of variation in the length of the side chain attached to the aromatic ring. In THC, CBD, and CBN, this side chain is a pentyl (5 carbon) chain. In the most common homologue, the pentyl chain is replaced with a propyl (3 carbon) chain. Cannabinoids with the propyl side chain are named using the suffix "varin", and are designated, for example, THCV, CBDV, or CBNV. It appears that shorter chains increase the intensity and decrease the duration of the activity of the chemicals.
Cannabinoids were first discovered in the 1940s, when CBD and CBN were identified. The structure of THC was first determined in 1964. Due to molecular similarity and ease of synthetic conversion, it was originally believed that CBD was a natural precursor to THC. However, it is now known that CBD and THC are produced independently in the cannabis plant. Cannabinoid production starts when an enzyme causes geranyl pyrophosphate and olivetolic acid to combine and form CBG. Next, CBG is independently converted to either CBD or CBC by two separate synthase enzymes. CBC is then enzymatically cyclized to THC. For the propyl homologues (THCV, CBDV and CBNV), there is a similar pathway that is based on CBGV.
Cannabis plants can exhibit wide variation in the quantity and type of cannabinoids they produce. The mixture of cannabinoids produced by a plant is known as the plant's cannabinoid profile. Selective breeding has been used to control the genetics of plants and modify the cannabinoid profile. For example, strains which are used as fiber (commonly called hemp), are bred such that they are low in psychoactive chemicals like THC. Strains used in medicine are often bred for high CBD content, and strains used for recreational purposes are usually bred for high THC content, or for a specific chemical balance. Some strains of more than 20% THC have been created.
Quantitative analysis of a plant's cannabinoid profile is usually determined by gas chromatography (GC), or more reliably by gas chromatography combined with mass spectrometry (GC/MS). Liquid chromatography (LC) techniques are also possible, although these are often only semi-quantitative or qualitative. There have been systematic attempts to monitor the cannabinoid profile of cannabis over time, but their accuracy is impeded by the illegal status of the plant in many countries.
Cannabinoids can be administered by smoking, vaporizing, oral ingestion, transdermal patch, intravenous injection, sublingual absorption, or rectal suppository. Once in the body, most cannabinoids are metabolized in the liver, although some is stored in fat. Delta-9-THC is metabolized to 11-hydroxy-delta-9-THC, which is then metabolized to 9-carboxy-THC. Some cannabis metabolites can be detected in the body after several weeks.
Cannabinoids can be separated from the plant by extraction with organic solvents. Hydrocarbons and alcohols are often used as solvents. However, these solvents are flammable and many are toxic. Supercritical solvent extraction with carbon dioxide is an alternative technique. Although this process requires high pressures, there is minimal risk of fire or toxicity, solvent removal is simple and efficient, and extract quality can be well-controlled. Once extracted, cannabinoid blends can be separated into individual components using wiped film vacuum distillation or other distillation techniques. However, to produce high purity cannabinoids, chemical synthesis or semisynthesis is generally required.
Endogenous cannabinoids
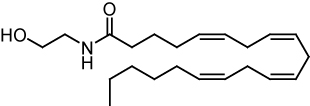
Endocannabinoids are naturally produced in the bodies of animals. After the first cannabinoid receptor was discovered in 1988, scientists began searching for natural compounds that activate these receptors.
In 1992, the first such compound was identified as arachidonoyl ethanolamide and named anandamide, a name derived from the Sanskrit word for bliss and amide. Anandamide is derived from the essential fatty acid arachidonic acid. It has a pharmacology similar to THC, although its chemical structure is different. Anandamide binds to both the central (CB1) and peripheral (CB2) cannabinoid receptors, and is found in nearly all tissues in a wide range of animals. It is about as potent as THC. Two analogs of anandamide, 7,10,13,16-docosatetraenoylethanolamide and homo-γ-linolenoylethanolamide, have similar pharmacology. All of these are members of a family of signalling lipids called N-acylethanolamides which also include the noncannabimimetic palmitoylethanolamide and oleoylethanolamide which have anti-inflammatory and orexigenic effects, respectively. Another endocannabinoid, 2-arachidonoyl glycerol, binds to both the CB1 and CB2 receptors, and is more abundant and a full efficacy agonist, clearly more potent than anandamide, in mediating CB, receptor-dependent G-protein activity in native membranes.[3] Many N-acylethanolamides have also been identified in plant seeds[4] and in molluscs.[5] In 2001 was reported a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (noladin ether), isolated from porcine brain.[6] It binds to the CB1 cannabinoid receptor (Ki = 21.2 nM) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds weakly to the CB2 receptor.
Endocannabinoids serve as intercellular 'lipid messengers', signaling molecules that are released from one cell and activate the cannabinoid receptors present on other nearby cells. Although in this intercellular signaling role they are similar to the well-known monoamine neurotransmitters, such as acetylcholine, GABA or dopamine, endocannabinoids differ in numerous ways from them. Neurotransmitters are commonly small, water-soluble molecules that are contained within, and released from, tiny membrane-bound vesicles inside cells. Vesicles are often found in the tips, ‘terminals’, of long cellular branches called axons, and complex morphological and biochemical specializations mark the location from which vesicular release occurs. Endocannabinoids are lipophilic molecules that are not very soluble in water. They are not stored in vesicles, and exist as integral constituents of the membrane bilayers that make up cells. They are believed to be synthesized 'on-demand' rather than made and stored for later use. The mechanisms and enzymes underlying the biosynthesis of endocannabinoids remain elusive and continue to be an area of active research.
Conventional neurotransmitters are released from a ‘presynaptic’ cell and activate appropriate receptors on a ‘postsynaptic’ cell, where presynaptic and postsynaptic designate the sending and receiving sides of a synapse, respectively. Endocannabinoids are described as ‘retrograde’ transmitters because they most commonly travel ‘backwards’ against the usual synaptic transmitter flow. They are in effect released from the postsynaptic cell and act on the presynaptic cell, where the target receptors are densely concentrated on axonal terminals in the zones from which conventional neurotransmitters are released. Activation of cannabinoid receptors temporarily reduces the amount of conventional neurotransmitter released. This endocannabinoid mediated system permits the postsynaptic cell to control its own incoming synaptic traffic. The ultimate effect on the endocannabinoid releasing cell depends on the nature of the conventional transmitter that is being controlled. When the release of the inhibitory transmitter, GABA, is reduced, the net effect is an increase in the excitability of the endocannabinoid-releasing cell. Conversely, when release of the excitatory neurotransmitter, glutamate, is reduced, the net effect is a decrease in the excitability of the endocannabinoid-releasing cell.
Endocannabinoids are hydrophobic molecules. They cannot travel unaided for long distances in the aqueous medium surrounding the cells from which they are released, and therefore act locally on nearby target cells. Hence, although emanating diffusely from their source cells, they have much more restricted spheres of influence than do hormones, which can affect cells throughout the body.
Endocannabinoids constitute a versatile system for affecting neuronal network properties in the nervous system.
Scientific American published an article in December of 2004, entitled "The Brain's Own Marijuana" discussing the endogenous cannabinoid system. [7]
The current understanding recognizes the role that endocannabinoids play in almost every major life function in the human body. Cannabinoids act as a bioregulatory mechanism for most life processes, which reveals why medical cannabis has been cited as treatments for many diseases and ailments in anecdotal reports and scientific literature. Some of these ailments include: pain, arthritic conditions, migraine headaches, anxiety, epileptic seizures, insomnia, loss of appetite, GERD (chronic heartburn), nausea, glaucoma, AIDS wasting syndrome, depression, bipolar disorder (particularly depression-manic-normal), multiple sclerosis, menstrual cramps, Parkinson's, trigeminal neuralgia (tic douloureux), high blood pressure, irritable bowel syndrome, and bladder incontinence.
Synthetic & Patented Cannabinoids
Historically, laboratory synthesis of cannabinoids were often based on the structure of herbal cannabinoids and a large number of analogs have been produced and tested, especially in a group led by Roger Adams as early as 1941 and later in a group led by Raphael Mechoulam. Newer compounds are no longer related to natural cannabinoids or are based on the structure of the endogenous cannabinoids.
Synthetic cannabinoids are particularly useful in experiments to determine the relationship between the structure and activity of cannabinoid compounds, by making systematic, incremental modifications of cannabinoid molecules.
Medications containing natural, synthetic, or cannabinoids analogs:
- Dronabinol (Marinol), an analog of Δ9-tetrahydrocannabinol (THC), used as an appetite stimulant, anti-emetic and analgesic.
- Nabilone (Cesamet), a synthetic cannabinoid and an analog of Marinol. It is Schedule II unlike Marinol which is Schedule III.
- Sativex, a cannabinoid extract oral spray containing both THC and CBD used for neuropathic pain and spasticity in Canada and Spain.
- Rimonabant (SR141716), a selective cannabinoid (CB1) receptor antagonist used as an anti-obesity drug under the proprietary name, Acomplia. It is also used for smoking cessation.
Other notable synthetic cannabinoids include:
- CP-55940, produced in 1974, this synthetic cannabinoid receptor agonist is many times more potent than THC
- HU-210, about 100 times as potent as THC[8].
- SR144528, a CB2 receptor antagonists
- WIN 55,212-2, a potent cannabinoid receptor agonist
- JWH-133, a potent selective CB2 receptor agonist.
- Levonantradol (Nantrodolum), an anti-emetic and analgesic but not currently in use in medicine.
Miscellaneous
- delta-9-Tetrahydrocannabinol (Δ9-THC, THC) and delta-8-tetrahydrocannabinol (Δ8-THC), mimic the action of anandamide, a neurotransmitter produced naturally in the body. The THCs produce the high associated with cannabis by binding to the CB1 cannabinoid receptors in the brain.
- Tetrahydrocannabivarin (THCV), prevalent in certain South African and Southeast Asian strains of Cannabis. It is an antagonist of THC at CB1 receptors and attenuates the psychoactive effects of THC.[9]
- Cannabidiol (CBD), non-psychoactive and not affecting psychoactivity of THC.[10] CBD has anti-inflammatory effects. CBD shares a precursor with THC and is the main cannabinoid in low-THC Cannabis strains.
- Cannabinol (CBN), a degradation product of THC, produces a depressant effect
- Cannabichromene (CBC), non-psychoactive and not affecting psychoactivity of THC,[10] a precursor of CBD and THC
- Cannabigerol (CBG), non-psychoactive
- Cannabinoids are good substrates for cytochrome P450 mixed-function oxidases, mainly CYP 2C9. Thus suplementing with CYP 2C9 inhibitors leads to extended intoxication.
Table of natural cannabinoids
External links
- [2] Homepage of the ICRS - the International Cannabinoid Research Society
- The Health and Psychological Effects of Cannabis Use (Australia - Monograph 44) - 2001 at Department of Health and Ageing (Australia)
- Marijuana and Medicine - Assessing the Science Base (Institute of Medicine) - 1999 at National Academies Press
- Overview of the Endocannabinoid signalling System at endocannabinoid.net
- Chemical Ecology of Cannabis (J. Intl. Hemp Assn. - 1994) at hempfood.com
- Therapeutic Potential in Spotlight at Cannabinoid Researchers' Meeting at California Cannabis Research Medical Group
- Medicinal marijuana laws, policies and news at cannabishq.com
Cited Sources
- ↑ Lambert DM, Fowler CJ (2005). "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". J. Med. Chem. 48 (16): 5059–87. doi:10.1021/jm058183t. PMID 16078824.
- ↑ Huffman JW (2000). "The search for selective ligands for the CB2 receptor". Curr. Pharm. Des. 6 (13): 1323–37. PMID 10903395.
- ↑ "British Journal of Pharmacology - Abstract of article: Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependent G-protein activation in rat cerebellar membranes". Retrieved 2007-06-24.
- ↑ "N-Acylethanolamines in Seeds. Quantification of Molecular Species and Their Degradation upon Imbibition -- Chapman et al. 120 (4): 1157 -- PLANT PHYSIOLOGY". Retrieved 2007-06-24.
- ↑ "ScienceDirect - Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism : Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs: Possible implications for mollusc physiology and sea food industry". Retrieved 2007-06-24.
- ↑ Hanus L, Abu-Lafi S, Fride E; et al. (2001). "2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor". Proc. Natl. Acad. Sci. U.S.A. 98 (7): 3662–5. doi:10.1073/pnas.061029898. PMID 11259648.
- ↑ Nicoll RA, Alger BE (2004). "The brain's own marijuana". Sci. Am. 291 (6): 68–75. PMID 15597982.
- ↑ http://www.marijuana.org/mydna10-12-05.htm
- ↑ "British Journal of Pharmacology - Abstract of article: Evidence that the plant cannabinoid [Delta]9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist". Retrieved 2007-06-24.
- ↑ 10.0 10.1 "Behavioural Pharmacology - Abstract: Volume 16(5-6) September 2005 p 487-496 Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids". Retrieved 2007-06-24.
References
- Elsohly MA, Slade D (2005). "Chemical constituents of marijuana: the complex mixture of natural cannabinoids". Life Sci. 78 (5): 539–48. doi:10.1016/j.lfs.2005.09.011. PMID 16199061.
- Hanus L (1987). "Biogenesis of cannabinoid substances in the plant". Acta Universitatis Palackianae Olomucensis Facultatis Medicae. 116: 47–53. PMID 2962461.
- Hanuš L., Krejčí Z. Isolation of two new cannabinoid acids from Cannabis sativa L. of Czechoslovak origin. Acta Univ. Olomuc., Fac. Med. 74, 161-166 (1975)
- Hanuš L., Krejčí Z., Hruban L. Isolation of cannabidiolic acid from Turkish variety of cannabis cultivated for fibre. Acta Univ. Olomuc., Fac. Med. 74, 167-172 (1975)
- Devane WA, Hanus L, Breuer A; et al. (1992). "Isolation and structure of a brain constituent that binds to the cannabinoid receptor". Science. 258 (5090): 1946–9. PMID 1470919.
- Hanus L, Gopher A, Almog S, Mechoulam R (1993). "Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor" (PDF). J. Med. Chem. 36 (20): 3032–4. PMID 8411021.
- Mechoulam R, Ben-Shabat S, Hanus L; et al. (1995). "Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors". Biochem. Pharmacol. 50 (1): 83–90. PMID 7605349.
cs:Endocannabinoidy
de:Cannabinoide
it:Cannabinoidi
nl:Cannabinoïde
fi:Kannabinoidi
sv:Cannabinoid
Template:Jb1